Heterotrophic Plate Count for Bottled Water Safety Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water Analysis
2.2. HPC Isolation and Identification
2.3. Assessment of Bacterial Adhesion
2.4. Statistical Methods
3. Results and Discussion
3.1. Bacteriological Analysis of Processed Water and Bottled Water
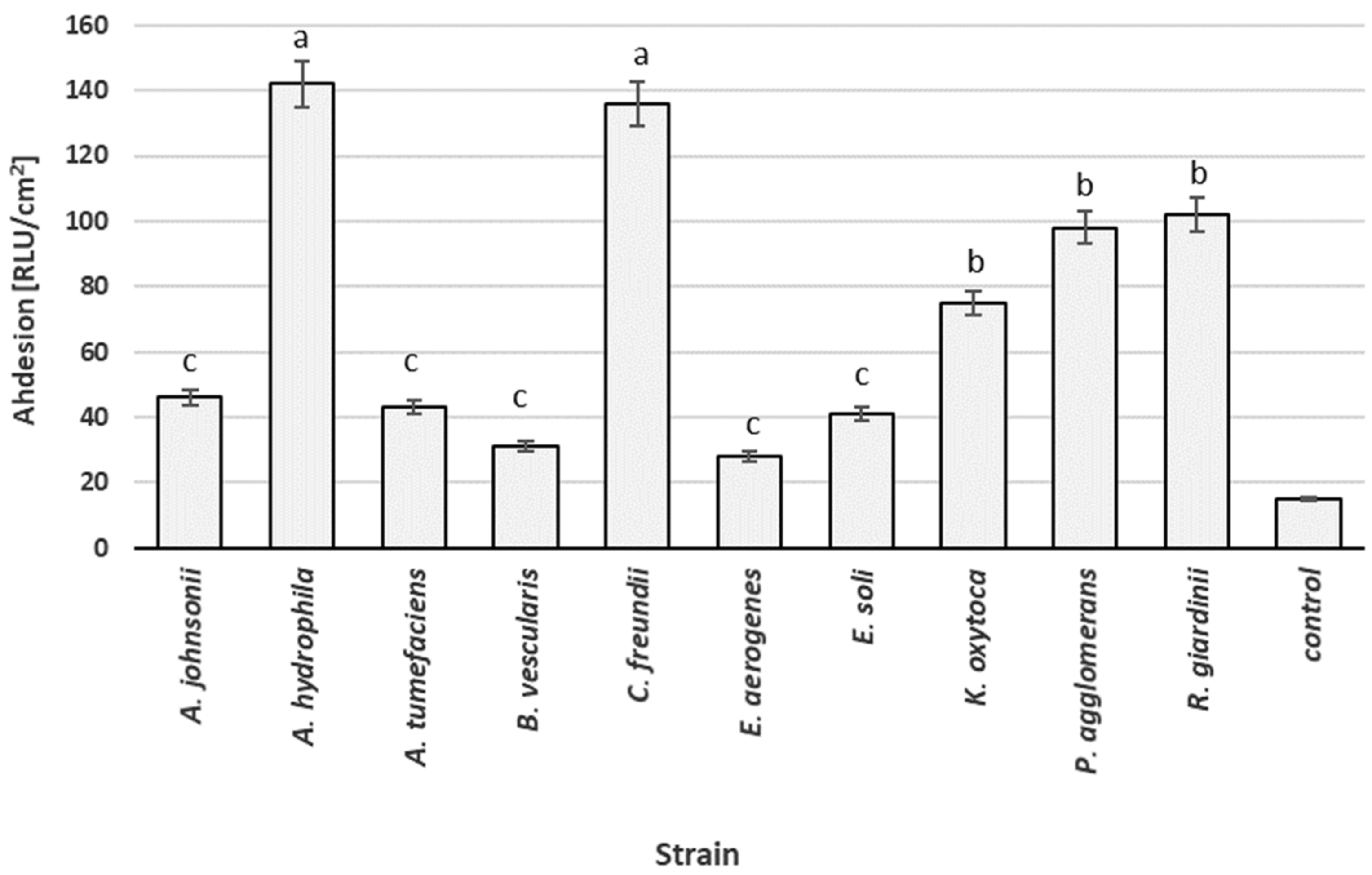
3.2. Adhesion Abilities
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Natural Waters: The Natural Choice for Hydration. Available online: https://www.efbw.org (accessed on 8 May 2020).
