Kinetics of Alkyl Lactate Formation from the Alcoholysis of Poly(Lactic Acid)
Abstract
1. Introduction
2. Materials & Methods
2.1. Materials
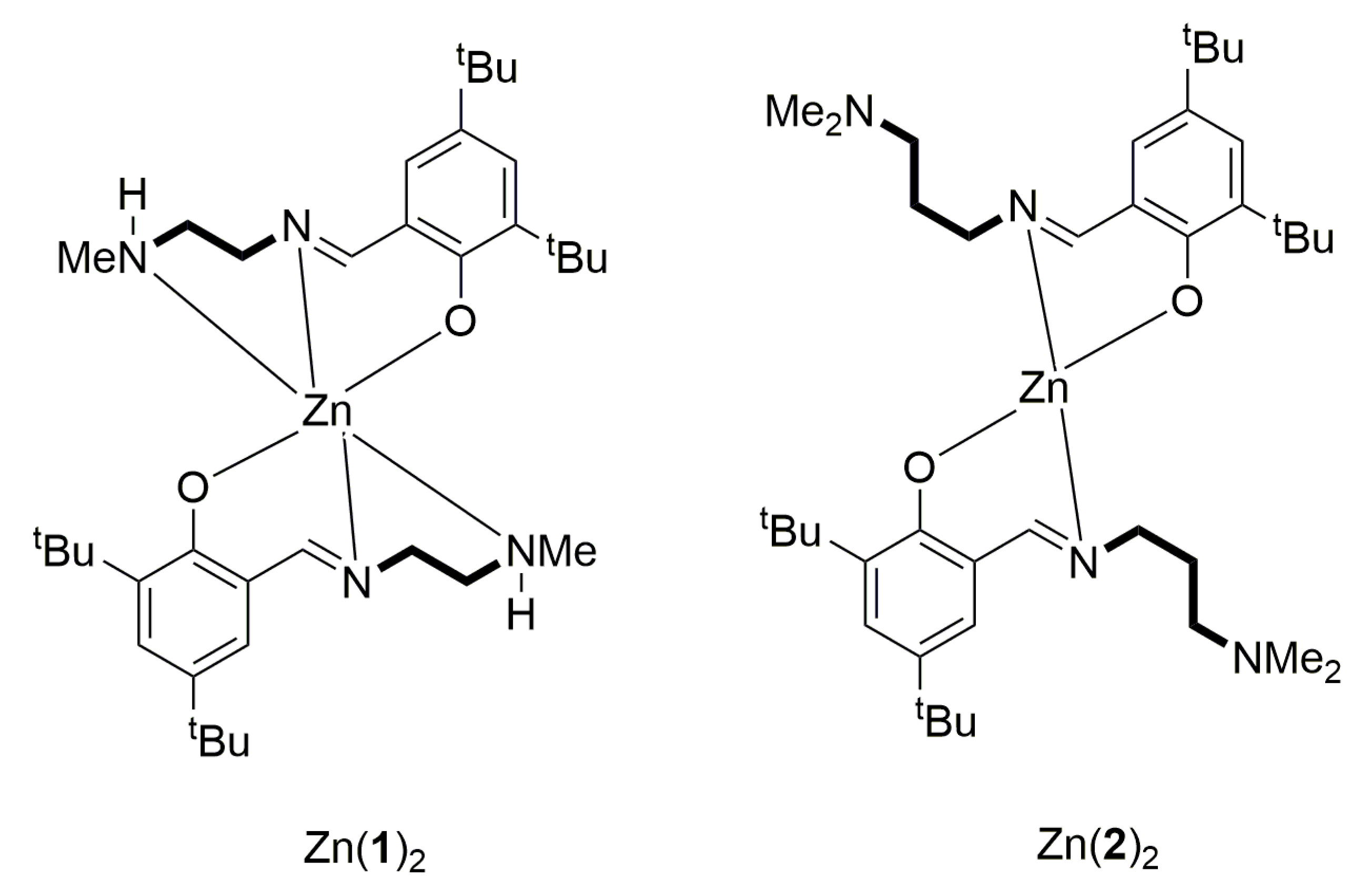
2.2. Catalyst Preparation
2.3. Apparatus and Procedure
2.4. GC and NMR
2.5. Kinetic Modelling
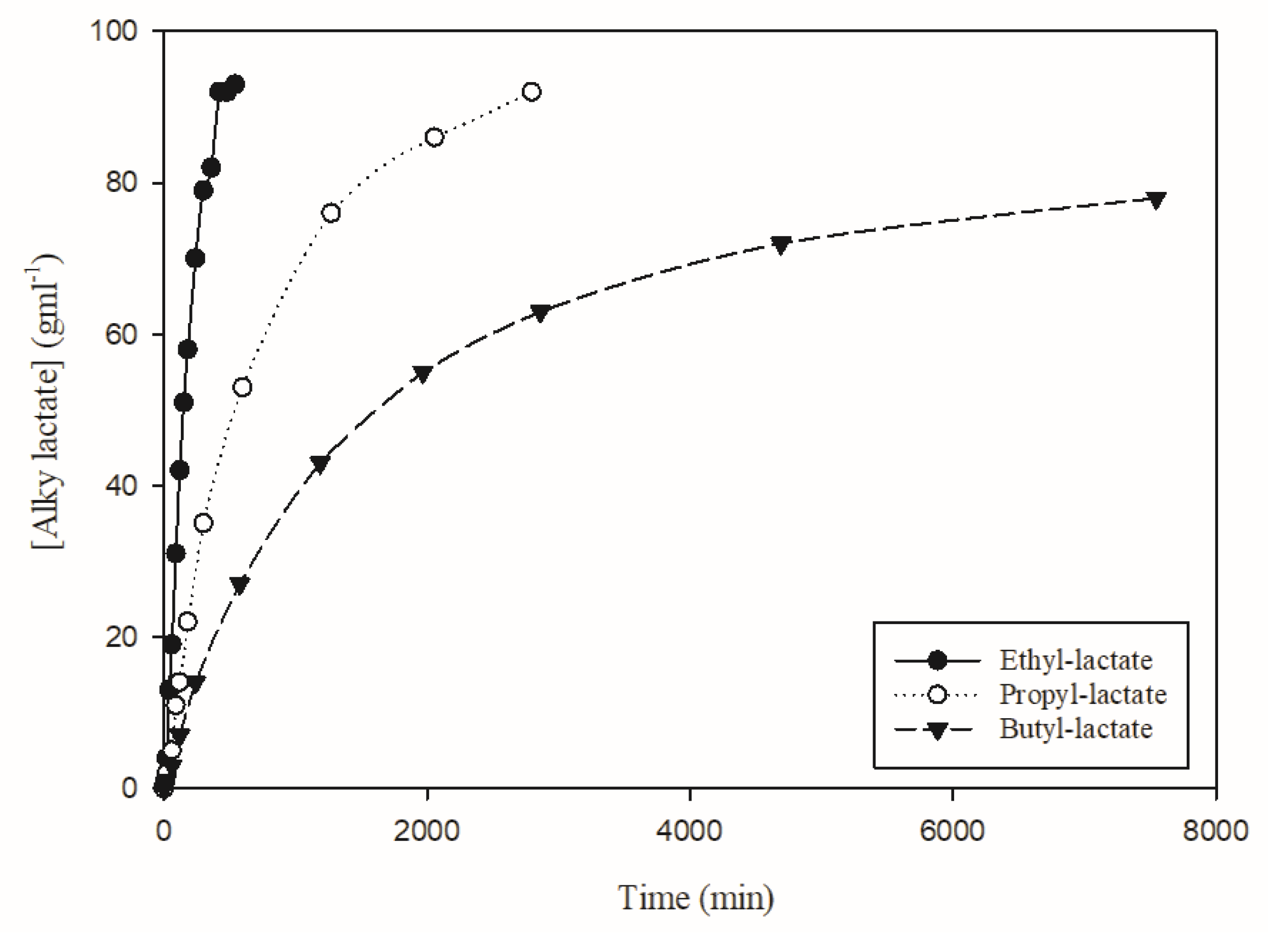
3. Results
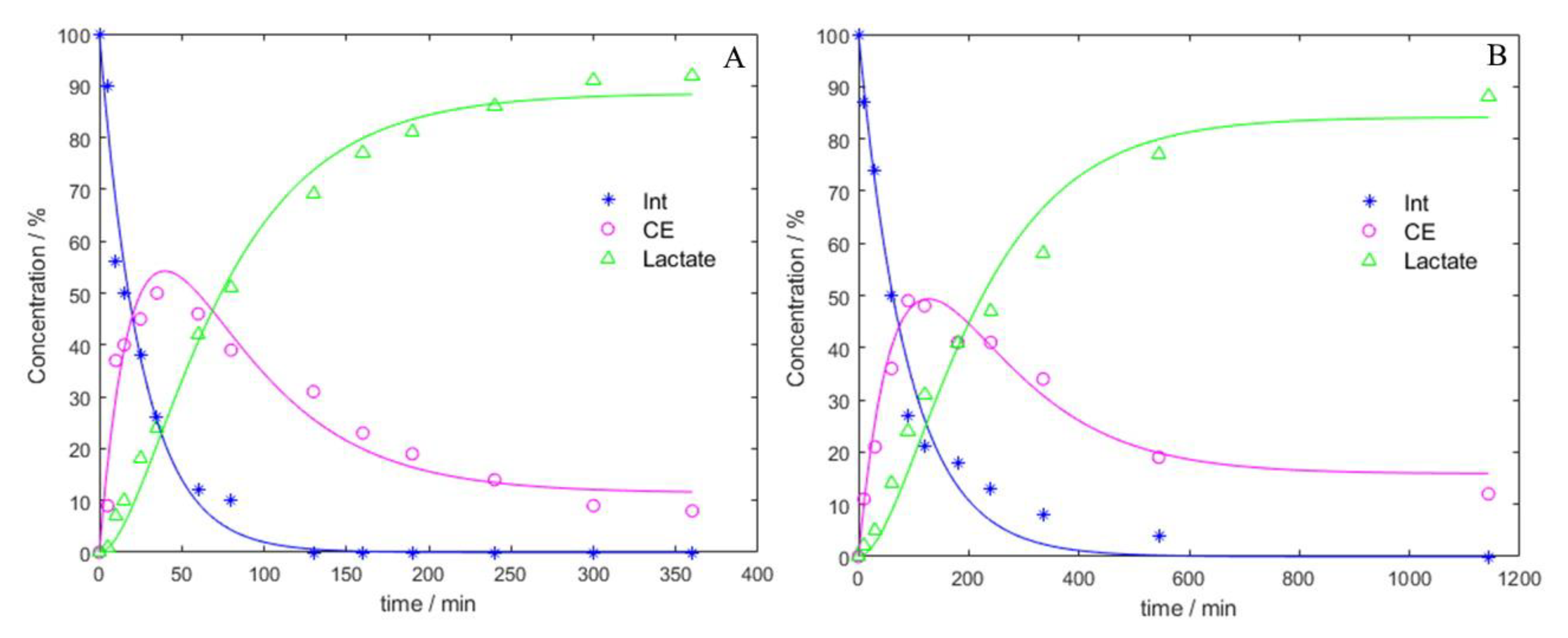
3.1. NMR Results
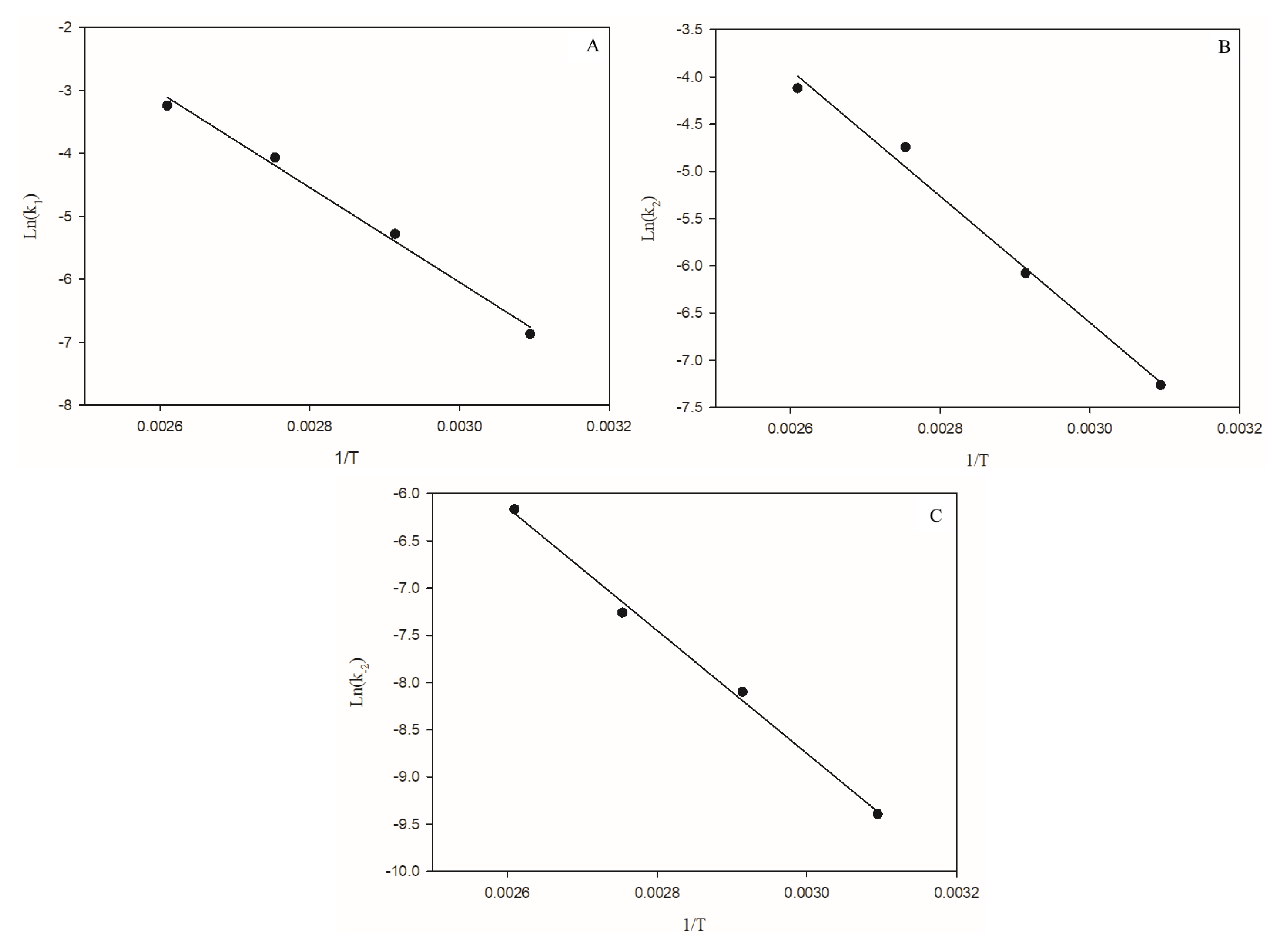
3.2. Arrhenius Temperature-Dependent Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data
References
- Calvo-Flores, F.G.; Monteagudo-Arrebola, M.J.; Dobado, J.A.; Isac-García, J. Green and Bio-Based Solvents. Top. Curr. Chem. 2018, 376, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.C.; Cannon, A.S.; Dye, K.M. Green chemistry. Environ. Impact Assess. Rev. 2004, 24, 775–799. [Google Scholar] [CrossRef]
- Jessop, P.G. Searching for green solvents. Green Chem. 2011, 13, 1391–1398. [Google Scholar] [CrossRef]
