Abstract
Wastewater from a cosmetic factory, with an initial total organic carbon (TOC) of 146.4 mg/L, was treated with Fe2O3/Fe0/H2O2, Fe3O4/Fe0/H2O2, light/Fe2O3/Fe0/H2O2, and light/Fe3O4/Fe0/H2O2 processes. The light-supported processes were more effective than the lightless processes. The fastest TOC removal was observed during the first 15 min of the process. Out of the four tested kinetic models, the best fit was obtained for the modified second-order reaction with respect to the TOC value. The best treatment efficiency was obtained for the light/Fe3O4/Fe0/H2O2 process with 250/750 mg/L Fe3O4/Fe0 reagent doses, a 1:1 hydrogen peroxide to Chemical Oxygen Demand (H2O2/COD) mass ratio, and a 120 min process time. These conditions allowed 75.7% TOC removal to a final TOC of 35.52 mg/L and 90.5% total nitrogen removal to a final content of 4.9 mg/L. The five-day Biochemical Oxygen Demand to Chemical Oxygen Demand (BOD5/COD) ratio was increased slightly from 0.124 to 0.161. Application of Head Space Solid-Phase Microextraction Gas Chromatography Mass Spectrometry (HS-SPME-GC-MS) analysis allows for the detection and identification of 23 compounds contained in the raw wastewater. The identified compounds were eliminated during the applied process. The HS-SPME-GC-MS results confirmed the high efficiency of the treatment processes.
1. Introduction
Cosmetic wastewater (CW) is generated as a result of cleaning production lines with water and surfactants. As a result, CW contains all cosmetic ingredients, and it is usually characterized by a high content of organic compounds and suspended solids. Most cosmetic factories use dissolved air flotation (DAF) to remove suspensions and to decrease the concentration of organic compounds. After that, as a next treatment step, biological treatment is employed [1,2].
Although DAF very effectively removes most of the initial total organic carbon (TOC) and suspended solids, toxic, persistent and disruptive compounds remain in the wastewater [3]. Therefore, there is an urgent need to add another treatment step between DAF pretreatment and the biological one. The aim of this middle step is to prepare wastewater for biological treatment. It could be obtained on the one hand by maintaining high susceptibility to biological treatment, which is usually expressed as a high five-day Biochemical Oxygen Demand to Chemical Oxygen Demand (BOD5/COD) ratio, and on the other hand, by the removal of toxic compounds, especially the ones described as endocrine disruptors: mostly UV filters and synthetic musks [4,5], heavy metals [6], or microplastic [5,7,8].
A lot of research on CW treatment have been done. Some of them include conventional wastewater treatment methods including coagulation [9,10,11], DAF [9,12], or electro-coagulation [13]. Furthermore, alternative treatment methods including membrane [14], dissolved ozone flotation (DOF) [15], and advanced oxidation processes (AOP), such as Fenton [10,16,17,18,19], zero-valent Fenton [14,20,21], photo-Fenton [22], TiO2 [23], or magnetite [24] have been tested. Moreover, biological methods including both aerobic and anaerobic processes were developed [25,26,27,28]. Hybrid processes combining both chemical and biological treatment methods have been also proposed [29,30,31]. A similar approach was applied for solid cosmetic wastes treatment [32].
Processes such as coagulation or membrane application allow for effective pollutants removal, but they do not change the structure of the compounds contained in the wastewater. An effective alternative for them could be AOPs consisting in the generation of radicals with significant redox potential, which non-selectively oxidizes almost all compounds found in wastewater. Many AOPs are known, among which one of the oldest and most popular is the Fenton process, which consists of the catalytic transformation of hydrogen peroxide to a hydroxyl radical, in the presence of iron (II) ions in the acidic conditions, as shown in Reaction (1):
After a certain time, the reaction is stopped by increasing the pH to 8.5–9.5, resulting in coagulation and the precipitation of iron hydroxide. Additionally, on the hydroxides’ surface, pollutants’ sorption occurs. This complicated, multi-threaded mechanism of the Fenton process is responsible for its high efficiency. However, the Fenton process has many disadvantages, including the production of large amounts of sludge or significant salinity increase. Due to this, many modifications were developed, among which, one can indicate the use of an alternative source of iron: Fe0 (metallic iron, zero-valent iron, ZVI) [33,34]. When ZVI is introduced into an acidic environment, it corrodes according to Reaction (2):
The corrosion process is faster the lower the pH. As the ZVI dissolves, the Fenton reaction starts. Another often modification is the use of light, allowing for faster regeneration of the catalyst, Fe (II) ions, according to Reaction (3) [35,36]:
[Fe(OH)]2+ + hν → Fe2+ + •OH.
The problem of sludge formation is attempted to be solved by using iron-based, acid-resistant compounds, transferring the form of catalyst from homogeneous to heterogeneous [37,38,39,40].
The pH value at which the process is carried out is of key importance, whether it is a classic Fenton process or a modification. The pH value determines the behavior of the most important components involved in the Fenton reaction: divalent iron ions as well as hydrogen peroxide. The optimal pH for the process is around 3.0 [41]. Hydrogen peroxide is a weak acid, and therefore, its stability at low pH increases. Typically, when lowering the pH below 3.0, there is a problem with the catalytic cleavage of the hydrogen peroxide to the hydroxyl radical and the hydroxyl ion, which negatively affects the efficiency of the process. On the other hand, at high pH, especially in an alkaline environment, hydrogen peroxide rapidly loses its stability and decomposes. This mechanism is commonly used (as an alternative to the use of catalase) to remove unreacted hydrogen peroxide after the application of AOP to reliably determine the COD content. The divalent iron form is preferred under the conditions of the experiment. However, the situation changes as the pH changes. In a slightly acidic, neutral, and alkaline environment, iron is oxidized to its trivalent form. However, the pH further influences the solubility of iron. It is believed that the minimum solubility of iron is at a pH of approximately 6.0 in the acid range and approximately 8.5 in the alkaline range. This phenomenon is used to precipitate iron compounds in the form of hydroxides during coagulation. The precipitate that appears is undesirable to the process for two reasons. Firstly, it causes radical scavenging, and secondly, it rapidly decreases the amount of available catalyst. In an alkaline environment, in addition, the oxygen generated by decomposing hydrogen peroxide is used to oxidize divalent to trivalent iron. What is also important is that trivalent iron hydroxide has a much lower solubility compared to divalent iron. The factors mentioned above and widely discussed in numerous articles determined the way we conducted the experiment. The pH value was set as most often considered to be optimal. The process was quenched by alkalizing to pH 9.0, thanks to which the decomposition of unreacted hydrogen peroxide, oxidation of divalent to trivalent iron, and secondary coagulation were obtained.
The scientific novelty of our article is the use of a mixture of catalysts for the treatment of real wastewater from the cosmetics industry. To the best of our knowledge, this is the first article where hematite and magnetite were used to treat cosmetic wastewater as catalysts, supporting the simultaneous Fenton and photo-Fenton processes.
The specific aim of this study is to demonstrate the feasibility of magnetite and hematite combined oxidation in removing organic contaminants from real cosmetic factory wastewater. The secondary aim was to investigate the impact of operation parameters, explore the working mechanism of the combined catalytic process in removing the target contaminants, assess the efficiency of light support, and select the best heterogeneous catalyst.
This article shows that the use of such a combination of catalysts has two benefits. First, it allows a decreasing in the total amount of solid catalyst used, and second, it allows a decreasing in the amount of hydrogen peroxide used. Both observations translate into economic benefits when applying the process in practice.
2. Materials and Methods
2.1. Wastewater
A sample of CW was collected from the cosmetic factory located in central Poland. CW was pretreated in the cosmetic factory by coagulation with aluminum-based coagulants, coupled with dissolved air flotation (C/DAF) for sludge removal. The sample of pretreated CW was collected directly from the outlet of the C/DAF unit and stored at 4 °C until analyses. The parameters of the pretreated CW, used in the experiments, are shown in Table 1.

Table 1.
Parameters of wastewater pretreated in the cosmetic factory by aluminum-based coagulants coupled with dissolved air flotation (C/DAF).
2.2. Treatment Process
The Fe0, Ferox Target, 325 mesh, was supplied by Hepure (Hillsborough, NJ, USA). Hematite and magnetite, 10 µm, were supplied by Kremer (Aichstetten, Germany).
Treatment processes were carried out in a 1.5 L reactor filled with a 1 L sample. Doses of magnetite, hematite, Fe0, and 30% H2O2 (Stanlab, Lublin, Poland) solution used in the experiment were selected in preliminary tests. Wastewater samples were stirred (300 rpm) on a magnetic stirrer (Heidolph MR3000, Schwabach, Germany). The pH was adjusted to 3.0. After specified times (15, 30, 60, and 120 min), treatment processes were stopped by increasing the pH to 9.0 using 3 M NaOH (POCh, Gliwice, Poland). The samples were left overnight for the decomposition of unreacted H2O2 and for iron hydroxides sedimentation; then, TOC was determined in supernatant. The details of the preparation of the experiment are shown in Table S1.
Medium pressure Fe/Co 400W lamps type HPA 400/30 SDC, with 94W UVA power (Philips, Amsterdam, The Netherlands), were used as a source of light.
2.3. Analytical Methods
Total organic carbon (TOC) was determined according to the EN 1484:1999 standard with a TOC-L analyzer (Shimadzu, Kyoto, Japan) with an OCT-L8-port sampler (Shimadzu, Kyoto, Japan). The combustion temperature was set to 680 °C.
Chemical oxygen demand (COD), total Kjeldahl nitrogen (TKN), total phosphorus (TP), ammonia, five-day biochemical oxygen demand (BOD5), total suspended solids (TSS), and surfactants concentrations were determined according to the standard methods. Dichloromethane extractable organics (DCMEO) was gravimetrically determined by the extraction of a 1 L sample with dichloromethane (50 + 50 mL). All reagents used in the research were analytical grade.
The identification and quantification of organics in the raw wastewater samples and after the investigated processes were performed using Head Space Solid-Phase Microextraction Gas Chromatography Mass Spectrometry (HS-SPME-GC-MS). Microsorption was performed using 10 mL of sample at 75 °C for 15 min with Supelco (Bellefonte, PA, USA) Poly(DiMethylSiloxane) (PDMS) 100 µm fiber. Desorption was performed in a chromatograph injector at 280 °C for 2 min. The analysis was performed with an Agilent 7890A (Santa Clara, CA, USA) gas chromatograph with a Restek (Bellefonte, PA, USA) RTX-5MS 30 m, 0.25 mm, and 0.25 µm column. The following temperature program was applied: 50 °C for 2 min and 5 °C/min up to 280 °C before maintaining at 280 °C for 5 min. A mass spectrometer Leco TruTOF (St. Joseph, MI, USA) was used as a detector in ionization mode EI at 70 eV and 250 °C across a range of 50 to 600 amu. Mass spectra were compared to the NIST/EPA/NIH published spectra. ChromaTOF software (Leco, St. Joseph, MI, USA) was used for data acquisition and handling.
2.4. Treatment Processes Kinetics Calculation
The following equations were used to describe the kinetics of CW treatment processes:
-first-order reaction with respect to the TOC value:
TOC = TOC0 * e −kt
-second-order reaction with respect to the TOC value:
TOC = TOC0 * e −kt
-modified first-order reaction with respect to the TOC value:
TOC = TOC0 * e −kt
-modified second-order reaction with respect to the TOC value:
TOC = TOC0 * e −kt.
3. Results
3.1. Raw Wastewater
Low values of the measured parameters (TSS, COD, and TOC) in the C/DAF pretreated wastewater Table 1 indicate the efficient performance of the DAF unit. Although biodegradability, described as the BOD5/COD ratio, was not relatively high (0.124), the small share of CW introduced into the biological reactor could cause rapid and negative changes in its biocenosis and work efficiency [29]. It clearly indicates the need for further treatment.
3.2. Results Matching Model
The mechanism of the treatment process is very complicated. It includes both homo and heterogeneous catalysis reactions, as well as coagulation, sorption, and co-precipitation. These processes are at least somewhat independent of each other, sometimes even antagonistic. The first stage of the results analysis was the selection of a kinetic model describing the mechanism of the process. The least squares method was used to select the best model, as shown in Equations (4)–(7). The exemplary models matching results are shown in Figure 1. As the optimal model, second-order kinetics with modification was chosen. There was a slight discrepancy between the results obtained in the experiment and the results of kinetic modeling. In the case of longer process times, the TOC value from modeling is usually slightly higher than that obtained experimentally.
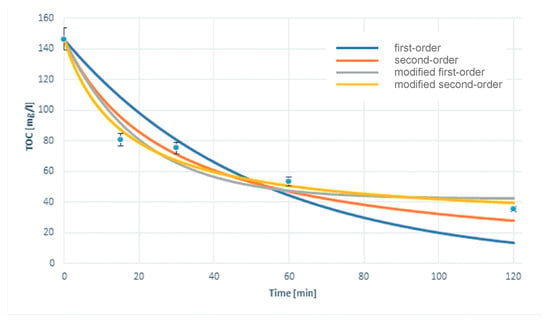
Figure 1.
Kinetic model matching results for cosmetic wastewater treatment using light/Fe2O3/Fe0/H2O2, 750 mg/L Fe0, 250 mg/L Fe2O3, and 1:1 H2O2/COD ratio.
3.3. Treatment Processes
Regardless of the solid catalyst used (Fe0, Fe3O4, Fe2O3), a rapid decrease in the pollutants content was observed in the initial part of the process, while with an extension of the process duration of up to 120 min, further pollutants removal occurred slowly. However, in case of Fe0, the final TOC after 120 min was similar, 74.45–78.04 mg/L, regardless of the hydrogen peroxide dose applied. A similar situation was not observed for iron oxides. A clear difference was observed depending on the H2O2 dose used. Magnetite used as a catalyst was more effective than hematite. A minimal TOC value of 61.86 mg/L was obtained for a 2:1 COD/H2O2 ratio. Similar to non-light-assisted processes, a rapid decrease in the pollutants content was observed in the initial part of the process. However, unlike them, a continuous further decrease in the content of pollutants was observed along with the extension of the process duration. In the Fe2O3/Fe0/H2O2 process, the minimum TOC value of 59.97 mg/L (69% removal) was obtained for 750 mg/L Fe0, 250 mg/L Fe2O3, and a 1:1 H2O2/COD ratio after 120 min. Over time, this value decreases in contrast to other doses, where after 30 min, the TOC value is similar. The lowest TOC removal is observed for H2O2 in a 4:1 H2O2/COD ratio. In the Fe3O4/Fe0/H2O2 process, the minimum TOC value of 44.55 mg/L (69.6% removal) was obtained for 750 mg/L Fe0, 250 mg/L Fe3O4, and a 1:1 H2O2/COD ratio after 120 min. There was no clear relationship between H2O2 concentration and decrease in TOC value.
Figure 2 shows the cosmetic wastewater treatment results, modified 2nd-order kinetic model matching results, light/Fe3O4/Fe0/H2O2 process with 250/750 mg/L Fe3O4/Fe0 reagent doses, and different H2O2/COD ratios, while Figure 3, Figure 4 and Figure 5 show the modified 2nd-order kinetic model matching results for different H2O2/COD ratios and catalyst ratios.
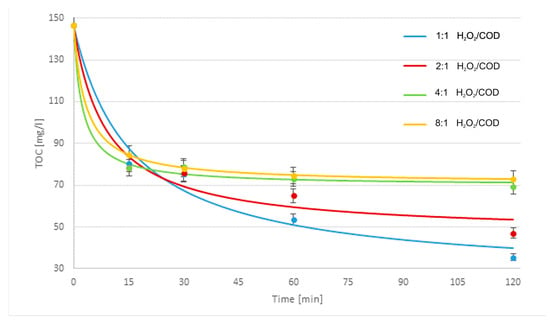
Figure 2.
Cosmetic wastewater treatment results, modified 2nd-order kinetic model matching results, light/Fe3O4/Fe0/H2O2 process with 250/750 mg/L Fe3O4/Fe0 reagent doses, and different H2O2/COD ratios.
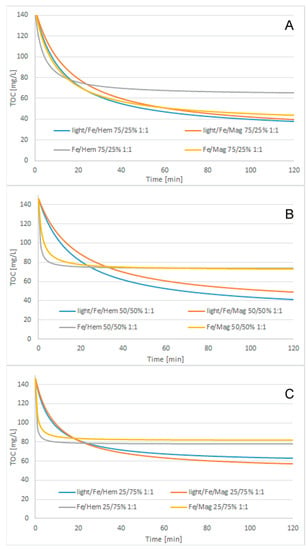
Figure 3.
Cosmetic wastewater treatment, modified 2nd-order kinetic model matching results, 1:1 H2O2/COD ratio, catalyst ratio: (A) 75/25 Fe/Iron oxide, (B) 50/50 Fe/Iron oxide, (C) 25/75 Fe/Iron oxide.
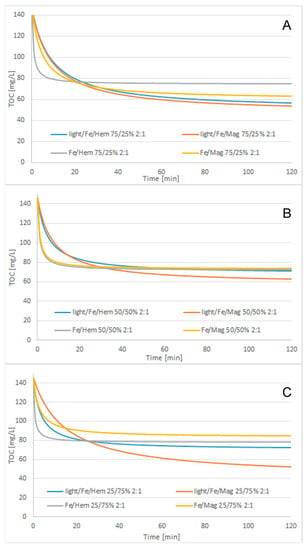
Figure 4.
Cosmetic wastewater treatment, modified 2nd-order kinetic model matching results, 2:1 H2O2/COD ratio, catalyst ratio: (A) 75/25 Fe/Iron oxide, (B) 50/50 Fe/Iron oxide, (C) 25/75 Fe/Iron oxide.
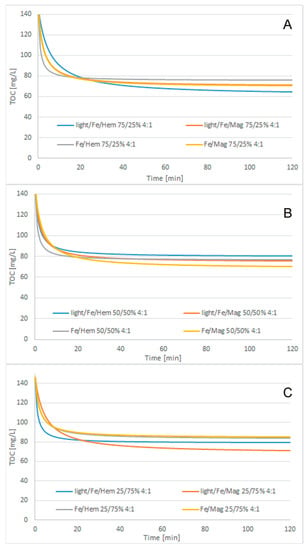
Figure 5.
Cosmetic wastewater treatment, modified 2nd-order kinetic model matching results, 4:1 H2O2/COD ratio, catalyst ratio: (A) 75/25 Fe/Iron oxide, (B) 50/50 Fe/Iron oxide, (C) 25/75 Fe/Iron oxide.
During light-supported processes performance, as in non-light-assisted processes, a rapid decrease in the pollutants content was observed in the initial part of the process. However, unlike them, a continuous further decrease in the content of pollutants was observed along with the extension of the process duration. In the light/Fe2O3/Fe0/H2O2, the highest TOC reduction efficiency is for 750 mg/L Fe0, 250 mg/L Fe2O3, and a 1:1 H2O2/COD ratio after 120 min. In this case, the TOC value is 40.46 mg/L, which is more than 72.2% compared to the initial value. The highest concentration of oxidant gives the lowest efficiency of reducing TOC values. The best treatment efficiency was obtained for the light/Fe3O4/Fe0/H2O2 process with 250/750 mg/L Fe3O4/Fe0 reagent doses, a 1:1 H2O2/COD mass ratio, and a 120 min process time to obtain 75.7% TOC removal for a final TOC of 35.52 mg/L. Analyzing reaction kinetics, the TOC for doses 4:1 and 8:1 H2O2/COD decreases faster at the beginning of the experiment, but after 60 min, the TOC values are similar. For doses 1:1 and 2:1 H2O2/COD, the decrease in TOC value is slower at the beginning but is long-lasting, and the TOC value decreases until the end of the experiment.
The excess hydrogen peroxide adversely affects the process. In addition, improperly selected proportions of the catalysts—the lack or too little amount of iron—does not have a positive effect on the advanced oxidation process. The worst treatment effect was in the case of using only hematite or magnetite as a catalyst—it is necessary to use a mixture of compounds in proportions comparable or with a predominance of metallic iron to obtain an effective method of cosmetic wastewater treatment. In the case of using a lower dose of hydrogen peroxide, the process is almost 30% more effective. This proves the need to properly select the dose of hydrogen peroxide, which in excessive amounts does not bring beneficial results. The excess of hydrogen peroxide makes it impossible to decompose it, which contributes to the inhibition of the process.
If only metallic iron is used, the required excess of hydrogen peroxide is associated with the need to start the Fenton reaction. Initially, there are no divalent iron ions in an acidified environment. On the one hand, they appear due to the slow corrosion reaction, and on the other hand, they appear due to the much faster reaction with the peroxide. Hence, hydrogen peroxide is consumed to obtain (partially) divalent iron ions and to generate hydroxyl radicals. When an additional catalyst (magnetite or hematite) is used, the presence of an additional catalyst decreases the amount of metallic iron necessary for the proper operation of the process, which then reduces the demand for hydrogen peroxide. It is indeed extremely interesting when an excess of one of the substrates inhibits the process or significantly decreases its effectiveness. The use of a higher concentration of the oxidant causes a lower efficiency in decreasing the TOC value in cosmetic wastewater. Then, the reaction of hydrogen peroxide with the resulting hydroxyl radical may take place, the product of which is a hydroperoxide radical with a lower oxidation-reduction potential and lower Reaction (8) [36].
FeIVOaq + H2O2 → FeIIaq + O2 + H2O
Secondly, in the process of enhanced heterocatalytic oxidation, one of the intermediates is oxoiron, which can react with excess hydrogen peroxide to form water and oxygen molecules, which additionally decreases the efficiency of the process.
As the duration of the process increases, the efficiency of the treatment can be increased. The processes that took the longest to run were the most effective—120 min. The treatment process lasting 15 min was the least effective. The values of TOC concentration after the 60 min and 120 min process durations did not differ significantly. Due to the length of the process (a long research time is associated with higher costs of the process) and the small difference in the concentration of total organic carbon after 60 min of treatment, the use of a process lasting 120 min is not recommended. The process shows the greatest efficiency in relation to its duration during the first 15 min of its duration; then, the concentration of TOC decreases the most in a short period of time. Sequential determinations show a slower speed of the treatment process. In the next 15 min, the value decreases by a similar amount, as it was during the first minutes of the process.
The pH value, which is of key importance for the effectiveness of the Fenton process and its modification, may change during the process. This phenomenon occurs particularly often when the process is carried out on unbuffered aqueous solutions of single compounds, but it loses its significance when working on complex matrices such as real industrial wastewater. It should be noted that cosmetic wastewater, even when subjected to initial coagulation in the factory, has strong buffer properties. Compounds that appear during oxidation may lead to slight changes in pH, but it should be noted that these are weak acid/base organic compounds that are much weaker than the strong mineral acids used to adjust the pH. The small amount of these potentially emerging compounds should also be considered. There was no constant control of the pH during the experiment. The pH during the experiment was controlled only at the time of sampling after selected process times (15, 30, 60, and 120 min). The pH values did not change significantly over the process and did not exceed 3.2 even after 120 min.
Based on the review of the available literature, it can be concluded that in most publications concerning the use of metallic iron, the higher the dose used, the better the treatment result—likewise for the dose or proportion of hydrogen peroxide. Usually, its excess is required −2–4 times the COD. We obtained different results in our research and we believe that we are able to explain them in a credible way. In the case of decreasing the dose of hydrogen peroxide, one can refer to the statement that part of the hydrogen peroxide is consumed by the reaction with metallic iron in order to obtain iron (II) ions dissolved in the wastewater. Increasing the iron dose necessitates increasing the peroxide dose and often the ratio to COD in order to obtain an efficient manner of reaction. Meanwhile, the use of magnetite and hematite as supporting catalysts allows for the effective generation of radicals on their surface, without the need to react with the peroxide. This simultaneously lowers the required amount of metallic iron and hydrogen peroxide.
TOC has been found to be the most important parameter to control the effectiveness of the process. The main reason for choosing TOC as the basic parameter is the ease and simplicity of its implementation (TOC analyzer, determination time 10–15 min, depending on the sample). Additionally, an important criterion is a reliable measurement. In the case of COD determination, much more criteria must be met during the measurement to obtain a reliable result. Due to the known fact that the determination is based on the oxidation of compounds with a strong oxidant, potassium dichromate, the presence of additional oxidants/reducing agents in the system will influence the measurement. In the case of our experiment, the presence of metallic iron and hydrogen peroxide are the substances that may influence the COD measurement. In order to remove the effect of magnetic iron, a magnetic stirrer was used as a separating agent during the process. It was assumed that metallic iron would be attracted to the magnet and removed from the solution. In order to remove hydrogen peroxide, alkalization to pH 9.0 was employed, as hydrogen peroxide as a weak acid decomposes in an alkaline environment. As an additional effect, there is the coagulation of dissolved iron compounds. Therefore, the COD measurement can only be performed with a significant time delay. Before testing, it was made sure that all factors that might interfere with the correct COD determination were removed. The problem of the reliability of such a measurement was discussed in previous publications. Therefore, COD was only determined as an auxiliary, for control experiments after 120 min, as a check of consistency with the TOC results. COD was not measured at the intermediate times 15, 30, and 60 min. In the case of BOD, it is known to be a 5-day test. Therefore, BOD determinations were made only after the process time of 120 min, only in selected samples. COD and BOD5 determination results for the selected processes are shown in Table 2.

Table 2.
Parameters of wastewater after advanced oxidation processes (AOP) treatment.
The effect of applying the AOP is not only TOC decreasing but also BOD5 decreasing. The initial BOD5/COD ratio, 0.124, suggests that the raw wastewater may be persistent to biochemical decomposition. However, after AOP, a significant decrease in BOD5 was observed, up to 12 mg/L in the case of the light/Fe3O4/Fe0/H2O2 process in optimal conditions. After the process, the BOD5/COD ratio slightly increased to 0.161. The change in the BOD5/COD ratio increase results from the non-selectivity of the oxidant, which are radicals, oxidizing both potentially susceptible and non-susceptible compounds to biological treatment and at the same time the partial degradation of compounds with a more complex structure to compounds with a simpler structure, which are potentially more susceptible to biodegradation. The use of light-assisted processes allows increasing the total removal of pollutants; however, it causes a significant reduction in the content of compounds responsible for BOD5 compared to non-light-assisted processes. The assessment of whether the compounds remaining in the wastewater after AOP treatment will affect the biocenosis of the activated sludge can be made on the basis of toxicological tests, etc. or, more credibly, by subjecting the wastewater treated with the AOP method to actual biological treatment. The BOD/COD ratio—the parameter used in the article, which is usually understood as susceptibility to biological treatment—is an indirect and simplified parameter. Indeed, both the initial value and its change as a result of the clean-up are not optimistic. In order to confirm the hypothesis about the biodegradability of oxidation products formed in the AOP process, the pretreated wastewater should be subjected to a biological treatment process, as it was done in a previous article [29]. In this article, the scope was extended to the use of hematite or magnetite as a co-catalyst and to assessing its effects. Among the important conclusions, it can be stated that a similar purification effect is obtained, but with the use of lower doses of reagents, which potentially reduces the costs of the entire process. However, the mechanism of the process with and without the use of a co-catalyst is similar. This suggests that the aforementioned speculation is plausible. It is also worth paying attention to the fact that although a significant amount of organic compounds described as COD remains after the process, these are firstly oxidation products, i.e., compounds with much lower polarity and greater solubility in water. This should mean greater availability for microorganisms, but it is not guaranteed; it needs to be confirmed. Additionally, it should be remembered that in the case of a biological treatment process, the microorganisms should be provided with an appropriate amount of carbon source, which is usually converted to the COD value. The content of COD in treated wastewater is lower than usually required for the proper operation of biological systems—e.g., for the operation of the SBR reactor [29], 600–800 mg/L COD is required. In the case of its content in the wastewater being too low, an additional carbon source is required.
It can be noticed that the highest total nitrogen was recorded in the sample where the lowest dose of hydrogen peroxide and magnetite or hematite were used (data not shown) for the treatment process. The obtained result indicates an insufficient dose of reagents. In the case of increasing the dose of hydrogen peroxide, the total nitrogen content decreased significantly. This result proves the significant influence of the amount of hydrogen peroxide on the treatment process, even with an ineffective solid catalyst dose. In the case of wastewater samples treated with 75% metallic iron and 25% iron oxide, the higher the hydrogen peroxide dose, the worse the degree of treatment that was obtained. For the optimal conditions—the light/Fe3O4/Fe0/H2O2 process, with 250/750 mg/L Fe3O4/Fe0 reagent doses, 1:1 H2O2/COD mass ratio, and 120 min process time—the total nitrogen content was decreased to 4.9 mg/L, 90.5% removal.
3.4. HS-SPME-GC-MS Analysis
Application of HS-SPME-GC-MS analysis allows for the detection and identification of 23 compounds contained in the raw wastewater, as shown in Table 3. The identified compounds are mainly cosmetic bases and fragrances. The identified compounds were eliminated during the applied process, regardless of process type and reagent doses. No new compounds were detected after the process. The HS-SPME-GC-MS results confirmed the high efficiency of the treatment processes.

Table 3.
Head Space, Solid-Phase Microextraction, Gas Chromatography, and Mass Spectrometry (HS-SPME-GC-MS) analysis results, raw wastewater.
Attention should be paid to the structure and properties of the compounds detected. They are volatile and resistant to thermal decomposition (high temperature in the dispenser is required to change the aggregate state of the substances and to effectively mix them with the carrier gas). These are largely non-polar compounds and therefore can be detected using the HS-SPME-GC-MS technique. The treatment mechanism is known to be oxidation. This may mean that the organic matter in the wastewater is oxidized to the final end products—carbon dioxide and water—but this seems unlikely. Partial oxidation to intermediates, to compounds of simpler structure (in comparison with parent compounds), seems much more likely. Knowing the mechanisms of oxidation and the properties of compounds containing oxygen in their structure, it can be suspected that the compounds that are products of oxidation are more polar than the parent compounds. Due to the above-mentioned factors, the HS-SPME-GC-MS technique does not detect all compounds present in raw wastewater as well as new compounds after the process. New compounds potentially detected by this technique would have to be derived from a non-polar compound. On the other hand, there is a question about the presence and properties of compounds resulting from the transformation of matrix compounds. Due to the limitations of the HS-SPME-GC-MS technique, it cannot be used for this. Finding and optimizing an appropriate analytical technique seems extremely important here and is an important direction for further research.
4. Discussion
Hydrogen peroxide is a well-known COD disruptor. Although there are several methods to consider the H2O2 impact on COD and other methods for determining H2O2 content, the determined COD may be affected by a significant error. This is particularly important when the differences in process efficiency between individual doses of reagents are small. For this reason, TOC, not COD, was used as the primary indicator for assessing the effectiveness of the pollutants’ removal. In this research, the residual H2O2 concentration was not determined. As a result of alkalization, which stops the Fenton reaction after the desired process time, H2O2 was used for Fe ions oxidation, from divalent into trivalent. As a result, secondary Fe(OH)3 coagulation takes place. The created precipitate was orange, with no trace of blue or green color. Solid catalyst particles were visible in the sediment: magnetite (black) or hematite (red). As H2O2 is a weak acid, it undergoes decomposition in an alkaline condition. Gas bubbles evolving from the sample volume were observed. The gas bubbles moving upwards hindered iron hydroxide sedimentation, but on the other hand, they lengthened the contact time of the flocs with the solution, allowing time for effective pollutants’ sorption. Twelve hours delay (overnight) in TOC determination theoretically could cause measurement error, TOC underestimation, which is related with partial compounds decomposition. However, it must be noted that TOC-responsible compounds were present in solution during the Fenton reaction. Hydroxyl and other radicals were not able to decompose those pollutants. As it is well known, dissolved oxygen, present in the sample after process, is a much weaker oxidant than radicals. As a result, although TOC underestimation is unlikely, it is important to take into consideration. What is more, few bacteria that were present in the raw wastewater; as a result of carrying out the process should be deactivated.
The contribution of final coagulation to the overall treatment effect is hard to assess. Bogacki et al. [20] tried to determine this share using a classical FeCl3 coagulation at a pH equal to the value used to terminate the Fenton process or its modifications. The obtained values were small up to ca. 20%, which is expressed as COD removal. However, it should be noted that this method has its drawbacks. The compounds present in treated wastewater are compounds resulting from the partial oxidation of mother compounds—so they are characterized by lower molecular weight and greater polarity. Since CW has already been subjected to coagulation with aluminum-based coagulants in the production plant, analyzing raw CW parameters, it should be suspected that the initial coagulation has removed most colloids, suspensions, etc. This hypothesis is supported by the results of studies on raw wastewater collected from the factory, indicating the small amount of TSS, as shown in Table 1. Therefore, the effectiveness of the final coagulation depends largely on the sorption. Due to the greater polarity, compounds resulting from radical oxidation may be much less susceptible to sorption on sediment flocs in comparison with parent compounds. Therefore, it can be assumed that the main mechanism for pollutants removal is oxidation.
The analysis of variance in Table S2, depending on the proportions of catalysts and hydrogen peroxide for the measurements of TOC made after 120 min of the process duration, showed that the null hypothesis, which said that both factors had an equal influence on the process, could not be rejected. According to the analysis, the effect of the dose of H2O2 is not much greater than the amount of iron. This shows that the amount of oxidizing substance and catalysts depends on the purification effect. This is a confirmation that there is only one factor influencing the process. As previously demonstrated, the reduction of the TOC value was faster at higher doses of metallic iron and, depending on the composition of the catalyst mixture, a larger or smaller volume of oxidant was required. Both the excess and deficiency of hydrogen peroxide or iron ions can affect the reaction rate.
The numerous process modifications that can be made make the method even more versatile and efficient. For future scientific studies, it is desirable to look for catalyst particles derived from waste iron of different diameters—along with the decrease in the surface of the particles, the active surface on which the process may occur and the mass transfer rate increases. Waste iron can also be a cheap and easily available raw material, the use of which can be a favorable form of development. The disadvantages of the process include a large amount of sludge formed during coagulation after the process. However, if the sludge is properly managed, e.g., a reclaimed layer at a landfill, it is not a significant problem.
To put the newly developed technology into everyday use, the process needs to be adaptable to the requirements of cosmetics companies. It is important to maintain high treatment efficiency and effectiveness when using the process on an industrial scale—as it was achieved during laboratory tests. For this purpose, cooperation with cosmetics companies and investment in new technological systems is necessary. An important element of the introduced innovations is the replacement of expensive chemical compounds acting as catalysts with cheaper substitutes, e.g., ferrous waste, which are cheap and easily available. It is also important to adjust the process effectiveness—the conditions for carrying out the process to a given sewage composition—to obtain high process efficiency. To reduce the cost of the process and at the same time significantly increase its efficiency, it is worth using an easily available light source, whose intensity can be increased by focusing UV rays on the lens.
In the case of replacing chemical compounds that act as catalysts with readily available iron waste, the process costs will be significantly reduced. It is necessary to replace the laboratory glass placed in the laboratory with larger reactors and mixers adapted to the wastewater flow.
5. Conclusions
The process of cosmetic wastewater treatment requires the introduction of new innovative ways to reduce the concentration of chemical compounds and eliminate toxic substances that can pass the sewage system and impact the wastewater treatment plant work. The following conclusions were drawn from the research on the effectiveness of the method:
- The wastewater treatment process should be carried out in three stages. The effectiveness of the first stage—coagulation with aluminum-based coagulants—combined with flotation in dissolved air is indicated by relatively low values of the parameters tested (TSS, COD, TOC). With the removal of colloids and suspensions, high TOC/COD remains after the initial process. The second process, i.e. highly effective catalytic oxidation, in which metallic iron, hematite, magnetite, and hydrogen peroxide were used, according to the type of study, allows the removal of potentially toxic organic compounds from wastewater. After eliminating substances that could negatively affect biocenosis and the growth of microorganisms in activated sludge, wastewater can be treated by biological methods in a municipal wastewater treatment plant.
- Cosmetic wastewater can be effectively treated with the use of Fe2O3/Fe0/H2O2, Fe3O4/Fe0/H2O2, light/Fe2O3/Fe0/H2O2, or light/Fe3O4/Fe0/H2O2 processes. Light-supported processes were more effective than lightless processes. The highest efficiency of the catalytic oxidation process was achieved when using a catalyst whose role was played by UV light. If an additional catalyst in the form of UV light is used, the process takes place in the initial stage of purification. Although purification without UV light continues until the end of purification, it is less efficient and effective than the process in which an additional catalyst was used. In most samples, magnetite turned out to be a better catalyst in combination with metallic iron than hematite, but the differences in COD concentration in the purified compounds were not significant. The fastest TOC removal was observed during the first 15 min of the process. The best treatment efficiency was obtained for the light/Fe3O4/Fe0/H2O2 process with 250/750 mg/L Fe3O4/Fe0 reagent doses, 1:1 H2O2/COD mass ratio, and 120 min process time. Under these conditions, 75.7% TOC removal to a final TOC of 35.52 mg/L and 90.5% total nitrogen removal to a final content of 4.9 mg/L were obtained. The BOD5/COD ratio was increased slightly from 0.124 to 0.161.
- The process of catalytic oxidation is an effective method for the treatment of cosmetic wastewater. Numerous studies confirm its effectiveness and encourage scientific teams to expand comprehensive solutions and introduce new innovations to achieve the highest efficiency. Thanks to a properly carried out treatment process, the wastewater flowing into the sewage system will not significantly affect the biological process by significantly interfering with biocenosis and the life processes of microorganisms in the activated sludge.
- Application of HS-SPME-GC-MS analysis allows for the detection and identification of 23 compounds contained in the raw wastewater. The identified compounds were eliminated during the applied process, regardless of process type and reagent doses. No new compounds were detected after the process. The HS-SPME-GC-MS results confirmed the high efficiency of the treatment processes.
- The best fit was obtained for the modified second-order reaction with respect to the TOC value.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9717/8/11/1343/s1, Table S1. Experimental setup, Table S2. Two-way analysis of variance without replications for UV/Fe3O4/Fe0 process, 250 mg/750 mg/L Fe3O4/Fe0 catalyst doses, 1:1 H2O2/COD mass ratio and 120 min process time.
Author Contributions
Conceptualization, methodology, writing—original draft preparation, review and editing, supervision J.B. and P.M.; laboratory work and data processing J.B., P.M., D.B., M.K., D.Ś., P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thank Hepure for providing ZVI Ferox Target samples as research material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ma, G.; Chen, J. Nitrogen and Phosphorus Pollutants in Cosmetics Wastewater and Its Treatment Process of a Certain Brand. IOP Conf. Ser. Earth Environ. Sci. 2018, 113, 012051. [Google Scholar] [CrossRef]
- Bello, L.A.; Omoboye, A.J.; Abiola, T.O.; Oyetade, J.A.; Udorah, D.O.; Ayeola, E.R. Treatment Technologies for Wastewater from Cosmetic Industry—A Review. Int. J. Chem. Biol. Sci. 2018, 4, 69–80. [Google Scholar]
- De Melo, E.D.; Mounteer, A.H.; de Souza Leão, L.H.; Bahia, R.C.B.; Campos, I.M.F. Toxicity identification evaluation of cosmetics industry wastewater. J. Hazard. Mater. 2013, 244–245, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Biel-Maeso, M.; Corada-Fernández, C.; Lara-Martín, P.A. Removal of personal care products (PCPs) in wastewater and sludge treatment and their occurrence in receiving soils. Water Res. 2019, 150, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Juliano, C.; Magrini, G.A. Cosmetic Ingredients as Emerging Pollutants of Environmental and Health Concern. A Mini-Review. Cosmetics 2017, 4, 11. [Google Scholar] [CrossRef]
- Amneklev, J.; Augustsson, A.; Sorme, L.; Bergback, B. Bismuth and Silver in Cosmetic Products A Source of Environmental and Resource Concern? J. Ind. Ecol. 2015, 20, 99–106. [Google Scholar] [CrossRef]
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. [Google Scholar] [CrossRef]
- Kalcíkova, G.; Alic, B.; Skalar, T.; Bundschuh, M.; Zgajnar Gotvajn, A. Wastewater treatment plant effluents as source of cosmetic polyethylene microbeads to freshwater. Chemosphere 2017, 188, 25–31. [Google Scholar] [CrossRef]
- El-Gohary, F.; Tawfik, A.; Mahmoud, U. Comparative study between chemical coagulation/precipitation (C/P) versus coagulation/dissolved air flotation (C/DAF) for pre-treatment of personal care products (PCPs) wastewater. Desalination 2010, 252, 106–112. [Google Scholar] [CrossRef]
- Naumczyk, J.; Marcinowski, P.; Bogacki, J. Highly polluted cosmetic wastewater treatment. Environ. Prot. Eng. 2017, 44, 25–40. [Google Scholar] [CrossRef]
- Michel, M.M.; Tytkowska, M.; Reczek, L.; Trach, Y.; Siwiec, T. Technological Conditions for the Coagulation of Wastewater from Cosmetic Industry. Ecol. Eng. 2019, 20, 78–85. [Google Scholar] [CrossRef]
- Michel, M.M.; Siwiec, T.; Tytkowska, M.; Reczek, L. Analysis of flotation unit operation in coagulation of wastewater from a cosmetic factory. Przem. Chem. 2015, 11, 2000–2005. (In Polish) [Google Scholar]
- Aloui, F.; Kchaou, S.; Sayadi, S. Physicochemical treatments of anionic surfactants wastewater: Effect on aerobic biodegradability. J. Hazard. Mater. 2009, 164, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.C.; Nunes, M.; Gando-Ferreira, L.M.; Quinta-Ferreira, R.M. Nanofiltration and Fenton’s process over iron shavings for surfactants removal. Environ. Technol. 2014, 35, 2380–2388. [Google Scholar] [CrossRef]
- Wiliński, P.; Marcinowski, P.; Naumczyk, J.; Bogacki, J. Pretreatment of cosmetic wastewater by dissolved ozone flotation (DOF). Desalin. Water Treat. 2017, 71, 95–106. [Google Scholar] [CrossRef]
- Perdigon-Melon, J.; Carbajo, J.; Petre, A.; Rosal, R.; García-Calvo, E. Coagulation-Fenton coupled treatment for ecotoxicity reduction in highly polluted industrial wastewater. J. Hazard. Mater. 2010, 181, 127–132. [Google Scholar] [CrossRef]
- Bautista, P.; Mohedano, A.F.; Gilarranz, M.A.; Casas, J.A.; Rodriguez, J.J. Application of Fenton oxidation to cosmetic wastewaters treatment. J. Hazard. Mater. 2007, 143, 128–134. [Google Scholar] [CrossRef]
- Bautista, P.; Casas, J.A.; Zazo, J.A.; Rodriguez, J.J.; Mohedano, A.F. Comparison of Fenton and Fenton-like oxidation for the treatment of cosmetic wastewater. Water Sci. Technol. 2014, 70, 472–478. [Google Scholar] [CrossRef]
- Bayhan, Y.K.; Değermenci, G.D. Kozmetik atık sularından fenton prosesiyle organik madde gideriminin ve kinetiğinin incelenmesi. J. Fac. Eng. Archit. Gazi Univ. 2017, 32, 181–188. (In Turkish) [Google Scholar] [CrossRef]
- Bogacki, J.; Marcinowski, P.; Zapałowska, E.; Maksymiec, J.; Naumczyk, J. Cosmetic wastewater treatment by Fe0/H2O2 process. Environ. Technol. 2017, 38, 2589–2600. [Google Scholar] [CrossRef]
- Martins de Andrade, P.; Dufrayer, C.R.; de Brito, N.N. Treatment of Real Cosmetic Effluent Resulting from the Manufacture of Hair Conditioners by Reduction Degradation, Adsorption and the Fenton Reaction. Ozone Sci. Eng. 2019, 41, 221–230. [Google Scholar] [CrossRef]
- Ebrahiem, E.E.; Al-Maghrabi, M.N.; Mobarki, A.R. Removal of organic pollutants from industrial wastewater by applying photo-Fenton oxidation technology. Arab. J. Chem. 2017, 10, S1674–S1679. [Google Scholar] [CrossRef]
- Boroski, M.; Rodrigues, A.C.; Garcia, J.C.; Sampaio, L.C.; Nozaki, J.; Hioka, N. Combined electrocoagulation and TiO2 photoassisted treatment applied to wastewater effluents from pharmaceutical and cosmetic industries. J. Hazard. Mater. 2009, 162, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Maksymiec, J.; Marcinowski, P.; Bogacki, J.; Zapałowska, E.; Dzienio, K. Wstępne wyniki zastosowania magnetytu w oczyszczaniu ścieków z przemysłu kosmetycznego. Gaz Woda Techn. San. 2017, 91, 336–339. (In Polish) [Google Scholar] [CrossRef]
- Friha, I.; Feki, F.; Karray, F.; Sayadi, S. A pilot study for cosmetic wastewater using a submerged flat sheet membrane bioreactor. Procedia Eng. 2012, 44, 819–820. [Google Scholar] [CrossRef]
- Monsalvo, V.M.; Lopez, J.; Mohedano, A.F.; Rodriguez, J.J. Treatment of cosmetic wastewater by a full-scale membrane bioreactor. Environ. Sci. Pollut. 2014, 21, 12662–12670. [Google Scholar] [CrossRef]
- Zhang, C.; Ning, K.; Guo, Y.; Chen, J.; Liang, C.; Zhang, X.; Wang, R.; Guo, L. Cosmetic wastewater treatment by a combined anaerobic/aerobic (ABR + UBAF) biological system. Desal. Water Treat. 2015, 53, 1606–1612. [Google Scholar] [CrossRef]
- Puyol, D.; Monsalvo, V.M.; Mohedano, A.F.; Sanz, J.L.; Rodriguez, J.J. Cosmetic wastewater treatment by upflow anaerobic sludge blanket reactor. J. Hazard. Mater. 2011, 185, 1059–1065. [Google Scholar] [CrossRef]
- Muszyński, A.; Marcinowski, P.; Maksymiec, J.; Beskowska, K.; Kalwarczyk, E.; Bogacki, J. Cosmetic wastewater treatment with combined light/Fe0/H2O2 process coupled with activated sludge. J. Hazard. Mater. 2019, 378, 120732. [Google Scholar] [CrossRef]
- Chávez, A.M.; Gimeno, O.; Rey, A.; Pliego, G.; Oropesa, A.L.; Álvarez, P.M.; Beltrán, F.J. Treatment of highly polluted industrial wastewater by means of sequential aerobic biological oxidation-ozone based AOPs. Chem. Eng. J. 2019, 361, 89–98. [Google Scholar] [CrossRef]
- Banerjee, P.; Dey, T.; Sarkar, S.; Swarnakar, S.; Mukhopadhyay, A.; Ghosh, S. Treatment of cosmetic effluent in different configurations of ceramic UF membrane based bioreactor: Toxicity evaluation of the untreated and treated wastewater using catfish (Heteropneustes fossilis). Chemosphere 2016, 146, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Demichelis, F.; Fiore, S.; Onofrio, M. Pretreatments aimed at increasing the biodegradability of cosmetic industrial waste. Process. Saf. Environ. Prot. 2018, 118, 245–253. [Google Scholar] [CrossRef]
- Minella, M.; Bertinetti, S.; Hanna, K.; Minero, C.; Vione, D. Degradation of ibuprofen and phenol with a Fenton-like process triggered by zero-valent iron (ZVI-Fenton). Environ. Res. 2019, 179, 108750. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Vione, D. Effect of pH on Zero Valent Iron Performance in Heterogeneous Fenton and Fenton-Like Processes: A Review. Molecules 2018, 23, 3127. [Google Scholar] [CrossRef] [PubMed]
- Jack, R.S.; Ayoko, G.A.; Adebajo, M.O.; Frost, R.L. A review of iron species for visible-light photocatalytic water purification. Environ. Sci. Pollut. Res. 2015, 22, 7439–7449. [Google Scholar] [CrossRef] [PubMed]
- Vorontsov, A.V. Advancing Fenton and photo-Fenton water treatment through the catalyst design. J. Hazard. Mater. 2019, 372, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- He, J.; Yang, X.; Men, B.; Wang, D. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. Int. J. Environ. Sci. 2016, 39, 97–109. [Google Scholar] [CrossRef]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation–A review. Appl. Catal. B-Environ. 2015, 176–177, 249–265. [Google Scholar] [CrossRef]
- Demarchis, L.; Minella, M.; Nisticò, R.; Maurino, V.; Minero, C.; Vione, D. Photo–Fenton reaction in the presence of morphologically controlled hematite as iron source. J. Photochem. Photobiol. A 2015, 307, 99–107. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).