- Rosenberg, F.A. The microbiology of bottled water. Clin. Microbiol. Newsl. 2003, 25, 41–44. [Google Scholar] [CrossRef]
- Farhadkhani, M.; Nikaeen, M.; Akbari Adergani, B.; Hatamzadeh, M.; Nabavi, B.F.; Hassanzadeh, A. Assessment of drinking water quality from bottled water coolers. Iran J. Public Health. 2014, 43, 674–681. [Google Scholar] [PubMed]
- Heterotrophic Plate Count Measurement in Drinking Water Safety Management. Available online: https://www.who.int/water_sanitation_health/dwq/WSH02.10.pdf (accessed on 8 May 2020).
- Douterelo, I.; Boxall, J.B.; Deines, P.; Sekar, R.; Fish, K.E.; Biggs, C.A. Methodological approaches for studying the microbial ecology of drinking water distribution systems. Water Res. 2014, 65, 134–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diduch, M.; Polkowska, Ż.; Namieśnik, J. The role of heterotrophic plate count bacteria in bottled water quality assessment. Food Control 2016, 61, 188–195. [Google Scholar] [CrossRef]
- Kregiel, D.; Otlewska, A.; Antolak, H. Attachment of Asaia bogorensis originating in fruit-flavored water to packaging materials. Biomed. Res. Int. 2014, 2014, 514190. [Google Scholar] [CrossRef]
- Kregiel, D.; Antolak, H. Growth of Asaia spp. in flavored mineral water—evaluation of the volumetric “bottle effect”. Int. J. Food Process. Technol. 2016, 3, 62–65. [Google Scholar] [CrossRef] [Green Version]
- Zamberlan da Silva, M.E.; Santana, R.G.; Guilhermetti, M.; Filho, I.C.; Endo, E.H.; Ueda-Nakamura, T.; Nakamura, C.V.; Filho, B.P.D. Comparison of the bacteriological quality of tap water and bottled mineral water. Int. J. Hyg. Environ. Health 2008, 211, 504–509. [Google Scholar] [CrossRef]
- Fujioka, T.; Ueyama, T.; Mingliang, F.; Leddy, M. Online assessment of sand filter performance for bacterial removal in a full-scale drinking water treatment plant. Chemosphere 2019, 229, 509–514. [Google Scholar] [CrossRef]
- Directive 2009/54/EC of the European Parliament and of the Council on the Exploitation and Marketing of Natural Mineral Waters. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009L0054 (accessed on 8 May 2020).
- Kręgiel, D.; Rygała, A.; Libudzisz, Z.; Walczak, P.; Oltuszak-Walczak, E. Asaia lannensisthe spoilage acetic acid bacteria isolated from strawberry-flavored bottled water in Poland. Food Control 2012, 26, 147–150. [Google Scholar] [CrossRef]
- Kręgiel, D. Adhesion of Aeromonas hydrophila to modified glass surfaces with organosilanes. Food Technol. Biotechnol. 2013, 51, 345–351. [Google Scholar]
- Kregiel, D.; Niedzielska, K. Effect of plasma processing and organosilane modifications of polyethylene on Aeromonas hydrophila biofilm formation. BioMed Res. Int. 2014, 2014, 23251. [Google Scholar] [CrossRef]
- Heterotrophic Plate Counts and Drinking-Water Safety. The Significance of HPCs for Water Quality and Human Health. Available online: http://www.who.int/water_sanitation_health/dwq/HPCFull.pdf (accessed on 15 May 2020).
