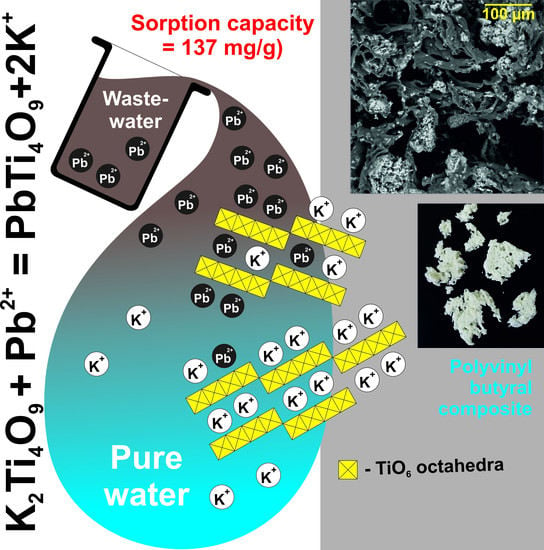
Sorbent Based on Polyvinyl Butyral and Potassium Polytitanate for Purifying Wastewater from Heavy Metal Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparing a Highly Filled Porous Composite Based on Potassium Polytitanates
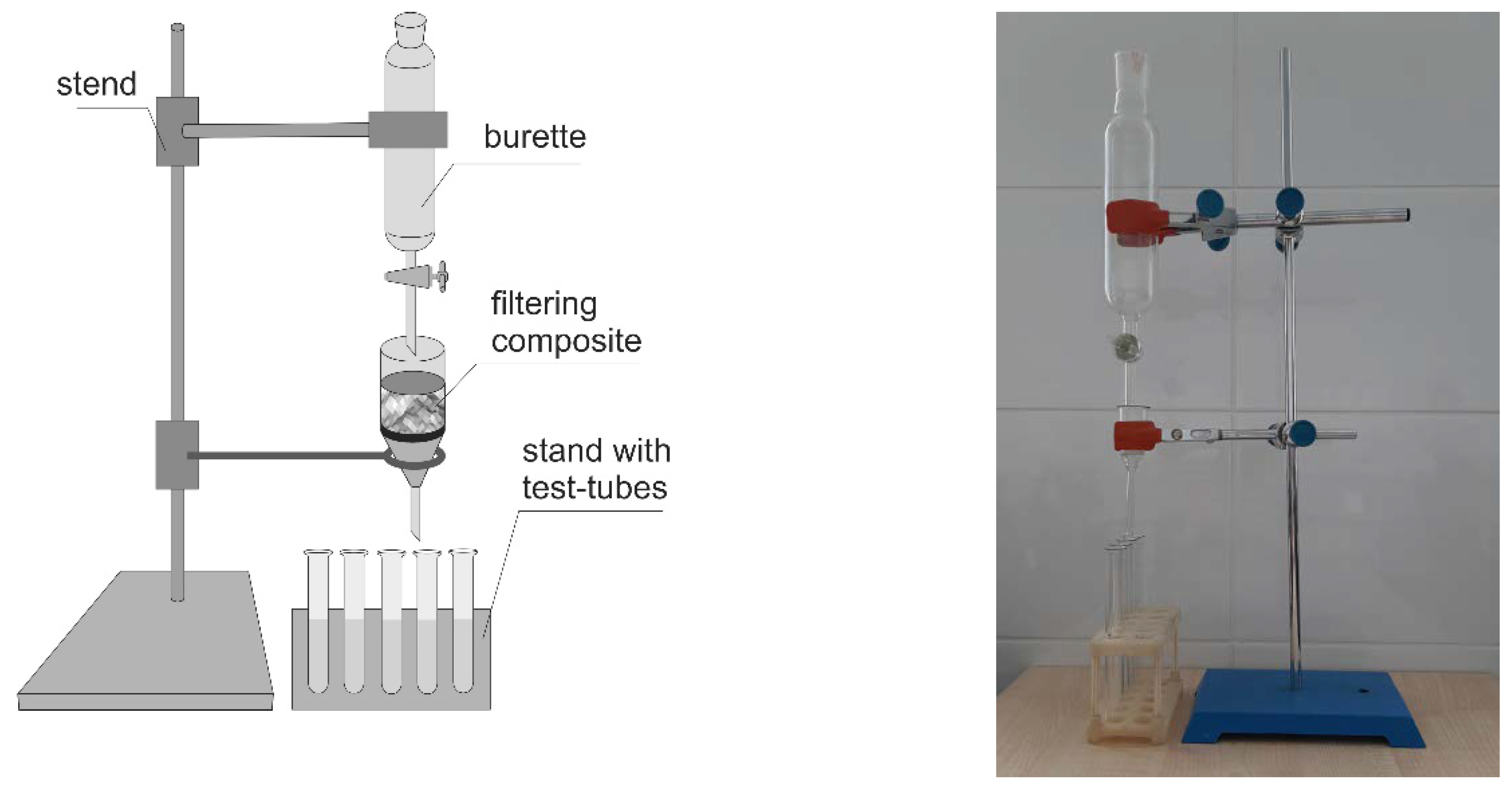
2.3. Sorption Capacity Assessment and Kinetic Analysis
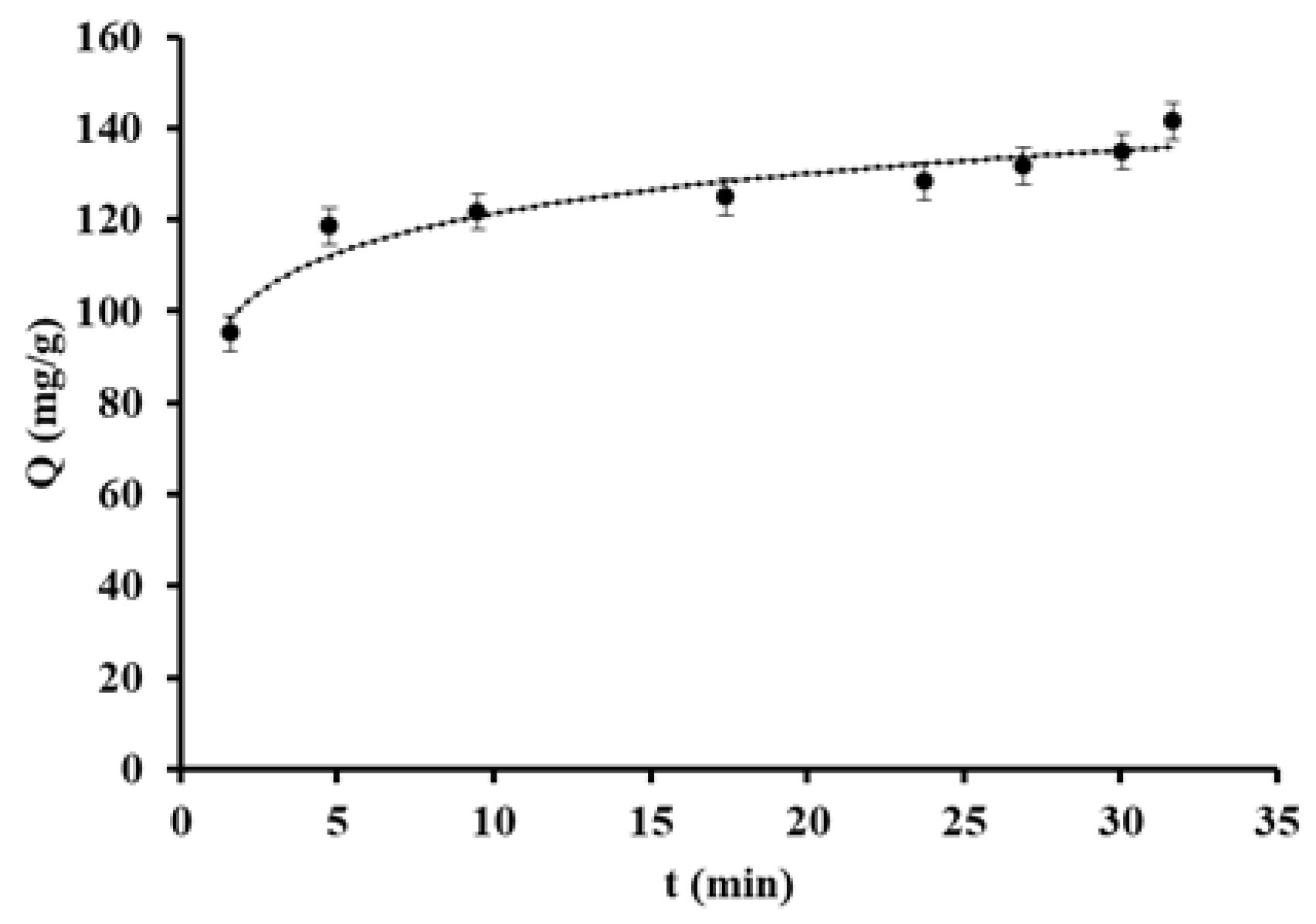
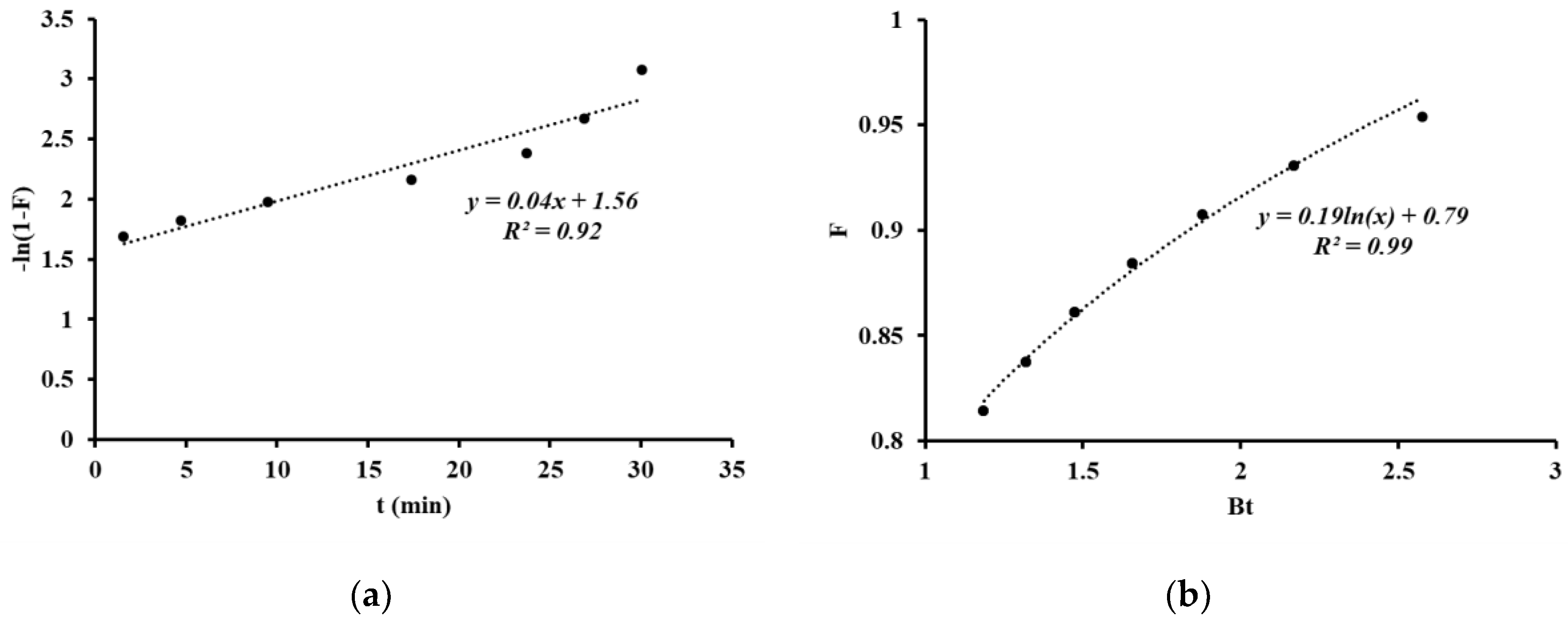
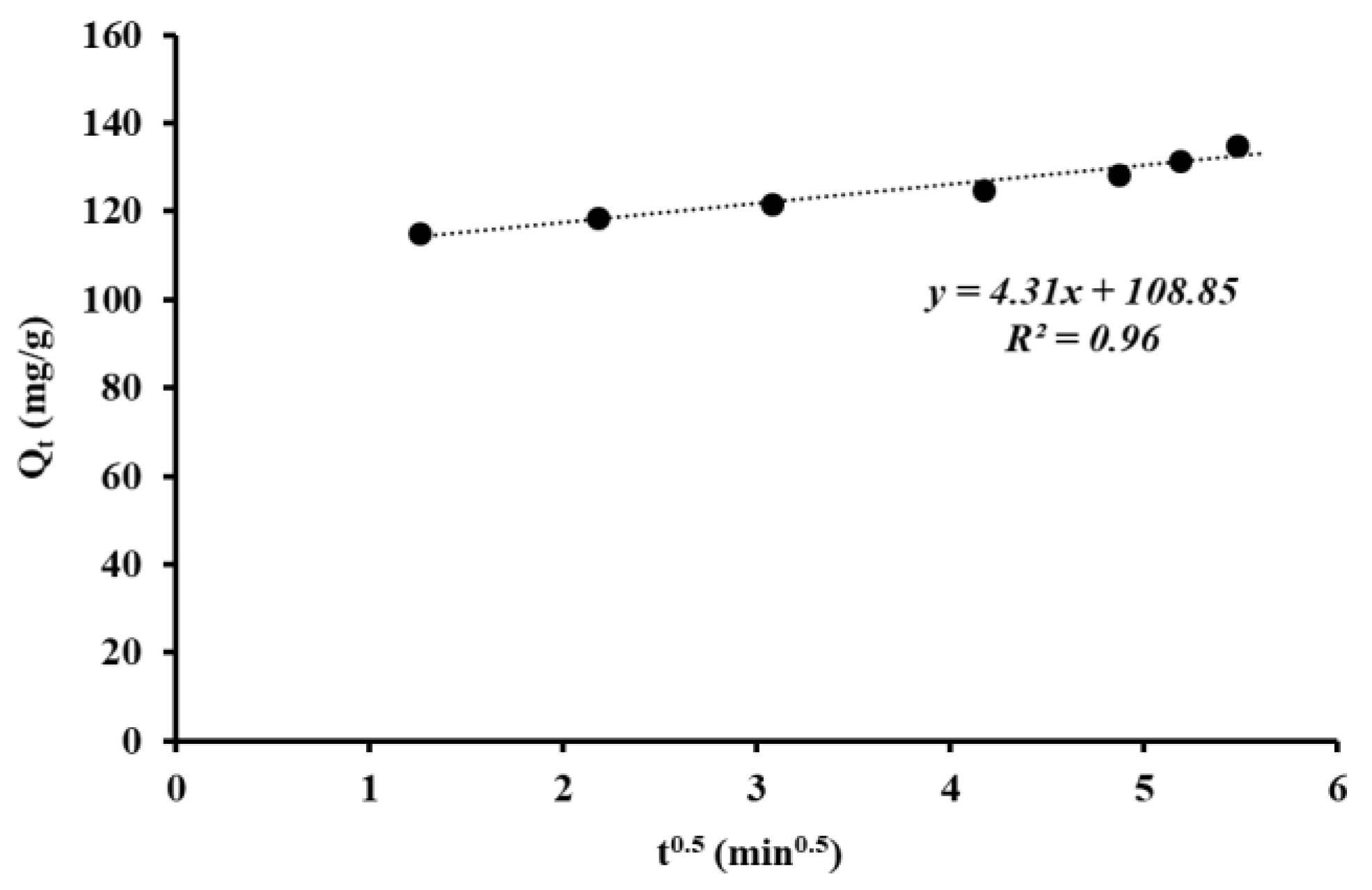
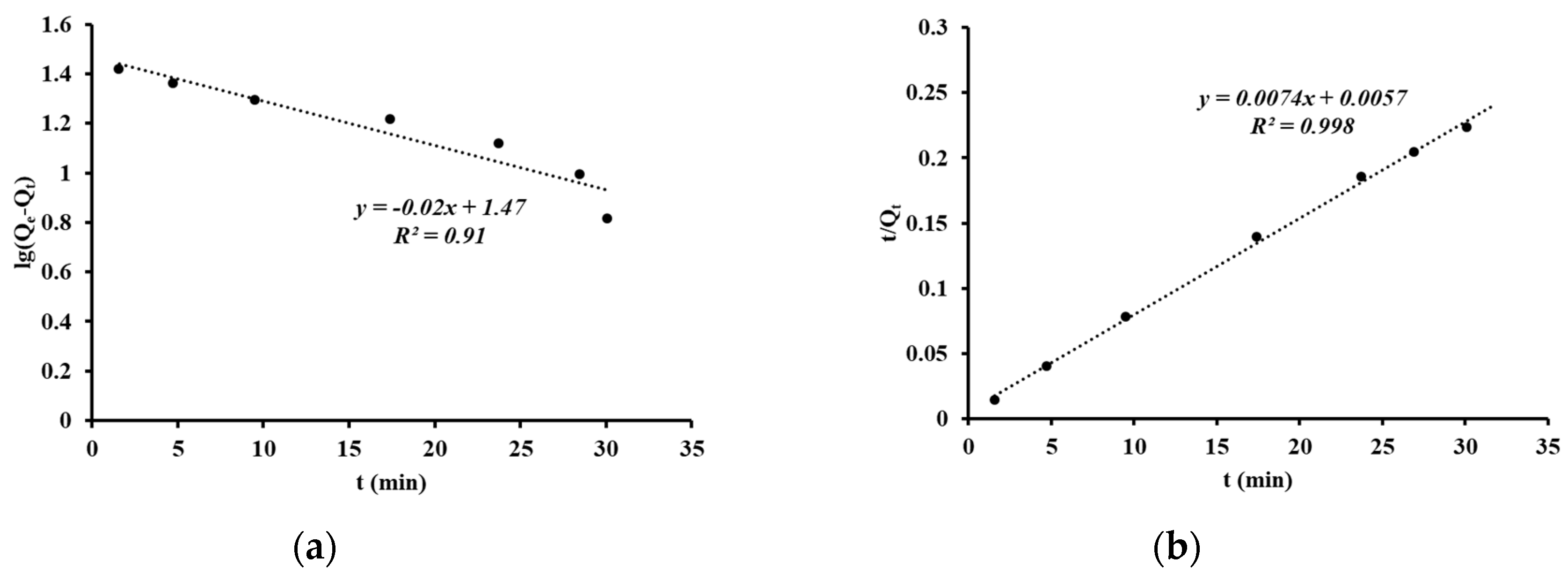
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Masindi, V.; Muedi, K.L. Environmental Contamination by Heavy Metals. In Heavy Metals; Hosam El-Din, M., Saleh, R.A., Eds.; IntechOpen: London, UK, 2018; pp. 1151–1235. [Google Scholar]
- Wen, Y.; Schoups, G.; van de Giesen, N. Organic pollution of rivers: Combined threats of urbanization, livestock farming and global climate change. Sci. Rep. 2017, 7, 43289. [Google Scholar] [CrossRef] [PubMed]