- Clary, J.J.; Feron, V.J.; Van Velthuijsen, J.A. Safety assessment of lactate esters. Regul. Toxicol. Pharmacol. 1998, 27, 88–97. [Google Scholar] [CrossRef]
- Bowmer, C.T.; Hooftman, R.N.; Hanstveit, A.O.; Venderbosch, P.W.M.; Van Der Hoeven, N. The ecotoxicity and the biodegradability of lactic acid, alkyl lactate esters and lactate salts. Chemosphere 1998, 37, 1317–1333. [Google Scholar] [CrossRef]
- Zuriaga, E.; Giner, B.; Ribate, M.P.; García, C.B.; Lomba, L. Exploring the usefulness of key green physicochemical properties: Quantitative structure–activity relationship for solvents from biomass. Environ. Toxicol. Chem. 2018, 37, 1014–1023. [Google Scholar] [CrossRef]
- Biddy, M.J.; Scarlata, C.J.; Kinchin, C.M. Chemicals from biomass: A market assessment of bioproducts with near-term potential. NREL Rep. 2016. [Google Scholar] [CrossRef]
- 360 Market Updates Global Ethyl Lactate Market 2019 by Manufacturers, Regions, Type and Application, Forecast to 2024. Available online: https://www.360marketupdates.com/global-ethyl-lactate-market-13806819 (accessed on 21 May 2020).
- Dorosz, U.; Barteczko, N.; Latos, P.; Erfurt, K.; Pankalla, E.; Chrobok, A. Highly efficient biphasic system for the synthesis of alkyl lactates in the presence of acidic ionic liquids. Catalysts 2020, 10, 37. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- Kua, Y.L.; Gan, S.; Morris, A.; Ng, H.K. Ethyl lactate as a potential green solvent to extract hydrophilic (polar) and lipophilic (non-polar) phytonutrients simultaneously from fruit and vegetable by-products. Sustain. Chem. Pharm. 2016, 4, 21–31. [Google Scholar] [CrossRef]
