Process Optimization of Palm Oil Mill Effluent-Based Biosurfactant of Halomonas meridiana BK-AB4 Originated from Bledug Kuwu Mud Volcano in Central Java for Microbial Enhanced Oil Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Media and Culture Conditions
2.3. Adaptation of H. meridiana BK-AB4 in POME
2.4. Preparation and Characterization of Palm Oil Mill Effluent (POME)
2.5. Optimization of Biosurfactant Production
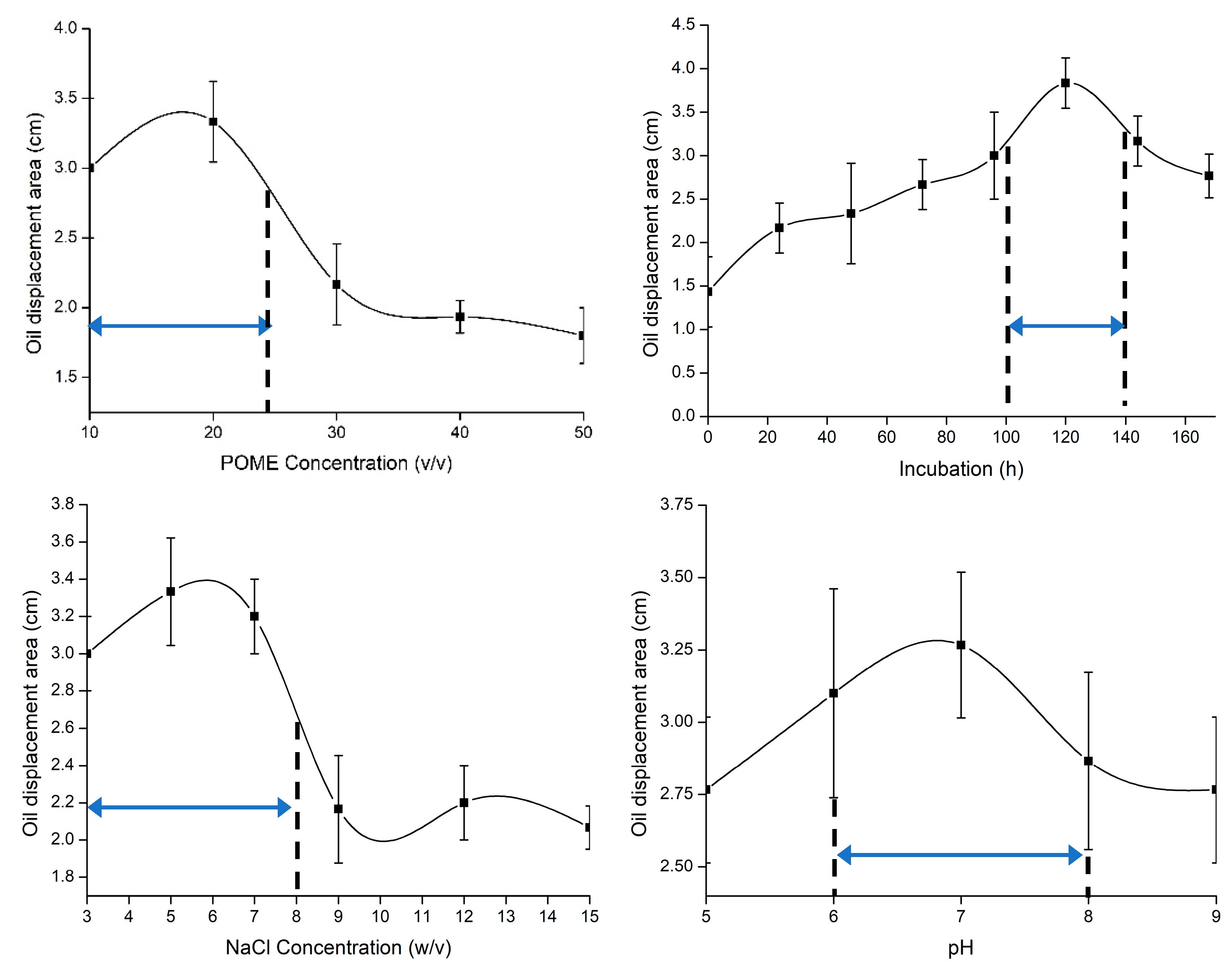
2.5.1. Single Factor Experiment (SFE)
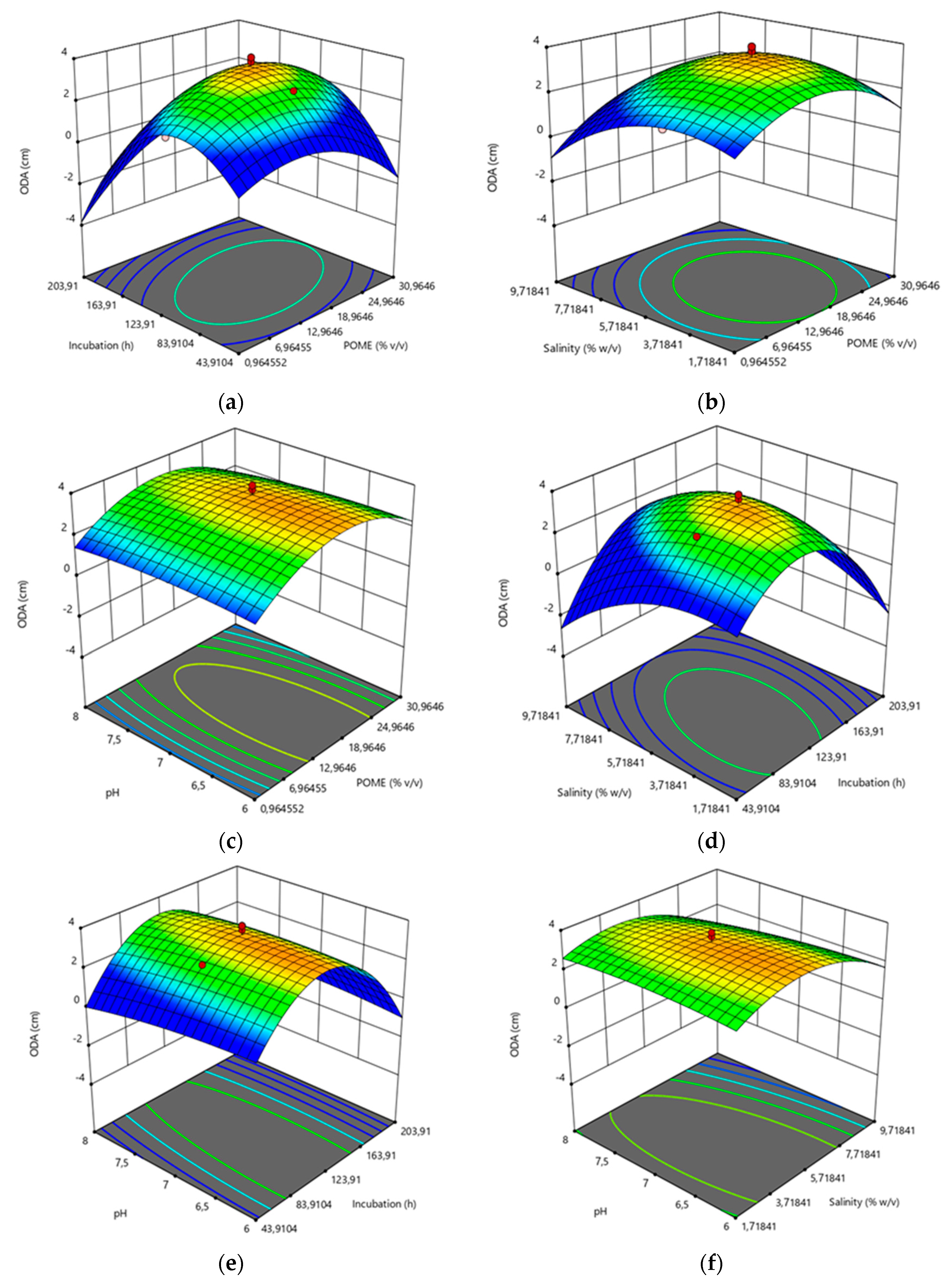
2.5.2. Response Surface Methodology (RSM)
2.6. Biosurfactant Extraction
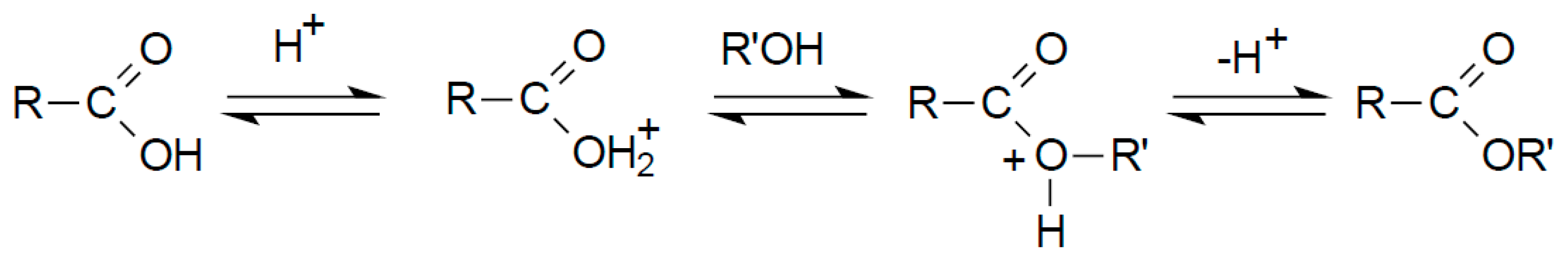
2.7. Identification of the Biosurfactants Structure
2.8. Surface Properties
2.8.1. Surface Tension (ST) and Critical Micelle Concentration (CMC)
2.8.2. Interfacial Tension (IFT)
2.8.3. Emulsification Index (E24)
2.9. Enhanced Oil Recovery Experiment
3. Results and Discussion
3.1. Characterization of POME
3.2. Optimization of Biosurfactant by Response Surface Methodology
3.3. Structural Characterization
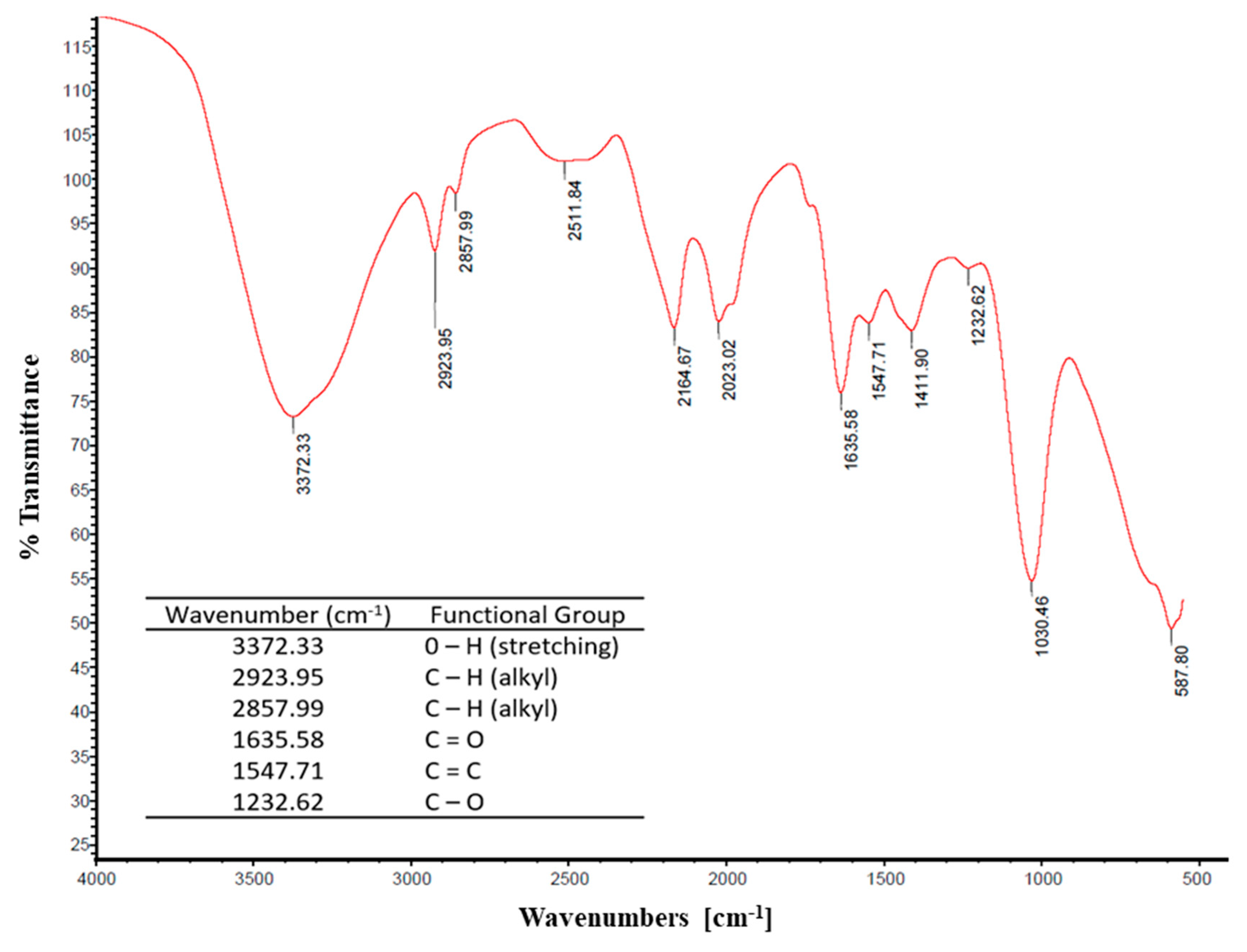
3.3.1. FTIR Analysis of Biosurfactant Molecule
3.3.2. LC-MS Analysis of Biosurfactant Molecule
3.4. Analysis of Physico-Chemical Properties of Biosurfactant
3.5. Imbibition Test Result
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| API | American Petroleum Institute |
| CCD | Central composite design |
| CMC | Critical micelle concentration |
| E24 | Emulsification Index |
| EOR | Enhanced oil recovery |
| FAMEs | Fatty acid methyl esters |
| IFT | Interfacial tension |
| MEOR | Microbial enhanced oil recovery |
| ODA | Oil displacement area |
| OOIP | Original oil in place |
| OST | Oil spreading test |
| POME | Palm oil mill effluent |
| RSM | Response surface methodology |
References
- Azarmi, R.; Ashjaran, A. Type and application of some common surfactants. J. Chem. Pharm. Res. 2015, 7, 632–640. [Google Scholar]
- Gudiña, E.J.; Pereira, J.F.B.; Rodrigues, L.R.; Coutinho, J.A.P.; Teixeira, J.A. Isolation and study of microorganisms from oil samples for application in Microbial Enhanced Oil Recovery. Int. Biodeterior. Biodegrad. 2012, 68, 56–64. [Google Scholar] [CrossRef]
- Sari, I.P.; Basyiruddin, M.I.; Hertadi, R. Bioconversion of palm oil into biosurfactant by Halomonas meridiana BK-AB4 for the application of corrosion inhibitor. Indones. J. Chem. 2018, 18, 718–723. [Google Scholar] [CrossRef]
- Patel, J.; Borgohain, S.; Kumar, M.; Rangarajan, V.; Somasundaran, P.; Sen, R. Recent developments in microbial enhanced oil recovery. Renew. Sustain. Energy Rev. 2015, 52, 1539–1558. [Google Scholar] [CrossRef]
- Tang, G.Q.; Morrow, N.R. Salinity, temperature, oil composition, and oil recovery by waterflooding. SPE Reserv. Eng. 1997, 12, 269–276. [Google Scholar] [CrossRef]
- Siegert, M.; Sitte, J.; Galushko, A.; Krueger, M. Starting up microbial enhanced oil recovery. Plant Cells 2013, 142, 1–94. [Google Scholar] [CrossRef]
- Edbeib, M.F.; Wahab, R.A.; Huyop, F. Halophiles: Biology, adaptation, and their role in decontamination of hypersaline environments. World J. Microbiol. Biotechnol. 2016, 32, 135. [Google Scholar] [CrossRef]
- James, S.; Dobson, S.J.; Franzmann, P.D.; McMeekin, T. Halomonas meridiana, a New Species of Extremely Halotolerant Bacteria Isolated from Antarctic Saline Lakes. Syst. Appl. Microbiol. 1990, 13, 270–278. [Google Scholar] [CrossRef]
- Asy’ari, M.; Parwata, I.P.; Aditiawati, P.P.; Akhmaloka, A.; Hertadi, R. Isolation and identification of halostable lipase producing bacteria from the Bledug Kuwu mud crater located at Purwodadi-Grobogan, Central Java, Indonesia. J. Pure Appl. Microbiol. 2014, 8, 3387–3396. [Google Scholar]
- Mazzini, A.; Etiope, G. Mud volcanism: An updated review. Earth Sci. Rev. 2017, 168, 81–112. [Google Scholar] [CrossRef]
- Firdaus, N.; Prasetyo, B.T.; Sofyan, Y.; Siregar, F. Part I of II: Palm oil mill effluent (POME): Biogas power plant. Distrib. Gener. Altern. Energy J. 2017, 32, 73–79. [Google Scholar] [CrossRef]
- Iskandar, M.J.; Baharum, A.; Anuar, F.H.; Othaman, R. Palm oil industry in South East Asia and the effluent treatment technology—A review. Environ. Technol. Innov. 2018, 9, 169–185. [Google Scholar] [CrossRef]
- Gozan, M.; Aulawy, N.; Rahman, S.F.; Budiarto, R. Techno-Economic analysis of biogas power plant from POME (Palm Oil Mill Effluent). Int. J. Appl. Eng. Res. 2018, 13, 6151–6157. [Google Scholar]
- Salihu, A.; Alam, M.Z.; Karim, M.A.; Hamzah, M.S. Characterization of Candida cylindracea lipase produced from palm oil mill effluent based medium. Int. J. Chem. Biochem. Sci. 2012, 2, 24–31. [Google Scholar]
- Cheng, J.; Zhu, X.; Ni, J.; Borthwick, A. Palm oil mill effluent treatment using a Two-Stage microbial fuel cells system integrated with immobilized biological aerated filters. Bioresour. Technol. 2010, 101, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Kusrini, E.; Lukita, M.; Gozan, M.; Susanto, B.H.; Widodo, T.W.; Nasution, D.A.; Wu, S.; Rahman, A.; Siregar, Y.D.I. Biogas from palm oil mill effluent: Characterization and removal of CO2 using modified clinoptilolite zeolites in a Fixed-Bed column. Int. J. Technol. 2016, 7, 625. [Google Scholar] [CrossRef]
- Radzuan, M.N.; Banat, I.M.; Winterburn, J. Production and characterization of rhamnolipid using palm oil agricultural refinery waste. Bioresour. Technol. 2017, 225, 99–105. [Google Scholar] [CrossRef]
- Morikawa, M.; Hirata, Y.; Imanaka, T. A study on the structure–Function relationship of lipopeptide biosurfactants. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2000, 1488, 211–218. [Google Scholar] [CrossRef]
- Sadhukhan, B.; Mondal, N.K.; Chattoraj, S. Optimisation using central composite design (CCD) and the desirability function for sorption of methylene blue from aqueous solution onto Lemna major. Karbala Int. J. Mod. Sci. 2016, 2, 145–155. [Google Scholar] [CrossRef]
- Chattoraj, S.; Mondal, N.K.; Das, B.; Roy, P.; Sadhukhan, B. Carbaryl removal from aqueous solution by Lemna major biomass using response surface methodology and artificial neural network. J. Environ. Chem. Eng. 2014, 2, 1920–1928. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Davis, R.; Mauer, L.J. Fourier transform infrared (FT-IR) spectroscopy: A rapid tool for detection and analysis of foodborne pathogenic bacteria. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 2, 1582–1594. [Google Scholar]
- Coates, J.P. The Interpretation of Infrared Spectra: Published Reference Sources. Appl. Spectrosc. Rev. 1996, 31, 179–192. [Google Scholar] [CrossRef]
- Solomons, T.W.G.; Fryhle, C.B. Organic Chemistry, 10th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011; pp. 779–821. [Google Scholar]
- Rufino, R.D.; De Luna, J.M.; De Campos-Takaki, G.M.; Sarubbo, L.A. Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron. J. Biotechnol. 2014, 17, 34–38. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-Active Agents from Two Bacillus Species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef]
- Christie, W.W. Preparation of ester derivatives of fatty acids for chromatographic analysis. Adv. Lipid Methodol. 1993, 2, 69–111. [Google Scholar]
- Abouseoud, M.; Maachi, R.; Amrane, A. Biosurfactant production from olive oil by Pseudomonas fluorescens. Comm. Curr. Res. Educ. Top. Trends Appl. Microbiol. 2007, 1, 340–347. [Google Scholar]
- Lam, M.-K.; Lee, K.T. Renewable and sustainable bioenergies production from palm oil mill effluent (POME): Win–win strategies toward better environmental protection. Biotechnol. Adv. 2011, 29, 124–141. [Google Scholar] [CrossRef]
- Emad, A.A. Production and purification of lipase from Pseudomonas sp. AB 2 with potential application in biodiesel production. J. Pure Appl. Microbiol. 2014, 8, 47–54. [Google Scholar]
- Hope, N.; Gideon, A. Biosurfactant production from palm oil mill effluent (POME) for applications as oil field chemical in Nigeria. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition; Society of Petroleum Engineers (SPE), Lagos, Nigeria, 4–6 August 2015. [Google Scholar]
- Singh, R.P.; Ibrahim, M.H.; Esa, N.; Iliyana, M.S. Composting of waste from palm oil mill: A sustainable waste management practice. Rev. Environ. Sci. Bio/Technol. 2010, 9, 331–344. [Google Scholar] [CrossRef]
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Derguine-Mecheri, L.; Kebbouche-Gana, S.; Khemili-Talbi, S.; Djenane, D. Screening and biosurfactant/bioemulsifier production from a high-salt-tolerant halophilic Cryptococcus strain YLF isolated from crude oil. J. Pet. Sci. Eng. 2018, 162, 712–724. [Google Scholar] [CrossRef]
- Satpute, S.K.; Banpurkar, A.G.; Dhakephalkar, P.K.; Banat, I.M.; Chopade, B.A. Methods for investigating biosurfactants and bioemulsifiers: A review. Crit. Rev. Biotechnol. 2010, 30, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Santhappan, R.; Pandian, M.R. Characterization of novel biosurfactants produced by the strain Fusarium oxysporum. J. Bioremediat. Biodegrad. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- DeZiel, E.; Lépine, F.; Dennie, D.; Boismenu, D.; Mamer, O.; Villemur, R. Liquid chromatography/mass spectrometry analysis of mixtures of rhamnolipids produced by Pseudomonas aeruginosa strain 57RP grown on mannitol or naphthalene. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 1999, 1440, 244–252. [Google Scholar] [CrossRef]
- Pereira, J.F.B.; Gudiña, E.J.; Costa, R.; Vitorino, R.; Teixeira, J.A.; Coutinho, J.A.P.; Rodrigues, L.R. Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications. Fuel 2013, 111, 259–268. [Google Scholar] [CrossRef]
- Sarafin, Y.; Donio, M.B.S.; Velmurugan, S.; Babu, M.; Citarasu, T. Kocuria marina BS-15 a biosurfactant producing halophilic bacteria isolated from solar salt works in India. Saudi J. Biol. Sci. 2014, 21, 511–519. [Google Scholar] [CrossRef]
- Fazli, R.R.; Hertadi, R. Optimization of rhamnolipid production from bioconversion of palm oil mill effluent (POME) waste by Pseudomonas stutzeri BK-AB12 using response surface methodology. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bogor, Indonesia, 1–2 August 2018; Volume 209, p. 012024. [Google Scholar]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef]
- Astuti, D.I.; Purwasena, I.A.; Putri, R.E.; Amaniyah, M.; Sugai, Y. Screening and characterization of biosurfactant produced by Pseudoxanthomonas sp. G3 and its applicability for enhanced oil recovery. J. Pet. Explor. Prod. Technol. 2019, 9, 2279–2289. [Google Scholar] [CrossRef]
- Perfumo, A.; Rancich, I.; Banat, I.M. Possibilities and challenges for biosurfactants use in petroleum industry. In Advances in Experimental Medicine and Biology; Springer Science and Business Media LLC: Berlin, Germany, 2010; Volume 672, pp. 135–145. [Google Scholar]