- Karpi, J.; Kotowska, U. Removal of Organic Pollution in the Water Environment. Water 2019, 11, 2017. [Google Scholar]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Wang, L.K.; Hung, Y.T.; Shammas, N.K. Chemical precipitation physicochemical treatment processes. Hum. Press 2005, 3, 141–197. [Google Scholar]
- Xing, Y.; Chen, X.; Wang, D. Electrically regenerated ion exchange for removal and recovery of Cr (VI) from wastewater. Environ. Sci. Technol. 2007, 41, 1439–1443. [Google Scholar] [CrossRef]
- Bakalar, T.; Bugel, M.; Gadoysova, L. Heavy metal removal using reverse osmosis. Acta Montan. Slovaca 2009, 14, 250–253. [Google Scholar]
- Rahul, K.J. Application of electro-dialysis (ED) to remove divalent metals ions from wastewater. Int. J. Chem. Sci. Appl. 2013, 4, 68–72. [Google Scholar]
- Ersahin, M.E.; Ozgun, H.; Dereli, R.K.; Ozturk, I.; Roest, K. A review on dynamic membrane filtration: Materials, applications and future perspectives. Bioresource Technol. 2012, 122, 196–206. [Google Scholar] [CrossRef]
- Wanees, S.A.; Ahmed, A.M.M.; Adam, M.S.; Mohamed, M.A. Adsorption studies on the removal of hexavalent chromium-contaminated wastewater using activated carbon and bentonite. Chem. J. 2012, 2, 95–105. [Google Scholar] [CrossRef]
- Vindoh, R.; Padmavathi, R.; Sangeetha, D. Separation of heavy metals from water samples using anion exchange polymers by adsorption process. Desalination 2011, 267, 267–276. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; Issa, A.A.; Khraisheh, M.A.; Walker, G.M. Sorption of Zn(II), Pb(II) and Co(II) using natural sorbents: Equilibrium and kinetic studies. Water Res. 2006, 40, 2645–2658. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wu, W.; Li, S. Removal of Cd2+ from aqueous solution by adsorption using Fe-montmorillonite. J. Hazard. Mater. 