- Kua, Y.L.; Gan, S.; Morris, A.; Ng, H.K. Liquid-Liquid Equilibrium Data for the Ternary Systems of Palm Oil + Ethyl Lactate + Phytonutrients (Carotenes and Tocols) at 303.15 K. Int. J. Chem. Eng. Appl. 2017, 8, 215–220. [Google Scholar] [CrossRef]
- Kua, Y.L.; Gan, S.; Morris, A.; Ng, H.K. Simultaneous Recovery of Carotenes and Tocols from Crude Palm Olein Using Ethyl Lactate and Ethanol. J. Phys. Conf. Ser. 2018, 989. [Google Scholar] [CrossRef]
- Planer, S.; Jana, A.; Grela, K. Ethyl Lactate: A Green Solvent for Olefin Metathesis. ChemSusChem 2019, 12, 4655–4661. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Inaba, K.; Sumida, H.; Kurihara, K.; Tochigi, K.; Ochi, K. Vapor-liquid equilibria of binary and ternary mixtures containing ethyl lactate and effect of ethyl lactate as entrainer. Fluid Phase Equilib. 2016, 420, 50–57. [Google Scholar] [CrossRef]
- EPA Propyl L-Lactate. Available online: https://comptox.epa.gov/dashboard/dsstoxdb/results?search=DTXSID3042344#exposure (accessed on 23 June 2020).
- Woo, D.; Shin, J.; Sam, M.; Bae, W.; Kim, H. High-pressure phase behavior of propyl lactate and butyl lactate in supercritical carbon dioxide. J. Chem. Thermodyn. 2012, 47, 177–182. [Google Scholar] [CrossRef]
- Zheng, S.; Cheng, H.; Chen, L.; Qi, Z. Feasibility of bio-based lactate esters as extractant for biobutanol recovery: (Liquid + liquid) equilibria. J. Chem. Thermodyn. 2016, 93, 127–131. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Pinho, S.P.; Silva, V.M.T.M.; Rodrigues, A.E. Thermodynamic equilibrium and reaction kinetics for the esterification of lactic acid with ethanol catalyzed by acid ion-exchange resin. Ind. Eng. Chem. Res. 2008, 47, 1453–1463. [Google Scholar] [CrossRef]
- Stadler, B.M.; Wulf, C.; Werner, T.; Tin, S.; De Vries, J.G. Catalytic Approaches to Monomers for Polymers Based on Renewables. ACS Catal. 2019, 9, 8012–8067. [Google Scholar] [CrossRef]
- Solas, M.; Suárez-Pantiga, S.; Sanz, R. Ethyl lactate as a renewable carbonyl source for the synthesis of diynones. Green Chem. 2019, 21, 213–218. [Google Scholar] [CrossRef]
- Dusselier, M.; Van Wouwe, P.; Dewaele, A.; Makshina, E.; Sels, B.F. Lactic acid as a platform chemical in the biobased economy: The role of chemocatalysis. Energy Environ. Sci. 2013, 6, 1415–1442. [Google Scholar] [CrossRef]
- Bykowski, D.; Grala, A.; Sobota, P. Conversion of lactides into ethyl lactates and value-added products. Tetrahedron Lett. 2014, 55, 5286–5289. [Google Scholar] [CrossRef]
- Shuklov, I.A.; Dubrovina, N.V.; Kühlein, K.; Börner, A. Chemo-Catalyzed Pathways to Lactic Acid and Lactates. Adv. Synth. Catal. 2016, 358, 3910–3931. [Google Scholar] [CrossRef]
- Grala, A.; Ejfler, J.; Jerzykiewicz, L.B.; Sobota, P. Chemoselective alcoholysis of lactide mediated by a magnesium catalyst: An efficient route to alkyl lactyllactate. Dalt. Trans. 2011, 40, 4042–4044. [Google Scholar] [CrossRef] [PubMed]
- Maki-Arvela, P.; Simakova, I.L.; Salmi, T.; Murzin, D.Y. Production of Lactic Acid/Lactates from Biomass and Their Catalytic Transformations to Commodities. Chem. Rev. 2014, 114, 1909–1971. [Google Scholar] [CrossRef] [PubMed]
- Vivian, A.; Fusaro, L.; Debecker, D.P.; Aprile, C. Mesoporous Methyl-Functionalized Sn-Silicates Generated by the Aerosol Process for the Sustainable Production of Ethyl Lactate. ACS Sustain. Chem. Eng. 2018, 6, 14095–14103. [Google Scholar] [CrossRef]
- Cosate de Andrade, M.F.; Souza, P.M.S.; Cavalett, O.; Morales, A.R. Life Cycle Assessment of Poly(Lactic Acid) (PLA): Comparison Between Chemical Recycling, Mechanical Recycling and Composting. J. Polym. Environ. 2016, 24, 372–384. [Google Scholar] [CrossRef]
- Song, X.; Zhang, X.; Wang, H.; Liu, F.; Yu, S.; Liu, S. Methanolysis of poly(lactic acid) (PLA) catalyzed by ionic liquids. Polym. Degrad. Stab. 2013, 98, 2760–2764. [Google Scholar] [CrossRef]
- Petrus, R.; Bykowski, D.; Sobota, P. Solvothermal Alcoholysis Routes for Recycling Polylactide Waste as Lactic Acid Esters. ACS Catal. 2016, 6, 5222–5235. [Google Scholar] [CrossRef]
- Alberti, C.; Damps, N.; Meißner, R.R.R.; Enthaler, S. Depolymerization of End-of-Life Poly(lactide) via 4-Dimethylaminopyridine-Catalyzed Methanolysis. ChemistrySelect 2019, 4, 6845–6848. [Google Scholar] [CrossRef]
- Alberti, C.; Damps, N.; Meißner, R.R.R.; Hofmann, M.; Rijono, D.; Enthaler, S. Selective Degradation of End-of-Life Poly (lactide) via Alkali-Metal-Halide Catalysis. Adv. Sustain. Syst. 2020, 4, 1900081–1900090. [Google Scholar] [CrossRef]
- Mckeown, P.; Roman-Ramírez, L.A.; Bates, S.; Wood, J. Zinc Complexes for PLA Formation and Chemical Recycling: Towards a Circular Economy. ChemSusChem 2019, 12, 5233–5238. [Google Scholar] [CrossRef] [PubMed]
- Román-Ramírez, L.A.; Mckeown, P.; Jones, M.D.; Wood, J. Poly(lactic acid) degradation into methyl lactate catalyzed by a well-defined Zn(II) complex. ACS Catal. 2019, 9, 409–416. [Google Scholar] [CrossRef]
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Kolstad, J.J.; Vink, E.T.H.; De Wilde, B.; Debeer, L. Assessment of anaerobic degradation of Ingeo polylactides under accelerated landfill conditions. Polym. Degrad. Stab. 2012, 97, 1131–1141. [Google Scholar] [CrossRef]
- Shogren, R.; Wood, D.; Orts, W.; Glenn, G. Plant-based materials and transitioning to a circular economy. Sustain. Prod. Consum. 2019, 19, 194–215. [Google Scholar] [CrossRef]
- Volodymr, B.; Varvarin, A.; Svetlana, L.; Ya, G. Vapor-phase synthesis of lactide from ethyl lactate over TiO2/SiO2 catalyst. Ukr. Chem. J. 2019, 85. [Google Scholar] [CrossRef]
- De Clercq, R.; Dusselier, M.; Makshina, E.; Sels, B.F. Catalytic Gas-Phase Production of Lactide from Renewable Alkyl Lactates. Angew. Chemie Int. Ed. 2018, 57, 3074–3078. [Google Scholar] [CrossRef]
- De Clercq, R.; Dusselier, M.; Poleunis, C.; Debecker, D.P.; Giebeler, L.; Oswald, S.; Makshina, E.; Sels, B.F. Titania-Silica Catalysts for Lactide Production from Renewable Alkyl Lactates: Structure-Activity Relations. ACS Catal. 2018, 8, 8130–8139. [Google Scholar] [CrossRef]
- Upare, P.P.; Hwang, Y.K.; Chang, J.S.; Hwang, D.W. Synthesis of lactide from alkyl lactate via a prepolymer route. Ind. Eng. Chem. Res. 2012, 51, 4837–4842. [Google Scholar] [CrossRef]
- Egiazaryan, T.A.; Makarov, V.M.; Moskalev, M.V.; Razborov, D.A.; Fedushkin, I.L. Synthesis of lactide from alkyl lactates catalyzed by lanthanide salts. Mendeleev Commun. 2019, 29, 648–650. [Google Scholar] [CrossRef]
- Payne, J.; Mckeown, P.; Mahon, M.F.; Emanuelsson, E.A.C.; Jones, M.D. Mono- and dimeric zinc(II) complexes for PLA production and degradation into methyl lactate—A chemical recycling method. Polym. Chem. 2020, 11, 2381–2389. [Google Scholar] [CrossRef]
- Román-Ramírez, L.A.; McKeown, P.; Shah, C.; Abraham, J.; Jones, M.D.; Wood, J. Chemical Degradation of End-of-Life Poly(lactic acid) into Methyl Lactate by a Zn(II) Complex. Ind. Eng. Chem. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Román-Ramírez, L.A.; McKeown, P.; Jones, M.D.; Wood, J. Kinetics of Methyl Lactate Formation from the Transesterification of Polylactic Acid Catalyzed by Zn(II) Complexes. ACS Omega 2020, 5, 5556–5564. [Google Scholar] [CrossRef] [PubMed]
- Alaerts, L.; Augustinus, M.; Van Acker, K. Impact of bio-based plastics on current recycling of plastics. Sustainability 2018, 10, 1487. [Google Scholar] [CrossRef]
- Cornell, D.D. Biopolymers in the existing postconsumer plastics recycling stream. J. Polym. Environ. 2007, 15, 295–299. [Google Scholar] [CrossRef]
- McKeown, P.; McCormick, S.N.; Mahon, M.F.; Jones, M.D. Highly active Mg(ii) and Zn(ii) complexes for the ring opening polymerisation of lactide. Polym. Chem. 2018, 9, 5339–5347. [Google Scholar] [CrossRef]
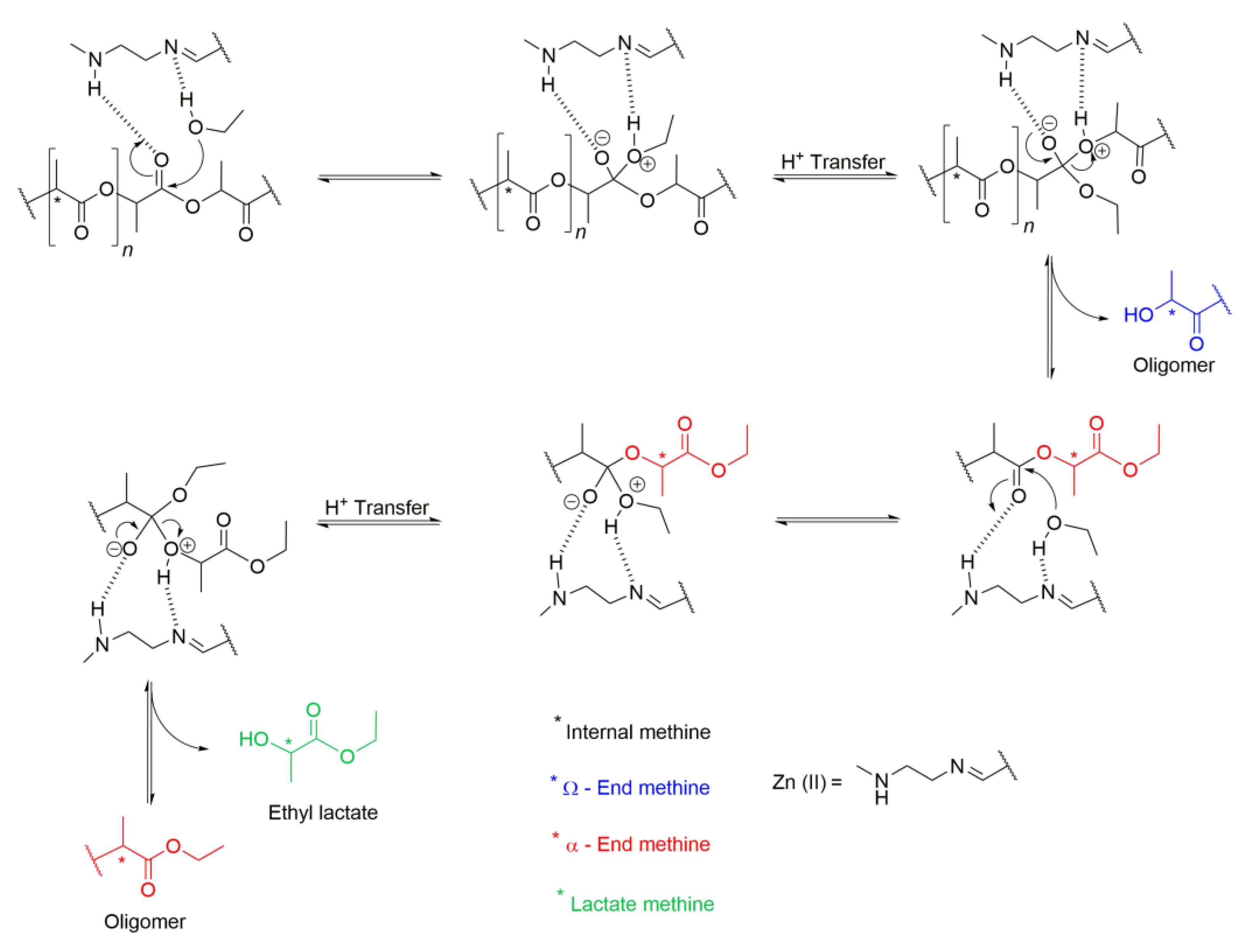
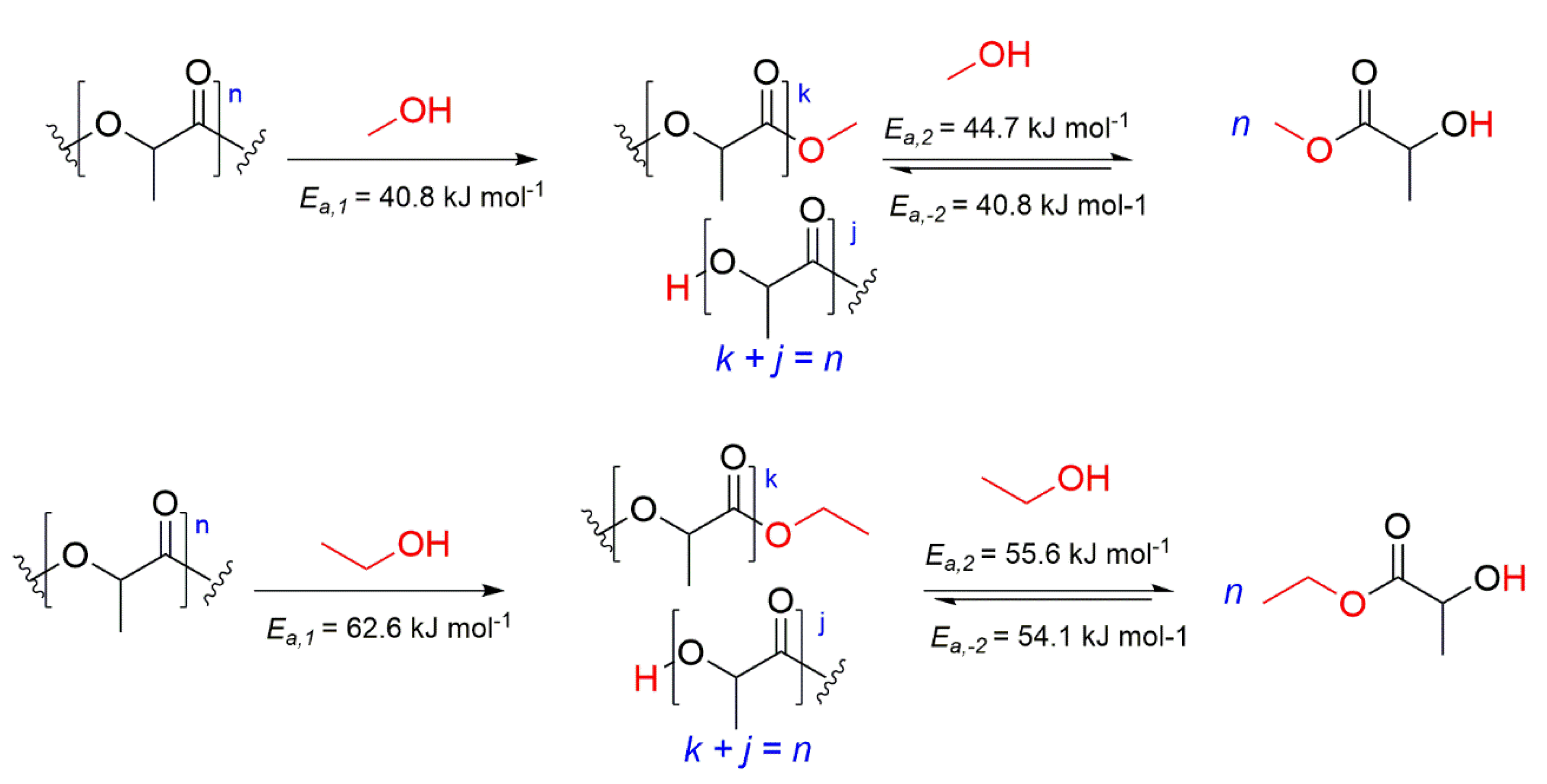
| Alcohol | Temperature (°C) | Alky Lactate Yield (%) | Final Time (min) | Time Taken to Reach 61% AL (min) | Initial Reaction Rate (g·mL−1·min−1) |
|---|---|---|---|---|---|
| EtOH | 50 | 81 | 4650 | 2748 | 6.66 × 10−6 |
| EtOHa | 50 | 86 | 1350 | 110 | 4.13 × 10−4 |
| EtOH | 70 | 91 | 1920 | 775 | 6 × 10−5 |
| EtOHa | 70 | 79 | 8700 | 5452 | 7.33 × 10−5 |
| EtOH | 90 | 93 | 540 | 195 | 2.8 × 10−4 |
| EtOHa | 90 | 83 | 38 70 | 1840 | 5.33 × 10−5 |
| EtOH | 110 | 96 | 420 | 108 | 4.53 × 10−4 |
| EtOHa | 110 | 96 | 1440 | 504 | 1.13 × 10−4 |
| PrOHa | 50 | 72 | 1509 | 194 | 4.35 × 10−4 |
| PrOH | 90 | 92 | 2796 | 828 | 8.25 × 10−5 |
| PrOH | 110 | 91 | 1260 | 343 | 2.1 × 10−4 |
| PrOHa | 110 | 61 | 1428 | 1428 | 3.75 × 10−5 |
| PrOH | 130 | 92 | 590 | 207 | 3 × 10−4 |
| BuOH | 90 | 78 | 7548 | 2647 | 5.8 × 10−5 |
| BuOH | 110 | 88 | 1143 | 369 | 2.56 × 10−4 |
| BuOHa | 110 | 75 | 1986 | 1286 | 3.3 × 10−5 |
| BuOH | 130 | 89 | 600 | 225 | 3.3 × 10−4 |
| Alcohol | Temp (°C) | k1 (min−1) | k2 (min−1) | k−2 (min−1) |
|---|---|---|---|---|
| EtOH | 110 | 0.0392 ± 0.0034 | 0.0163 ± 0.0021 | 0.0021 ± 0.0009 |
| EtOH | 90 | 0.0171 ± 0.0008 | 0.0087 ± 0.0005 | 0.0007 ± 0.0002 |
| EtOH | 70 | 0.0051 ± 0.0004 | 0.0023 ± 0.0003 | 0.0003 ± 0.0002 |
| EtOH | 50 | 0.0010 ± 0.0006 | 0.0007 ± 0.0008 | 0.0001 ± 0.0006 |
| PrOH | 130 | 0.0197 ± 0.0010 | 0.0081 ± 0.0006 | 0.0009 ± 0.0004 |
| PrOH | 110 | 0.0139 ± 0.0009 | 0.0049 ± 0.0005 | 0.0007 ± 0.0003 |
| PrOH | 90 | 0.0062 ± 0.0006 | 0.0028 ± 0.0005 | 0.0005 ± 0.0002 |
| BuOH | 130 | 0.0170 ± 0.0010 | 0.0072 ± 0.0006 | 0.0010 ± 0.0004 |
| BuOH | 110 | 0.0111 ± 0.0010 | 0.0061 ± 0.0010 | 0.0012 ± 0.0006 |
| BuOH | 90 | 0.0031 ± 0.0005 | 0.0011 ± 0.0003 | 0.0004 ± 0.0002 |
| Alcoholysis | Ea1 (kJ/mol) | Ea2 (kJ/mol) | Ea−2 (kJ/mol) |
|---|---|---|---|
| MeOH | 40.8 ± 2.3 | 44.7 ± 2.8 | 40.8 ± 21.1 |
| EtOH | 62.58 ± 16.94 | 55.61 ± 17.72 | 54.11 ± 10.92 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamberti, F.M.; Román-Ramírez, L.A.; Mckeown, P.; Jones, M.D.; Wood, J. Kinetics of Alkyl Lactate Formation from the Alcoholysis of Poly(Lactic Acid). Processes 2020, 8, 738. https://doi.org/10.3390/pr8060738
Lamberti FM, Román-Ramírez LA, Mckeown P, Jones MD, Wood J. Kinetics of Alkyl Lactate Formation from the Alcoholysis of Poly(Lactic Acid). Processes. 2020; 8(6):738. https://doi.org/10.3390/pr8060738
Chicago/Turabian StyleLamberti, Fabio M., Luis A. Román-Ramírez, Paul Mckeown, Matthew D. Jones, and Joseph Wood. 2020. "Kinetics of Alkyl Lactate Formation from the Alcoholysis of Poly(Lactic Acid)" Processes 8, no. 6: 738. https://doi.org/10.3390/pr8060738
APA StyleLamberti, F. M., Román-Ramírez, L. A., Mckeown, P., Jones, M. D., & Wood, J. (2020). Kinetics of Alkyl Lactate Formation from the Alcoholysis of Poly(Lactic Acid). Processes, 8(6), 738. https://doi.org/10.3390/pr8060738