- De, S.; Malik, S.; Ghosh, A.; Saha, R. A review on natural surfactants. RSC Adv. 2015, 5, 65757–65767. [Google Scholar] [CrossRef]
- Khajepour, H.; Mahmoodi, M.; Biria, D.; Ayatollahi, S. Investigation of wettability alteration through relative permeability measurement during MEOR process: A micromodel study. J. Pet. Sci. Eng. 2014, 120, 10–17. [Google Scholar] [CrossRef]
- Ghojavand, H.; Vahabzadeh, F.; Shahraki, A.K. Enhanced oil recovery from low permeability dolomite cores using biosurfactant produced by a Bacillus mojavensis (PTCC 1696) isolated from Masjed-I Soleyman field. J. Pet. Sci. Eng. 2012, 81, 24–30. [Google Scholar] [CrossRef]
- Câmara, J.M.; Sousa, M.A.S.B.; Neto, E.L.B.; Oliveira, M.C.A. Application of rhamnolipid biosurfactant produced by Pseudomonas aeruginosa in microbial-enhanced oil recovery (MEOR). J. Pet. Explor. Prod. Technol. 2019, 9, 2333–2341. [Google Scholar] [CrossRef]
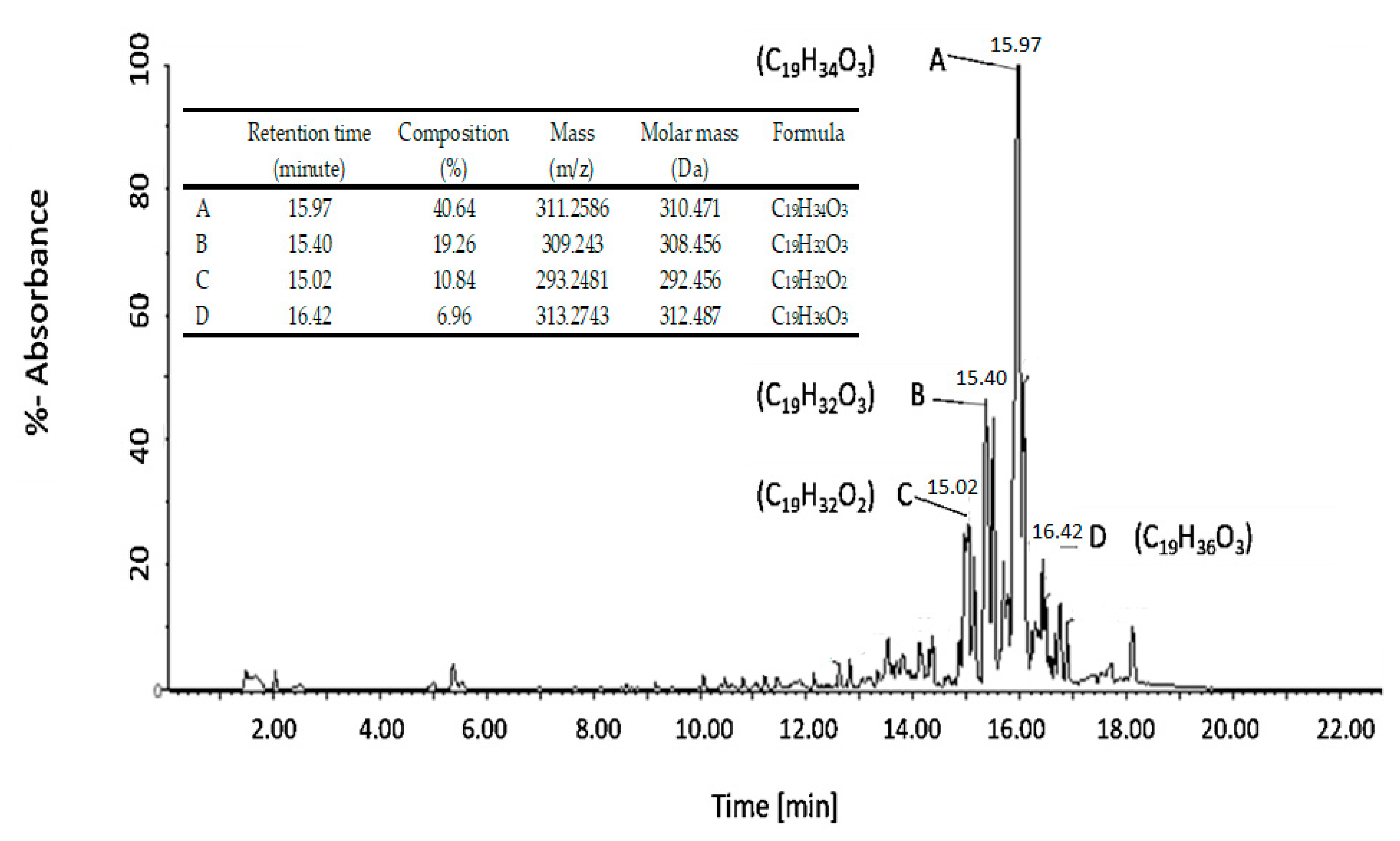
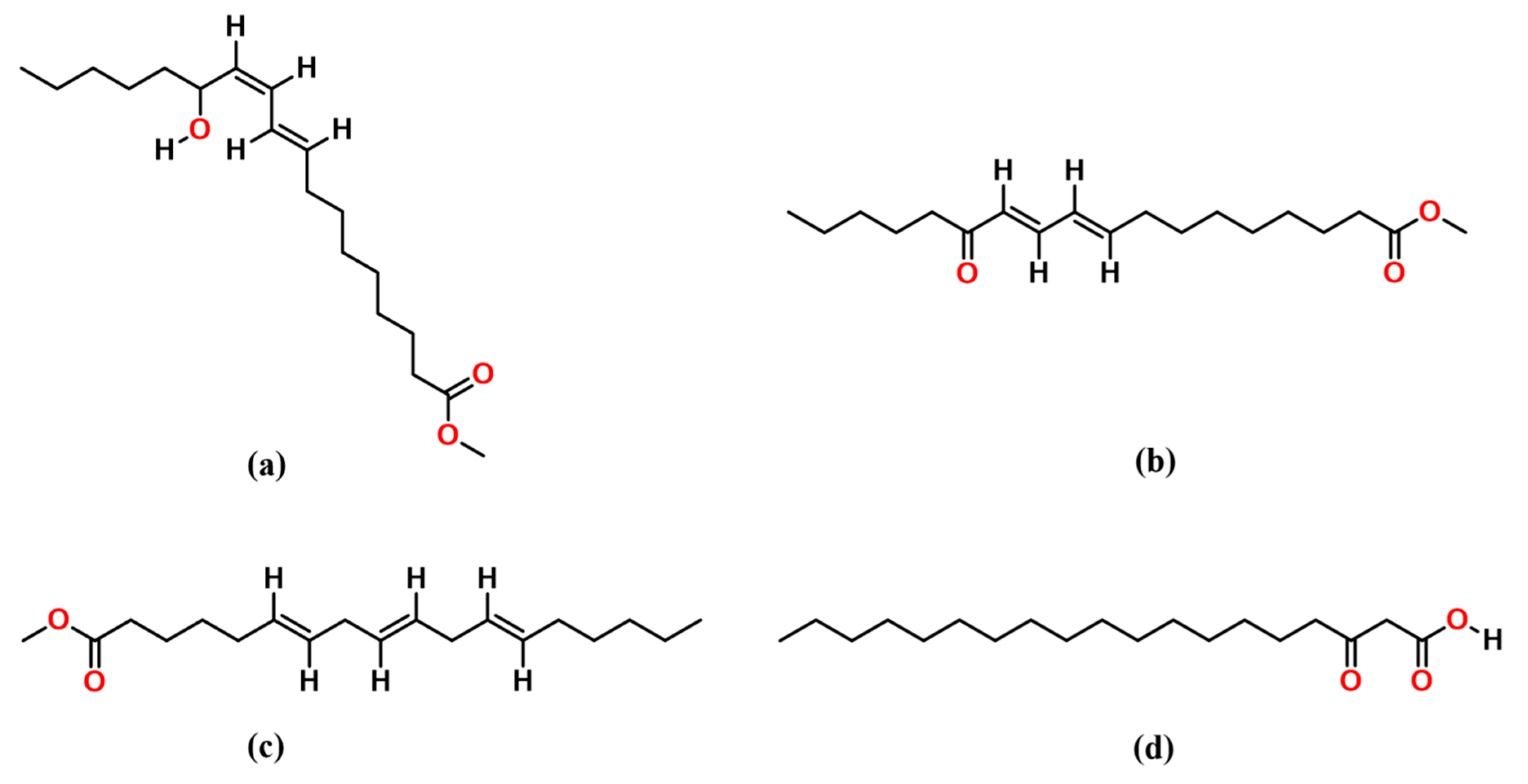
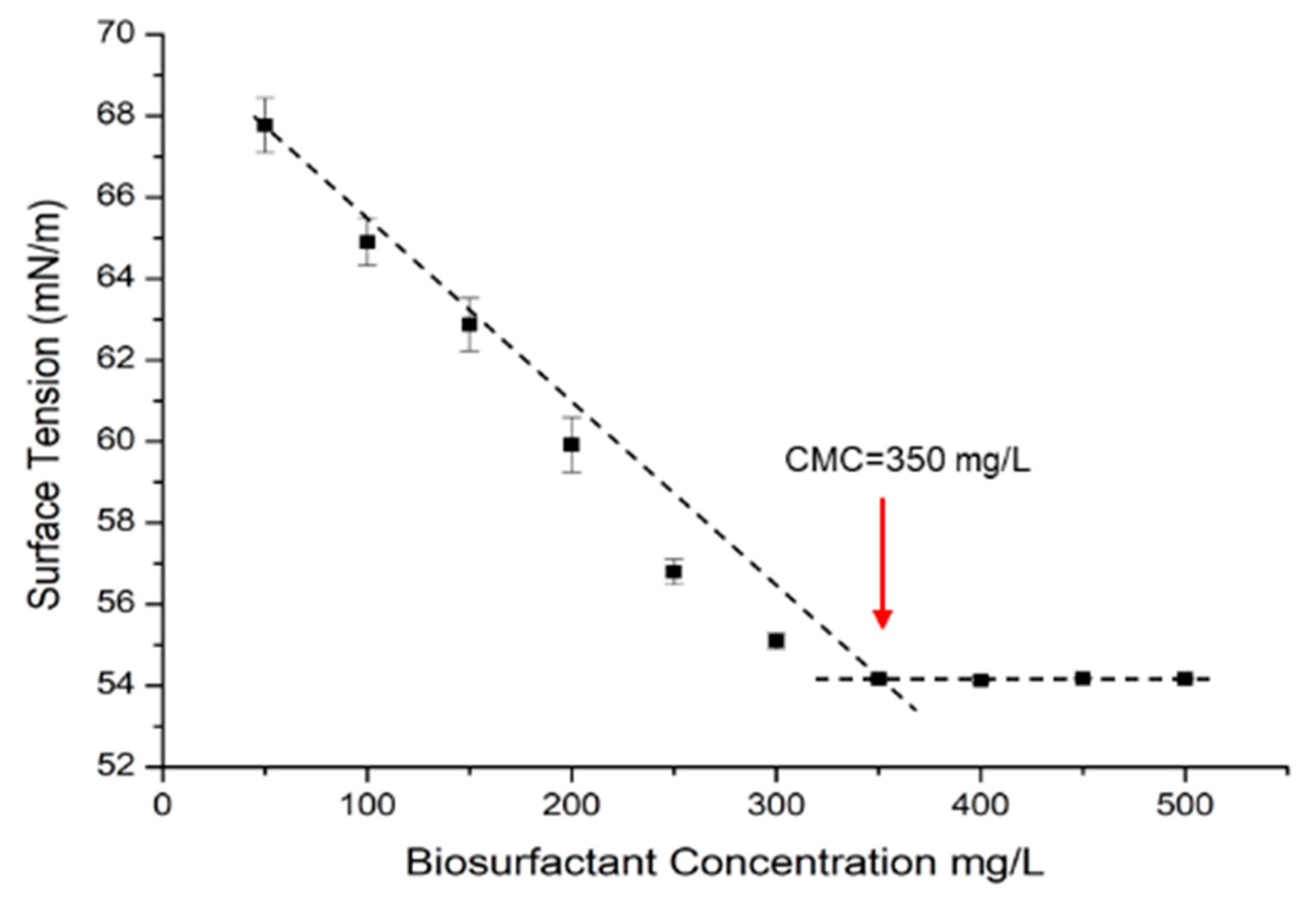
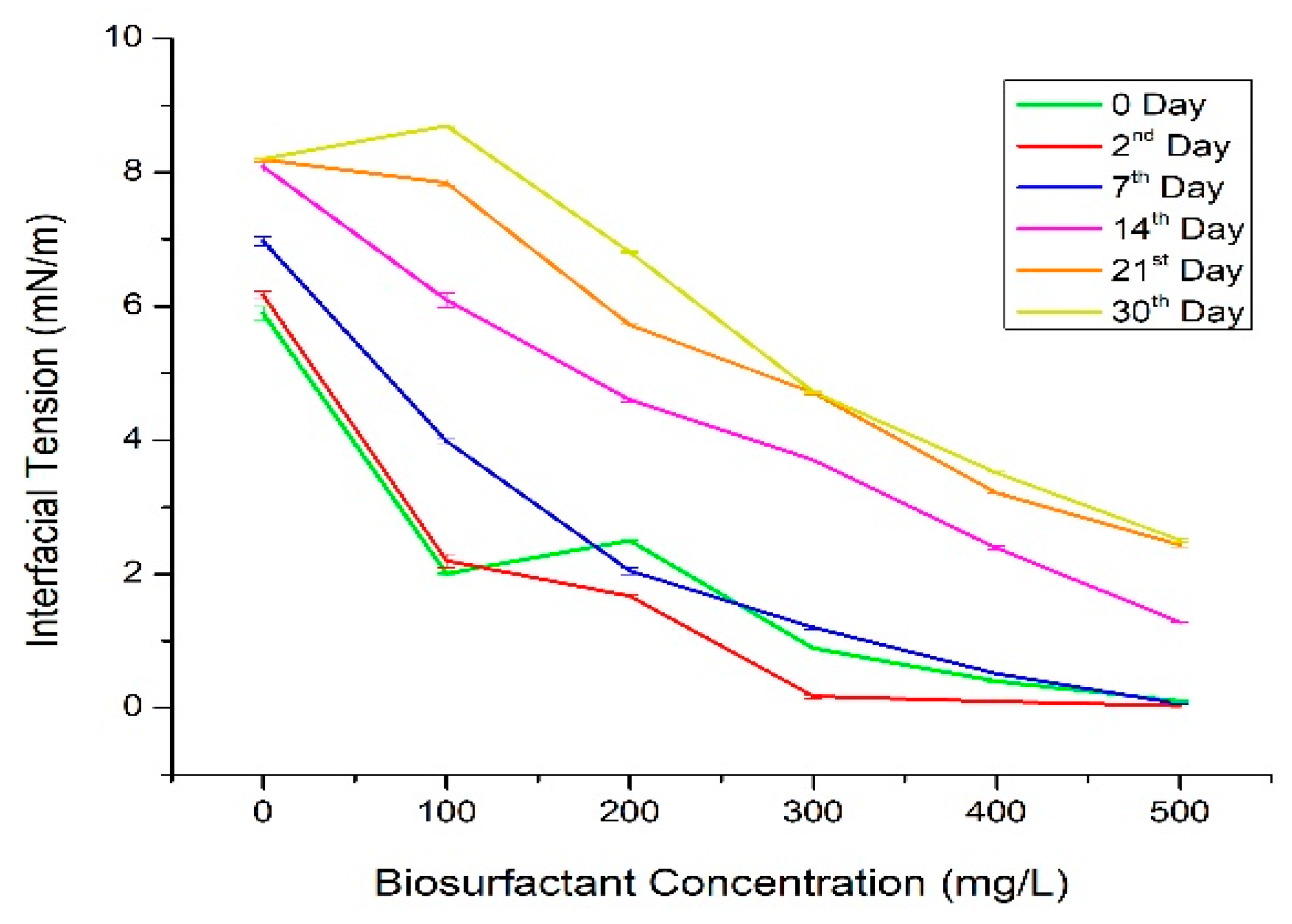

| Chemical Compound | Composition (%) | Chemical Formula | Retention Time (Minute) |
|---|---|---|---|
| 9(E)-Octadecenoic acid, methyl ester | 60.78 | C19H36O2 | 19.467 |
| Hexadecanoic acid, methyl ester | 30.78 | C17H34O2 | 13.361 |
| Octadecanoic acid, methyl ester | 1.95 | C19H38O2 | 26.833 |
| Tetradecanoic acid, methyl ester | 1.85 | C15H30O2 | 9.576 |
| Hexadecanoic acid, 14-methyl-, methyl ester | 1.03 | C18H36O2 | 32.529 |
| Heptadecanoic acid, methyl ester | 0.77 | C18H36O2 | 16.403 |
| Methylene Chloride | 0.34 | CH2Cl2 | 1.544 |
| Octadecanoic acid, 11-methyl-, methyl ester | 0.33 | C20H40O2 | 41.378 |
| 11-Eicosenoic acid, methyl ester | 0.25 | C21H40O2 | 30.866 |
| n-Hexadecanoic acid | 0.23 | C16H32O2 | 18.090 |
| 9(Z),11(E),13(E)-Octadecatrienoic acid methyl ester | 0.22 | C19H32O2 | 29.879 |
| 9(E)-Octadecenoic acid | 0.21 | C18H34O2 | 27.809 |
| Dodecanoic acid, methyl ester | 0.18 | C13H26O2 | 6.592 |
| Pentadecanoic acid, methyl ester | 0.14 | C16H32O2 | 11.270 |
| Std | Run | Experimental Variable | Oil Displacement Area (cm) | Residual | ||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Actual Value | Predicted Value | |||
| 30 | 1 | 17.5 | 120 | 5 | 7 | 4 | 3.565 | 0.435 |
| 15 | 2 | 10 | 140 | 7 | 8 | 2.4 | 1.968 | 0.432 |
| 20 | 3 | 17.5 | 160 | 5 | 7 | 1.8 | 2.534 | −0.734 |
| 27 | 4 | 17.5 | 120 | 5 | 7 | 3.5 | 3.565 | −0.065 |
| 8 | 5 | 25 | 140 | 7 | 6 | 3 | 2.885 | 0.115 |
| 3 | 6 | 10 | 140 | 3 | 6 | 2.2 | 2.168 | 0.032 |
| 5 | 7 | 10 | 100 | 7 | 6 | 2.8 | 2.703 | 0.097 |
| 21 | 8 | 17.5 | 120 | 1 | 7 | 2 | 2.402 | −0.402 |
| 29 | 9 | 17.5 | 120 | 5 | 7 | 3.4 | 3.565 | −0.165 |
| 4 | 10 | 25 | 140 | 3 | 6 | 2.8 | 2.339 | 0.461 |
| 14 | 11 | 25 | 100 | 7 | 8 | 2 | 1.653 | 0.347 |
| 11 | 12 | 10 | 140 | 3 | 8 | 2.7 | 2.322 | 0.378 |
| 12 | 13 | 25 | 140 | 3 | 8 | 2.5 | 2.218 | 0.282 |
| 9 | 14 | 10 | 100 | 3 | 8 | 3.2 | 2.936 | 0.264 |
| 2 | 15 | 25 | 100 | 3 | 6 | 2.6 | 2.653 | −0.053 |
| 1 | 16 | 10 | 100 | 3 | 6 | 3.4 | 3.107 | 0.293 |
| 13 | 17 | 10 | 100 | 7 | 8 | 1.8 | 2.007 | −0.207 |
| 16 | 18 | 25 | 140 | 7 | 8 | 2.2 | 2.239 | −0.039 |
| 7 | 19 | 10 | 140 | 7 | 6 | 2.8 | 2.339 | 0.461 |
| 23 | 20 | 17.5 | 120 | 5 | 5 | 2.8 | 3.119 | −0.319 |
| 25 | 21 | 17.5 | 120 | 5 | 7 | 3.5 | 3.565 | −0.065 |
| 19 | 22 | 17.5 | 80 | 5 | 7 | 3 | 2.887 | 0.113 |
| 26 | 23 | 17.5 | 120 | 5 | 7 | 3.8 | 3.565 | 0.235 |
| 22 | 24 | 17.5 | 120 | 9 | 7 | 1.8 | 2.019 | −0.219 |
| 28 | 25 | 17.5 | 120 | 5 | 7 | 3.2 | 3.565 | −0.365 |
| 24 | 26 | 17.5 | 120 | 5 | 9 | 2 | 2.302 | −0.302 |
| 10 | 27 | 25 | 100 | 3 | 8 | 2 | 2.207 | −0.207 |
| 18 | 28 | 32.5 | 120 | 5 | 7 | 1.8 | 1.869 | −0.069 |
| 6 | 29 | 25 | 100 | 7 | 6 | 2.5 | 2.624 | −0.124 |
| 17 | 30 | 2.5 | 120 | 5 | 7 | 1.5 | 2.052 | −0.552 |
| Source | Sum of Square | Mean of Square | F-Value | p-Value | |
|---|---|---|---|---|---|
| Model | 10.15 | 0.7251 | 3.73 | 0.0081 | significant |
| X1-POME | 0.0504 | 0.0504 | 0.2594 | 0.6180 | |
| X2-Incubation | 0.1838 | 0.1838 | 0.9453 | 0.3463 | |
| X3-Salinity | 0.2204 | 0.2204 | 1.13 | 0.3038 | |
| X4-pH | 1.00 | 1.00 | 5.15 | 0.0385 | |
| X1 X2 | 0.3906 | 0.3906 | 2.01 | 0.1768 | |
| X1 X3 | 0.1406 | 0.1406 | 0.7234 | 0.4084 | |
| X1 X4 | 0.0756 | 0.0756 | 0.3890 | 0.5422 | |
| X2 X3 | 0.3306 | 0.3306 | 1.70 | 0.2118 | |
| X2 X4 | 0.1056 | 0.1056 | 0.5434 | 0.4724 | |
| X3 X4 | 0.2756 | 0.2756 | 1.42 | 0.2523 | |
| X1² | 4.41 | 4.41 | 22.69 | 0.0003 | |
| X2² | 1.25 | 1.25 | 6.43 | 0.0228 | |
| X3² | 3.14 | 3.14 | 16.17 | 0.0011 | |
| X4² | 1.25 | 1.25 | 6.43 | 0.0228 | |
| Residual | 2.92 | 0.1944 | |||
| Lack of Fit | 2.50 | 0.2502 | 3.03 | 0.1166 | not significant |
| Pure Error | 0.4133 | 0.0827 | |||
| Cor Total | 13.07 |
| Crude Oil | Classification | ⁰API | E24 (%) | IFT (mN/m) |
|---|---|---|---|---|
| CR-01 | Intermediate–paraffinic | 39.1 | 50 | 1.56 |
| CR-02 | Intermediate–intermediate | 32.7 | 63 | 0.72 |
| CR-03 | Paraffinic–intermediate | 26.0 | 53 | 1.67 |
| CR-04 | Naphthenic–naphthenic | 38.7 | 76 | 0.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sari, C.N.; Hertadi, R.; Harahap, A.F.P.; Ramadhan, M.Y.A.; Gozan, M. Process Optimization of Palm Oil Mill Effluent-Based Biosurfactant of Halomonas meridiana BK-AB4 Originated from Bledug Kuwu Mud Volcano in Central Java for Microbial Enhanced Oil Recovery. Processes 2020, 8, 716. https://doi.org/10.3390/pr8060716
Sari CN, Hertadi R, Harahap AFP, Ramadhan MYA, Gozan M. Process Optimization of Palm Oil Mill Effluent-Based Biosurfactant of Halomonas meridiana BK-AB4 Originated from Bledug Kuwu Mud Volcano in Central Java for Microbial Enhanced Oil Recovery. Processes. 2020; 8(6):716. https://doi.org/10.3390/pr8060716
Chicago/Turabian StyleSari, Cut Nanda, Rukman Hertadi, Andre Fahriz Perdana Harahap, Muhammad Yusuf Arya Ramadhan, and Misri Gozan. 2020. "Process Optimization of Palm Oil Mill Effluent-Based Biosurfactant of Halomonas meridiana BK-AB4 Originated from Bledug Kuwu Mud Volcano in Central Java for Microbial Enhanced Oil Recovery" Processes 8, no. 6: 716. https://doi.org/10.3390/pr8060716
APA StyleSari, C. N., Hertadi, R., Harahap, A. F. P., Ramadhan, M. Y. A., & Gozan, M. (2020). Process Optimization of Palm Oil Mill Effluent-Based Biosurfactant of Halomonas meridiana BK-AB4 Originated from Bledug Kuwu Mud Volcano in Central Java for Microbial Enhanced Oil Recovery. Processes, 8(6), 716. https://doi.org/10.3390/pr8060716