2009, 169, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Gupta, V.K. Advances in water treatment by adsorption technology. Nat. Protoc. 2006, 1, 2661–2667. [Google Scholar] [CrossRef] [PubMed]
- Jiuhui, Q.U. Research progress of novel adsorption processes in water purification. J. Environ. Sci. 2008, 20, 1–13. [Google Scholar]
- Shahmirzadi, M.A.A.; Hosseini, S.S.; Luo, J.; Ortiz, I. Significance, evolution and recent advances in adsorption technology, materials and processes for desalination, water softening and salt removal. J. Environ. Manag. 2018, 215, 324–344. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Płaza, A.; Kołodyńska, D.; Hałas, P.; Gęca, M.; Franus, M.; Hubicki, Z. The zeolite modified by chitosan as an adsorbent for environmental applications. Adsorp. Sci. Technol. 2017, 35, 834–844. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Andal, N.M.; Anbalagan, K. Adsorption studies of iron (III) on chitin. J. Chem. Sci. 2005, 117, 663–672. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Anbalagan, K.; Andal, N.M. Adsorption dynamics and equilibrium studies of Zn (II) onto chitosan. J. Chem. Sci. 2004, 116, 119–127. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J. Environ. Manag. 2010, 91, 798–806. [Google Scholar] [CrossRef]
- Rao, G.P.; Lu, C.; Su, F. Sorption of divalent metal ions from aqueous solution by carbon nanotubes: A review. Sep. Purif. Technol. 2007, 58, 224–231. [Google Scholar] [CrossRef]
- Xue, C.; Qi, P.; Liu, Y. Adsorption of aquatic Cd2+ using a combination of bacteria and modified carbon fiber. Adsorp. Sci. Technol. 2018, 36, 857–871. [Google Scholar] [CrossRef]
- Guo, T.; Bulin, C.; Li, B.; Zhao, Z.; Yu, H.; Sun, H.; Ge, X.; Xing, R.; Zhang, B.; Zhang, B. Efficient removal of aqueous Pb(II) using partially reduced graphene oxide-Fe3O4. Adsorp. Sci. Technol. 2018, 36, 1031–1048. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Yang, L.; Han, M.; Zhao, J.; Cheng, X. Nanomaterials as sorbents to remove heavy metal ions in wastewater treatment. J. Environ. Anal. Toxicol. 2012, 2, 154–158. [Google Scholar] [CrossRef]
- Li, Y.H.; Ding, J.; Luan, Z.; Di, Z.; Zhu, Y.; Xu, C.; Wu, D.; Wei, B. Competitive adsorption of Pb2+, Cu2+ and Cd2+ ions from aqueous solutions by multiwalled carbon nanotubes. Carbon 2003, 41, 2787–2792. [Google Scholar] [CrossRef]
- Smart, S.K.; Cassady, A.I.; Lu, G.Q.; Martin, D.J. The biocompatibility of carbon nanotubes. Carbon 2006, 44, 1034–1047. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Adsorption of heavy metals on kaolinite and montmorillonite: A review. Phys. Chem. Chem. Phys. 2012, 14, 6698–6723. [Google Scholar] [CrossRef]
- Gorokhovsky, A.; Vikulova, M.; Escalante-Garcia, J.I.; Tretyachenko, E.; Burmistrov, I.; Kuznetsov, D.; Yuri, D. Utilization of nickel-electroplating wastewaters in manufacturing of photocatalysts for water purification. Process Saf. Environ. