On the Degradation of 17-β Estradiol Using Boron Doped Diamond Electrodes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
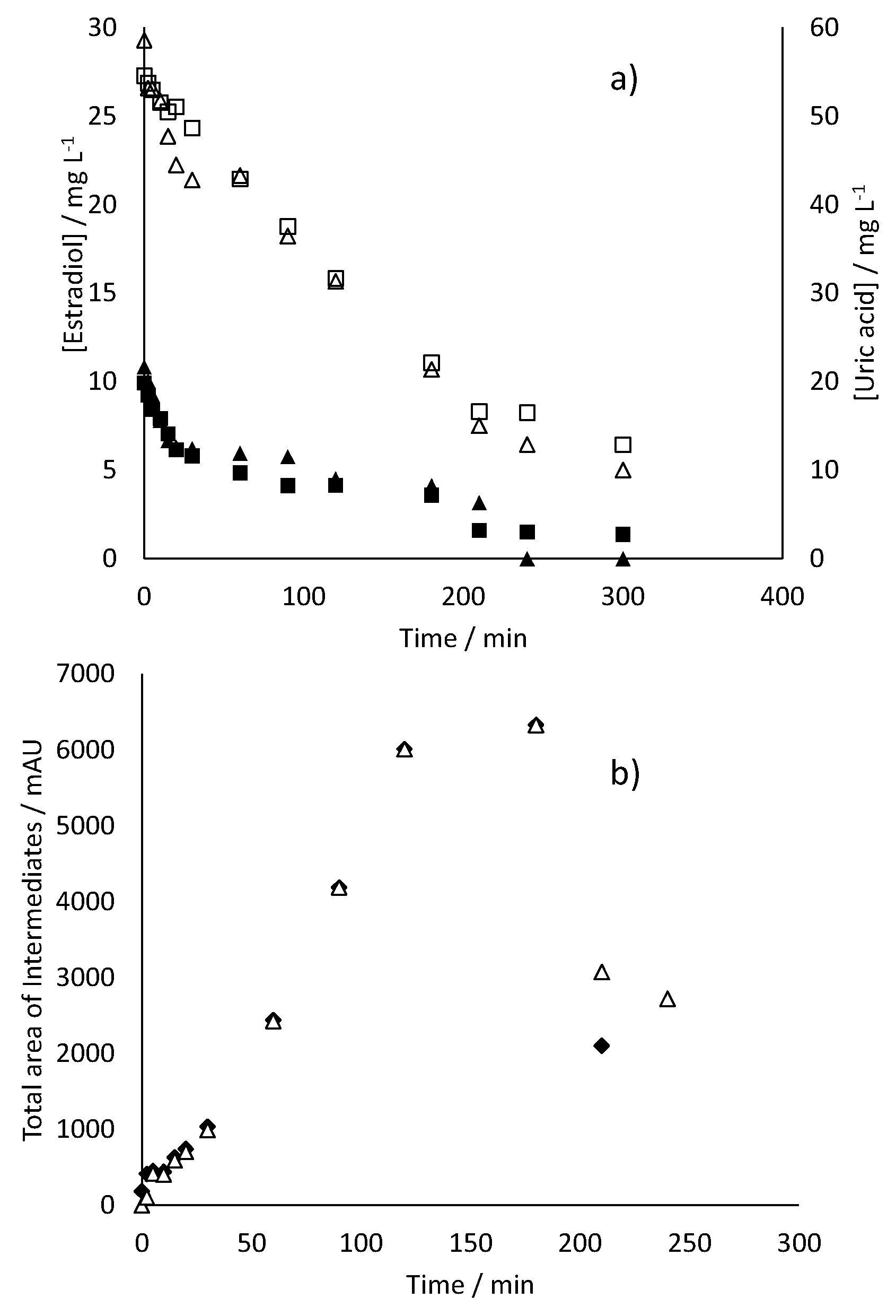
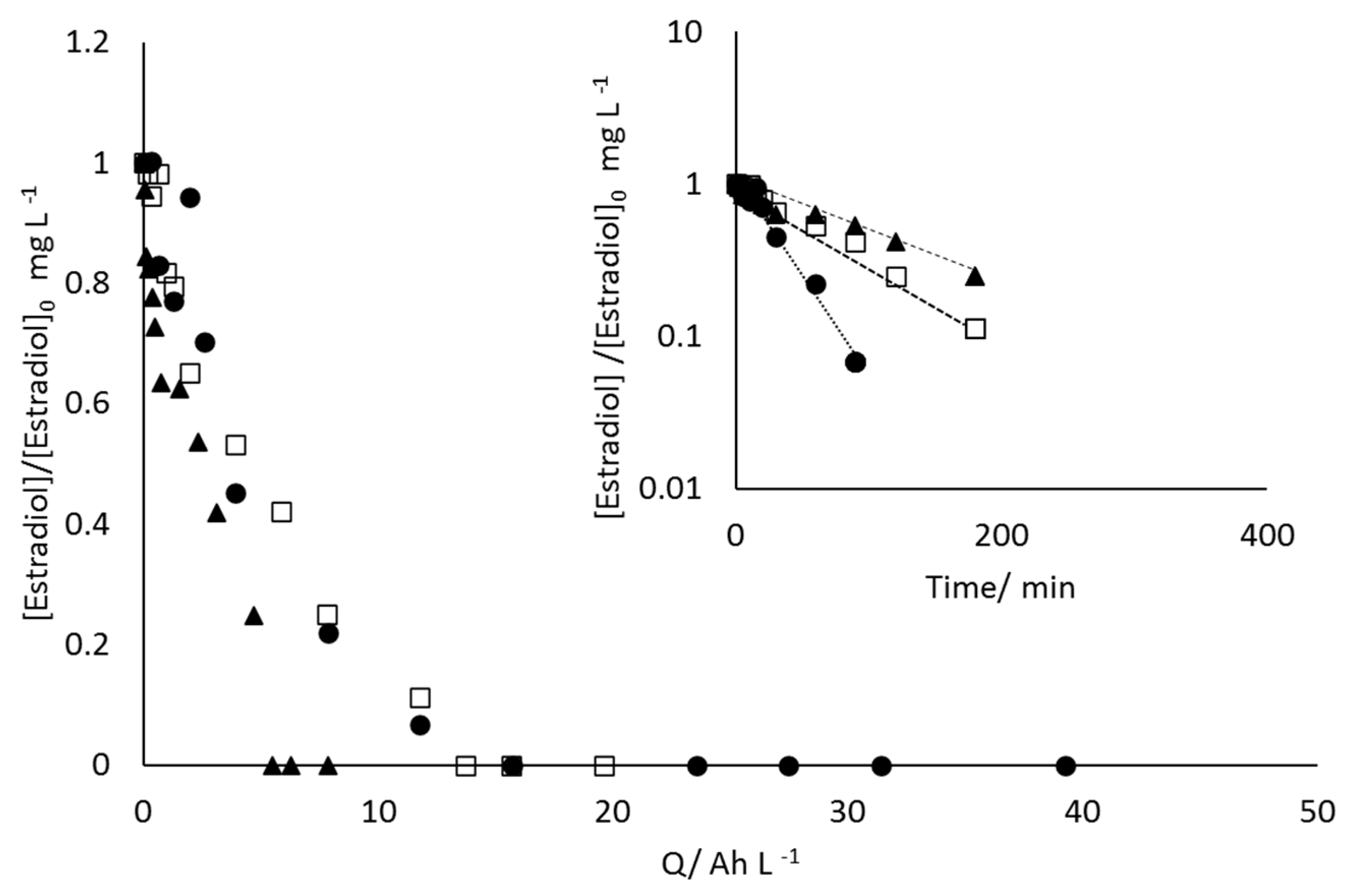
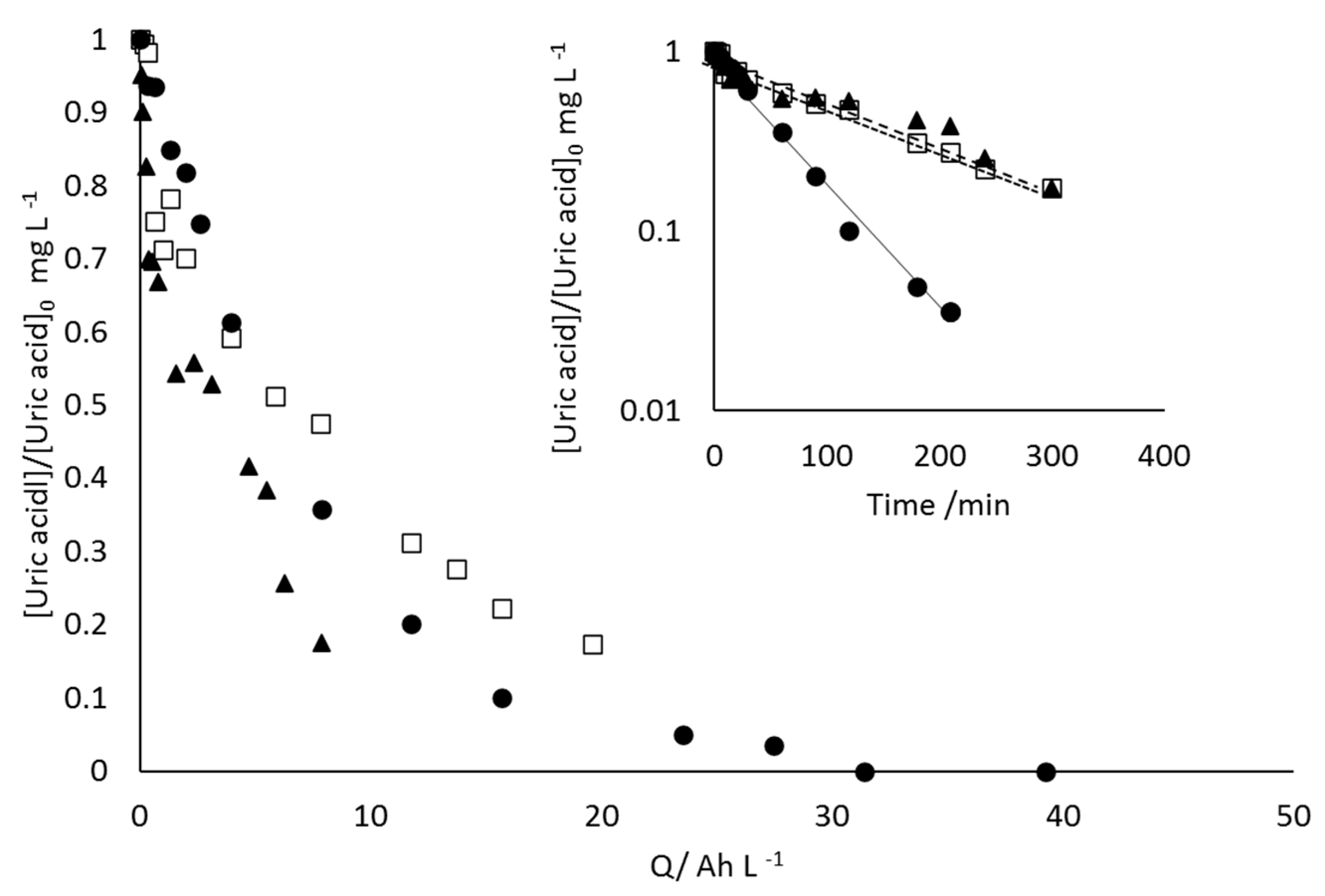
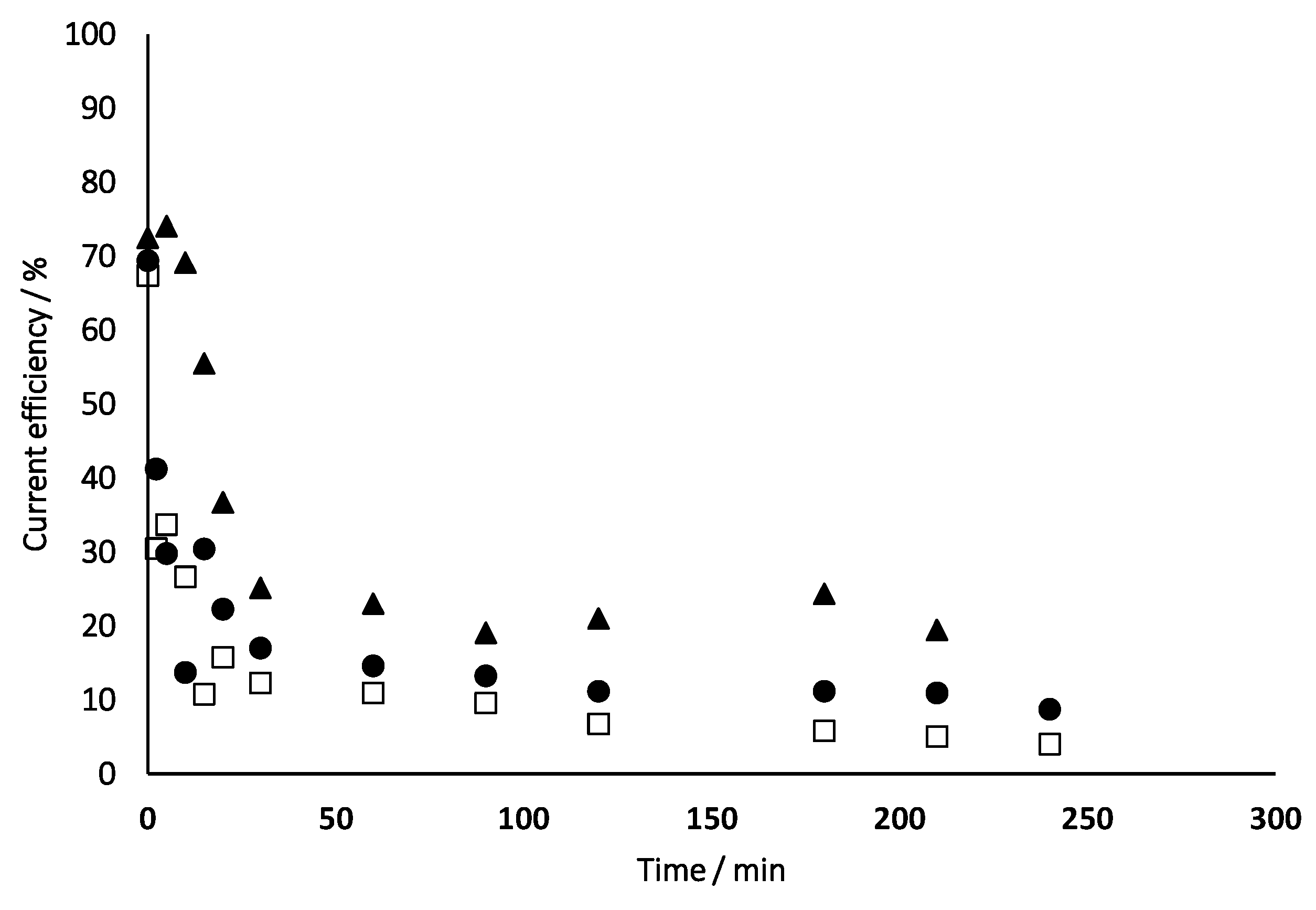
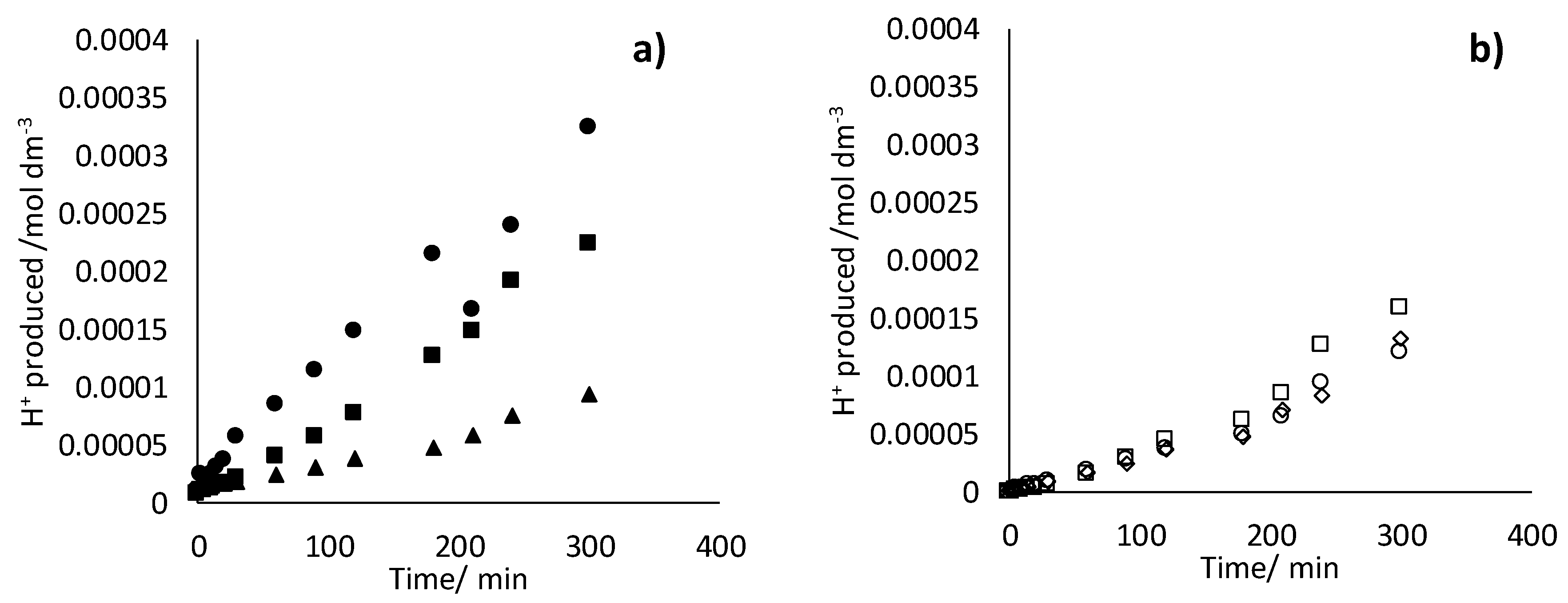
- 17β-estradiol can be efficiently destroyed from mixtures urine/methanol. This removal is simultaneous with the removal of uric acid and leads to the formation of other organic species that behave as intermediates. This opens the possibility of using a concentration strategy based on the adsorption of pollutants using granular activated carbon and their later desorption in methanol, at a much higher concentration. Despite methanol being a well-known radical scavenger, the electrolysis is found to be very efficient and, in the best case, current charge lower than 7 kAh·m−3 is enough to completely deplete the hormone from urine.
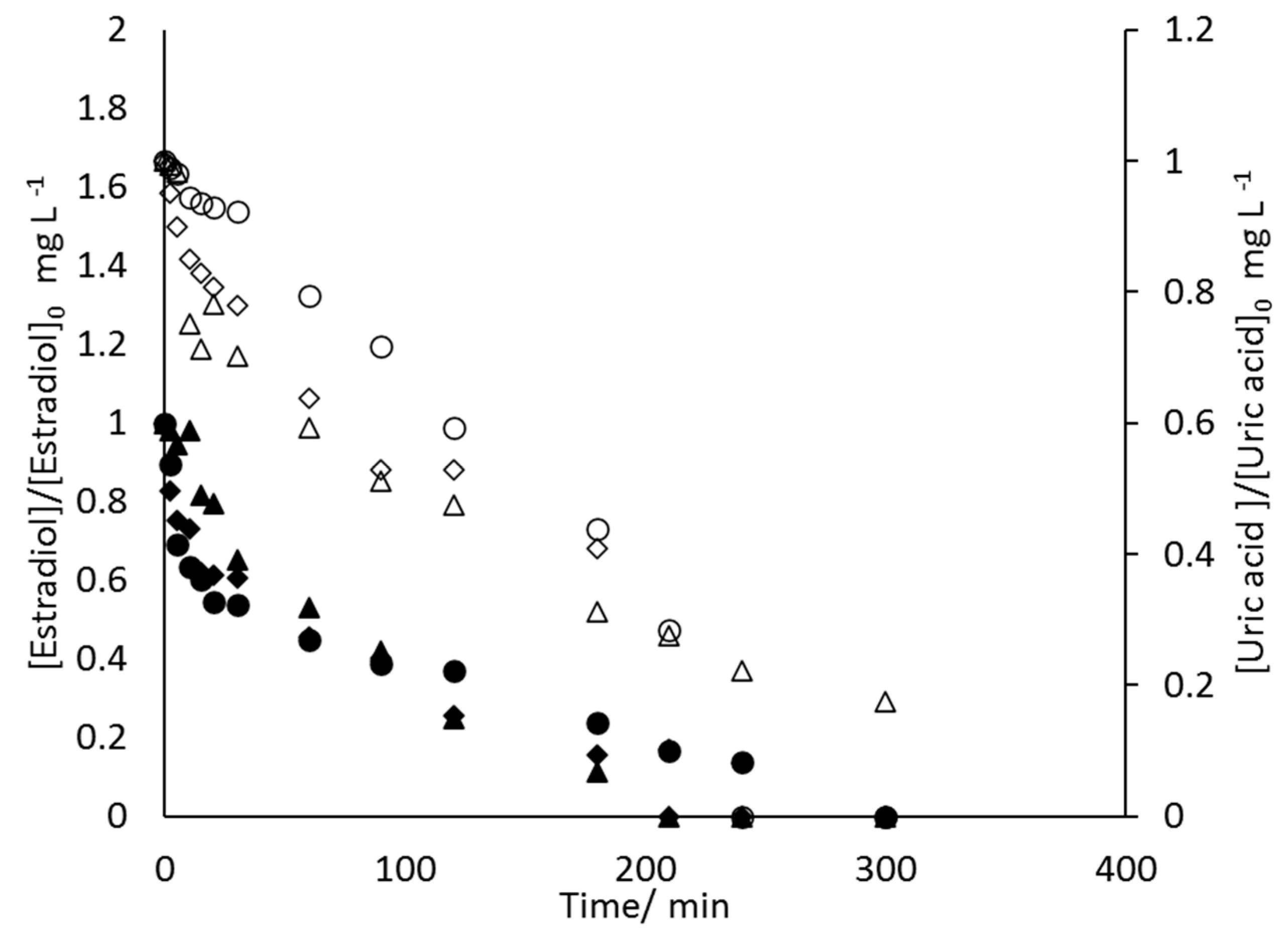
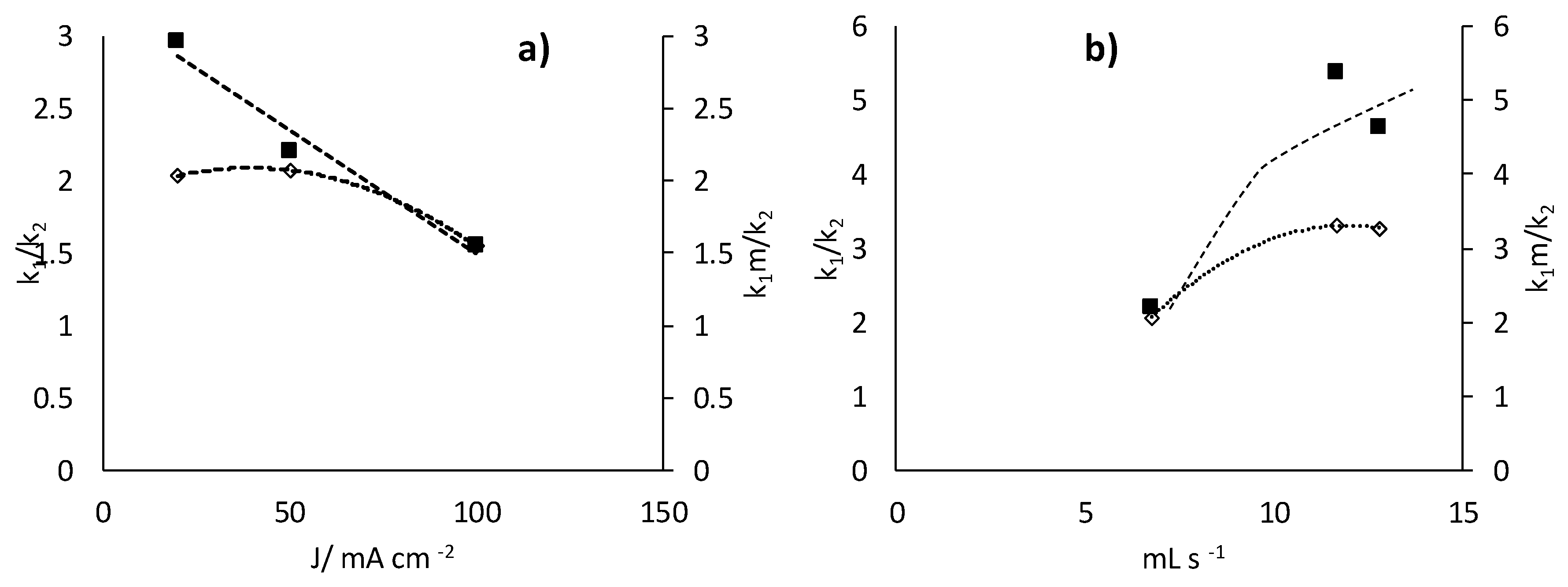
- Increases in the operation current density lead to faster but less efficient removal of the 17β-estradiol. Increases in the operation flowrate do not markedly affect the efficiency in the removal, indicating that the process is not mass transport controlled and that mediated oxidation plays a very important role.
- Degradation of 17β-estradiol and uric acid can fit first order models. However, in the case of 17β-estradiol, two zones are clearly discerned. In the first the removal is faster and it can be explained in terms of the lack of competition with the oxidation of intermediates or, alternatively/simultaneously, with the oxidation of the solubilized 17β-estradiol as it is contained in solution in the form of micelles and solubilized.
- Degradation of 17β-estradiol is favoured with respect to that of uric acid at low current densities and at high flowrates. In those conditions, direct oxidation processes on the surface of the anode are promoted. This means that these direct processes can have a higher influence on the degradability of the hazardous species and opens the possibility for the development of selective oxidation processes, with a great economic impact on the degradation of the hazardousness of hospitalary wastewater.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanz, G.; Ferreira Garcia, L.; Yepez, A.; Colletes de Carvalho, T.; Gontijo Vaz, B.; Romã, W.; Luque, R. TiO2@C Nanostructured Electrodes for the Anodic Removal of Cocaine. Electroanalysis 2018, 30, 2094–2098. [Google Scholar] [CrossRef]
- Weber, S.; Leuschner, P.; Kämpfer, P.; Dott, W.; Hollender, J. Degradation of estradiol and ethinyl estradiol by activated sludge and by a defined mixed culture. Appl. Microbiol. Biotechnol. 2005, 67, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, L.; Rousseau, D.P.L.; Lens, P.N.L. Removal of estrone, 17α-ethinylestradiol, and 17ß-estradiol in algae and duckweed-based wastewater treatment systems. Environ. Sci. Pollut. Res. 2010, 17, 824–833. [Google Scholar] [CrossRef]
- Gong, Y.; Li, J.; Zhang, Y.; Zhang, M.; Tian, X.; Wang, A. Partial degradation of levofloxacin for biodegradability improvement by electro-Fenton process using an activated carbon fiber felt cathode. J. Hazard. Mater. 2016, 5, 320–328. [Google Scholar] [CrossRef]
- Vallejo-Rodríguez, R.; Murillo-Tovar, M.A.; Hernández-Mena, L.; Saldarriaga-Noreña, H.; López-López, A. Compuestos emergentes: Implementación de métodos analíticos para extraer y cuantificar 17b-estradiol, 17a-etinilestradiol, ibuprofeno y naproxeno en agua. Tecnología y Ciencias Del Agua 2012, III, 101–110. [Google Scholar]
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Naimi, I.; Bellakhal, N. Removal of 17 β-Estradiol by Electro-Fenton Process. Mater. Sci. Appl. 2012, 3, 880–886. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.Y.; Tang, K.; Li, Q.S.; Song, Y.L.; Ni, Y.J.; Gao, N.Y. Parameters on 17β-Estradiol degradation by Ultrasound in an aqueous system. J. Chem. Technol. Biotechnol. 2014, 89, 322–327. [Google Scholar] [CrossRef]
- Brocenschi, R.F.; Rocha-Filho, R.C.; Bocchi, N.; Biaggio, S.R. Electrochemical degradation of estrone using a boron-doped diamond anode in a filter-press reactor. Electrochim. Acta 2016, 197, 186–193. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, L.; Chen, J.; Ji, H. Degradation of 17β-estradiol in aqueous solution by ozonation in the presence of manganese(II) and oxalic acid. Environ. Technol. 2013, 34, 131–138. [Google Scholar] [CrossRef]
- Reyhanian Caspillo, N.; Volkova, K.; Hallgren, S.; Olsson, P.E.; Porsch-Hällström, I. Short-term treatment of adult male zebrafish (Danio Rerio) with 17α-ethinyl estradiol affects the transcription of genes involved in development and male sex differentiation. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 164, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, A.J.; Nunez, M.F.; Machacek, D.; Ferguson, J.E.; Iossi, M.F.; Kao, P.C.; Landers, J.P. Separation of urinary estrogens by micellar electrokinetic chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1995, 669, 15–26. [Google Scholar] [CrossRef]
- Katzung, B.G.; Masters, S.B.; Trevor, A.J. Farmacología Básica y Clínica, 11th ed.; McGraw-Hill: New York, NY, USA, 2010. [Google Scholar]
- Antoniazzi, C.; de Lima, C.A.; Marangoni, R.; Spinelli, A.; de Castro, E.G. Voltammetric determination of 17β-estradiol in human urine and buttermilk samples using a simple copper (II) oxide-modified carbon paste electrode. J. Solid State Electrochem. 2017, 22, 1–11. [Google Scholar] [CrossRef]
- Moraes, F.C.; Rossi, B.; Donatoni, M.C.; de Oliveira, K.T.; Pereira, E.C. Sensitive determination of 17β-estradiol in river water using a graphene based electrochemical sensor. Anal. Chim. Acta 2015, 881, 37–43. [Google Scholar] [CrossRef]
- Pape-Zambito, D.A.; Magliaro, A.L.; Kensinger, R.S. 17β-Estradiol and Estrone Concentrations in Plasma and Milk during Bovine Pregnancy. J. Dairy Sci. 2007, 91, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Wei, T. Detection of 17β-estradiol in water samples by a novel double-layer molecularly imprinted film-based biosensor. Talanta 2015, 141, 279–287. [Google Scholar] [CrossRef]
- Shappell, N.W.; Vrabel, M.A.; Madsen, P.J.; Harrington, G.; Billey, L.O.; Hakk, H.; Collins, T.J. Destruction of estrogens using Fe-TAML/peroxide catalysis. Environ. Sci. Technol. 2008, 42, 1296–1300. [Google Scholar] [CrossRef]
- Fuerhacker, M.; Durauer, A.; Jungbauer, A. Adsorption isotherms of 17b-estradiol on granular activated carbon (GAC). Chemosphere 2001, 44, 1573–1579. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, H.; Shen, G.; Yuan, Z.; Xu, T.; Ji, R. Effects of 17Β-estradiol and 17A-ethinylestradiol on the embryonic development of the clearhead icefish (Protosalanx hyalocranius). Chemosphere 2017, 176, 18–24. [Google Scholar] [CrossRef]
- Gárriz, Á.; del Fresno, P.S.; Miranda, L.A. Exposure to E2and EE2environmental concentrations affect different components of the Brain-Pituitary-Gonadal axis in pejerrey fish (Odontesthes bonariensis). Ecotoxicol. Environ. Saf. 2017, 144, 45–53. [Google Scholar] [CrossRef]
- Ohko, Y.; Iuchi, K.I.; Niwa, C.; Tatsuma, T.; Nakashima, T.; Iguchi, T.; Fujishima, A. 17β-estradiol degradation by TiO2 photocatalysis as a means of reducing estrogenic activity. Environ. Sci. Technol. 2002, 36, 4175–4181. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, M.D.; Holthaus, K.I.E.; Johnson, A.C.; Smith, J.J.L.; Hetheridge, M.; Williams, R.J. The potential for estradiol and ethinylestradiol degradation in English rivers. Environ. Toxicol. Chem. 2002, 21, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, D.J.; Mastrocco, F.; Anderson, P.D.; Länge, R.; Sumpter, J.P. Predicted-no-effect concentrations for the steroid estrogens estrone, 17β-estradiol, estriol, and 17α-ethinylestradiol. Environ. Toxicol. Chem. 2012, 31, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Forero, J.; Ortiz, O.; Rios, F. Aplicacion de Procesos de Oxidacion Avanzada como tratamiento de fenol en aguas residuales industriales de refineria. Ciencia Tecnología y Futuro 2005, 3, 97–109. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef]
- Vieira, K.M.; Nascentes, C.C.; Motheo, A.J.; Augusti, R. Electrochemical Oxidation of Ethinylestradiol on a Commercial Ti/Ru 0.3 Ti 0.7 O 2 DSA Electrode. Int. Sch. Res. Not. 2013, 2013, 7. [Google Scholar] [CrossRef] [Green Version]
- Barrera-Díaz, C.; Cañizares, P.; Fernández, F.J.; Natividad, R.; Rodrigo, M.A. Electrochemical Advanced Oxidation Processes: An Overview of the Current Applications to Actual Industrial Effluents. Chem. Soc. J. Mex. Chem. Soc. 2014, 58, 256–275. [Google Scholar] [CrossRef] [Green Version]
- Moraes, F.C.; Gorup, L.F.; Rocha, R.S.; Lanza, M.R.V.; Pereira, E.C. Photoelectrochemical removal of 17β-estradiol using a RuO2-graphene electrode. Chemosphere 2016, 162, 99–104. [Google Scholar] [CrossRef]
- Feng, L.; van Hullebusch, E.D.; Rodrigo, M.A.; Esposito, G.; Oturan, M.A. Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chem. Eng. J. 2013, 228, 944–964. [Google Scholar] [CrossRef]
- Martínez, M.R.; Reyes, E.P.; Luna, J.S.; Villa, G.M.; Méndez, A.R. Degradación electroquímica de 4-clorofenol con electrodos de diamante dopado con boro. In Memorias del XXXVI Encuentro Nacional de la AMIDIQ; Academia Mexicana de Investigación y Docencia en Ingeniería Química: Cancún, Quintana Roo, México, 2015; pp. 810–813. [Google Scholar]
- Zhao, H.; Wei, G.; Gao, J.; Liu, Z.; Zhao, G. Ultrasonic Electrochemical Reaction on Boron-Doped Diamond Electrodes: Reaction Pathway and Mechanism. ChemElectroChem 2015, 2, 366–373. [Google Scholar] [CrossRef]
- Frontistis, Z.; Antonopoulou, M.; Yazirdagi, M.; Kilinc, Z.; Konstantinou, I.; Katsaounis, A.; Mantzavinos, D. Boron-doped diamond electrooxidation of ethyl paraben: The effect of electrolyte on by-products distribution and mechanisms. J. Environ. Manag. 2017, 195, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.; Compton, R.G.; Foord, J.S. The Voltammetry and Electroanalysis of Some Estrogenic Compounds at Modified Diamond Electrodes. Electroanalysis 2013, 25, 2423–2434. [Google Scholar] [CrossRef]
- Montilla, F.; Morallón, E.; Gamero-Quijano, A. Synthetic Boron-Doped Diamond Electrodes for Electrochemical Water Treatment Electrodos de Diamante Dopado con Boro para el tratamiento electroquímico de aguas. Bol. Grupo Español Carbón 2014, 31, 8–12. [Google Scholar]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Rodrigo, M.A.; Sirés, I.; Scialdone, O. Single and Coupled Electrochemical Processes and Reactors for the Abatement of Organic Water Pollutants: A Critical Review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef]
- Muñoz-Morales, M.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. A new strategy for the electrolytic removal of organics based on adsorption onto granular activated carbon. Electrochem. Commun. 2018, 90, 47–50. [Google Scholar] [CrossRef]
- Martín de Vidales, M.J.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. Electrolysis of progesterone with conductive-diamond electrodes. J. Chem. Technol. Biotechnol. 2012, 87, 1173–1178. [Google Scholar] [CrossRef]
- Muñoz-Morales, M.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. A new electrochemically-based process for the removal of perchloroethylene from gaseous effluents. Chem. Eng. J. 2019, 361, 609–614. [Google Scholar] [CrossRef]
- Muñoz-Morales, M.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. Enhanced electrolytic treatment for the removal of clopyralid and lindane. Chemosphere 2019, 234, 132–138. [Google Scholar] [CrossRef]
- Cotillas, S.; Lacasa, E.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. Disinfection of urine by conductive-diamond electrochemical oxidation. Appl. Catal. B Environ. 2018, 229, 63–70. [Google Scholar] [CrossRef]
- Pereira, R.D.O.; de Alda, M.L.; Joglar, J.; Daniel, L.A.; Barceló, D. Identification of new ozonation disinfection byproducts of 17β-estradiol and estrone in water. Chemosphere 2011, 84, 1535–1541. [Google Scholar] [CrossRef] [Green Version]
- Murugananthan, M.; Yoshihara, S.; Rakuma, T.; Uehara, N.; Shirakashi, T. Electrochemical degradation of 17β-estradiol (E2) at boron-doped diamond (Si/BDD) thin film electrode. Electrochim. Acta 2007, 52, 3242–3249. [Google Scholar] [CrossRef]
- Cañizares, P.; Sáez, C.; Lobato, J.; Paz, R.; Rodrigo, M.A. Effect of the Operating Conditions on the Oxidation Mechanisms in Conductive-Diamond Electrolyses. J. Electrochem. Soc. 2007, 154, E37–E44. [Google Scholar] [CrossRef]
- Dbira, S.; Bensalah, N.; Cañizares, P.; Rodrigo, M.A.; Bedoui, A. The electrolytic treatment of synthetic urine using DSA electrodes. J. Electroanal. Chem. 2015, 744, 62–68. [Google Scholar] [CrossRef]
- Nélieu, S.; Kerhoas, L.; Einhorn, J. Atrazine degradation by ozonation in the presence of methanol as scavenger. Int. J. Environ. Anal. Chem. 1996, 65, 297–311. [Google Scholar] [CrossRef]
- Li, Q.; Gao, N.; Deng, Y.; Ma, X.; Chu, W. Factors Affecting UV/H 2 O 2 Oxidation of 17α-Ethynyestradiol in Water. CLEAN Soil Air Water 2013, 41, 143–147. [Google Scholar] [CrossRef]
- Pereira, R.O.; Postigo, C.; de Alda, M.L.; Daniel, L.A.; Barceló, D. Removal of estrogens through water disinfection processes and formation of by-products. Chemosphere 2011, 82, 789–799. [Google Scholar] [CrossRef]
| Name | Structure | Concentration/mg·L−1 |
|---|---|---|
| Calcium phosphate | 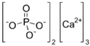 | 28.34 |
| Diammonium phosphate | 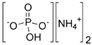 | 83.34 |
| Sodium carbonate | 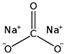 | 166.67 |
| Magnesium sulphate |  | 170 |
| Potassium chloride |  | 1000 |
| Uric acid | 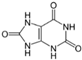 | 50 |
| Creatinine | 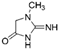 | 166.67 |
| Urea | 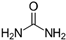 | 3333.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado, S.; Rodrigo, M.; Cañizares, P.; Roa, G.; Barrera, C.; Ramirez, J.; Sáez, C. On the Degradation of 17-β Estradiol Using Boron Doped Diamond Electrodes. Processes 2020, 8, 710. https://doi.org/10.3390/pr8060710
Maldonado S, Rodrigo M, Cañizares P, Roa G, Barrera C, Ramirez J, Sáez C. On the Degradation of 17-β Estradiol Using Boron Doped Diamond Electrodes. Processes. 2020; 8(6):710. https://doi.org/10.3390/pr8060710
Chicago/Turabian StyleMaldonado, Sandra, Manuel Rodrigo, Pablo Cañizares, Gabriela Roa, Carlos Barrera, Javier Ramirez, and Cristina Sáez. 2020. "On the Degradation of 17-β Estradiol Using Boron Doped Diamond Electrodes" Processes 8, no. 6: 710. https://doi.org/10.3390/pr8060710
APA StyleMaldonado, S., Rodrigo, M., Cañizares, P., Roa, G., Barrera, C., Ramirez, J., & Sáez, C. (2020). On the Degradation of 17-β Estradiol Using Boron Doped Diamond Electrodes. Processes, 8(6), 710. https://doi.org/10.3390/pr8060710