- Wang, F.; Li, W.; Zhang, J.; Qi, W.; Zhou, Y.; Xiang, Y.; Shi, N. Characterization of suspended bacteria from processing units in an advanced drinking water treatment plant of China. Environ. Sci. Pollut. Res. 2017, 24, 12176–12184. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Li, W.; Zhang, J.; Qi, W.; Lu, Y.; Ding, Z. Coupling changes of disinfectant and bacteria induced by the water stagnation and disinfection strategy. Chemosphere 2020, 242, 125190. [Google Scholar] [CrossRef] [PubMed]
- Zlatanović, L.; van der Hoek, J.P.; Vreeburg, J.H.G. An experimental study on the influence of water stagnation and temperature change on water quality in a full-scale domestic drinking water system. Water Res. 2017, 123, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Inoue, A. Viability of sublethally injured coliform bacteria on fresh-cut cabbage stored in high CO2 atmospheres following rinsing with electrolyzed water. Int. J. Food Microbiol. 2018, 266, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Debs-Louka, E.; Louka, N.; Abraham, G.; Chabot, V.; Allaf, K. Effect of compressed carbon dioxide on microbial cell viability. Appl. Environ. Microbiol. 1999, 65, 626–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaz-Moreira, I.; Nunes, O.C.; Manaia, C.M. Ubiquitous and persistent Proteobacteria and other Gram-negative bacteria in drinking water. Sci. Total Environ. 2017, 586, 1141–1149. [Google Scholar] [CrossRef]
- Guentzel, M.N. Escherichia, Klebsiella, Enterobacter, Serratia, Citrobacter, and Proteus. In Source Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Krenek, P.; Samajova, O.; Luptovciak, I.; Doskocilova, A.; Komis, G.; Samaj, J. Transient plant transformation mediated by Agrobacterium tumefaciens: Principles, methods and applications. Biotechnol. Adv. 2015, 33, 1024–1042. [Google Scholar] [CrossRef]
- Padikasan, I.A.; Chinnannan, K.; Kumar, S.; Subramaniyan, G. Agricultural Biotechnology: Engineering Plants for Improved Productivity and Quality. In Omics Technologies and Bio-Engineering; Barh, D., Azevedo, V., Eds.; Academic Press: London, UK, 2018; Volume 2, pp. 87–104. [Google Scholar]
- Myszka, K.; Czaczyk, K. Effect of starvation stress on morphological changes and production of adhesive exopolysaccharide (EPS) by Proteus vulgaris. Acta Sci. Pol. Technol. Aliment. 2011, 10, 305–312. [Google Scholar]
- Simões, L.C.; Simões, M.; Vieira, M.J. Adhesion and biofilm formation on polystyrene by drinking water-isolated bacteria. Antonie Leeuwenhoek 2010, 98, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Kregiel, D. Attachment of Asaia lannensis to materials commonly used in beverage industry. Food Control 2013, 32, 537–542. [Google Scholar] [CrossRef]
- Priority Pathogens List for R&D of New Antibiotics. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed on 5 May 2020).
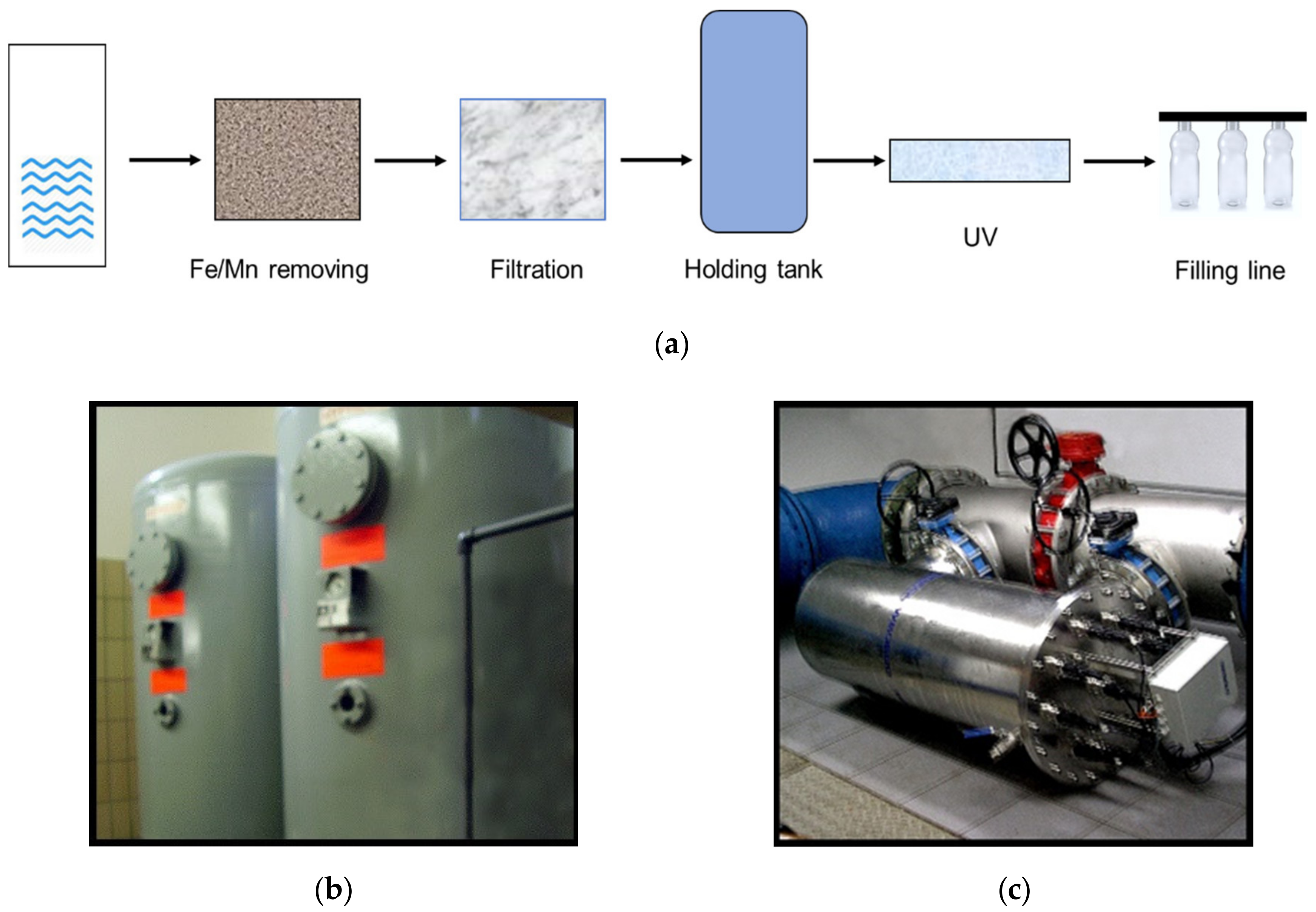
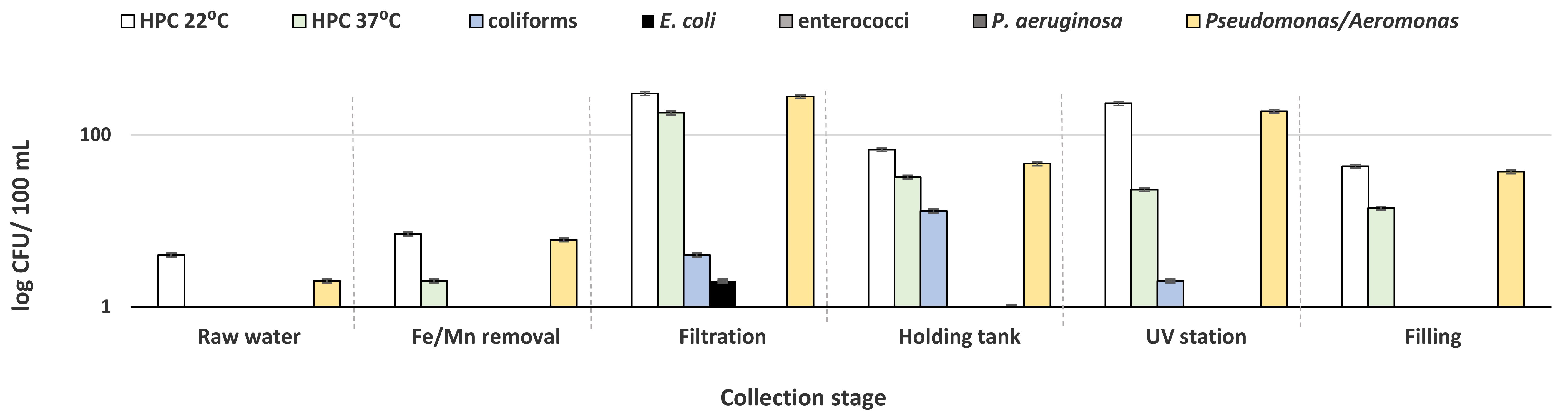
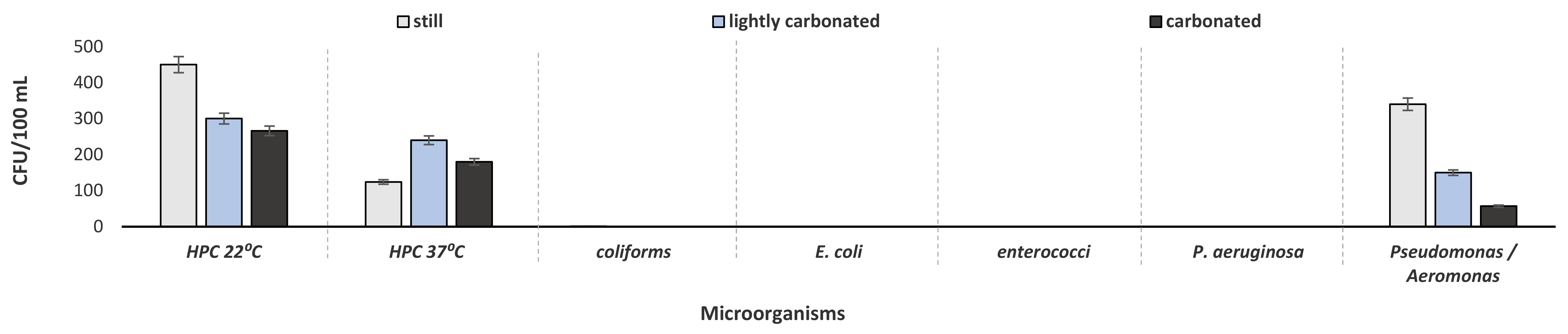
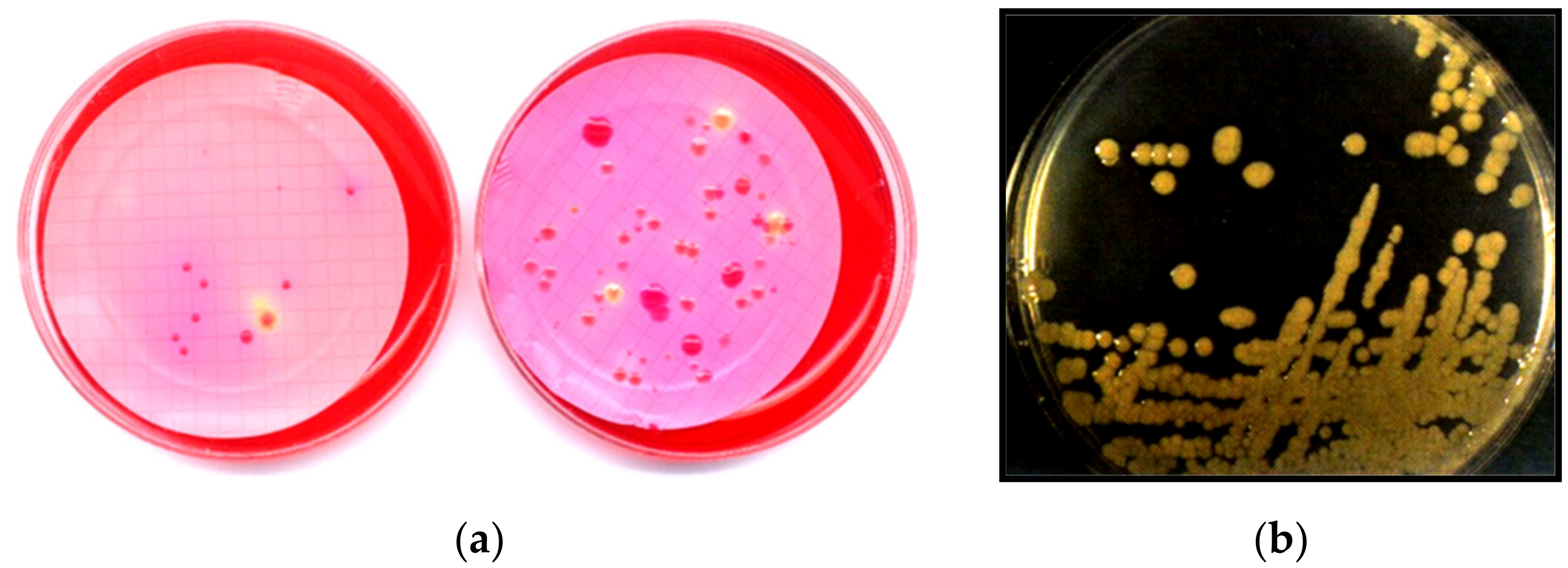
| Collection Stage | No of Samples |
|---|---|
| Raw water | 3 |
| Clarification (Fe/Mn removal) | 3 |
| Filtration | 3 |
| Holding tank | 5 |
| UV station | 5 |
| Filling | 4 |
| Detection | Agar Medium | Temperature [°C] | Time [Day] |
|---|---|---|---|
| HPC | R2 Agar, TSA Agar | 22, 37 | 3, 2 |
| Coliforms/E. coli | Chromocult Coliform Agar | 37 | 2 |
| Enterococci | Slanetz-Bartley Agar | 37 | 2 |
| P. aeruginosa | Cetrimide Agar | 37 | 2 |
| Pseudomonas /Aeromonas sp. | GSP Agar | 25 | 1 |
| Isolate | Cell Shape | Gram | Oxidase | GenBank Number |
|---|---|---|---|---|
| A. johnsonii | 1 R | 2 N | N | KT751294 |
| A. hydrophila | R | N | 3 P | KC756842 |
| A. tumefaciens | R | N | P | KJ719245 |
| B. vesicularis | R | N | P | KT751295 |
| C. freundii | R | N | N | KJ995856 |
| E. aerogenes | R | N | N | KJ995857 |
| E. soli | R | N | N | KJ995858 |
| K. oxytoca | R | N | N | KJ995859 |
| P. agglomerans | R | N | N | KJ995860 |
| R. giardinii | R | N | P | KT751297 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rygala, A.; Berlowska, J.; Kregiel, D. Heterotrophic Plate Count for Bottled Water Safety Management. Processes 2020, 8, 739. https://doi.org/10.3390/pr8060739
Rygala A, Berlowska J, Kregiel D. Heterotrophic Plate Count for Bottled Water Safety Management. Processes. 2020; 8(6):739. https://doi.org/10.3390/pr8060739
Chicago/Turabian StyleRygala, Anna, Joanna Berlowska, and Dorota Kregiel. 2020. "Heterotrophic Plate Count for Bottled Water Safety Management" Processes 8, no. 6: 739. https://doi.org/10.3390/pr8060739
APA StyleRygala, A., Berlowska, J., & Kregiel, D. (2020). Heterotrophic Plate Count for Bottled Water Safety Management. Processes, 8(6), 739. https://doi.org/10.3390/pr8060739







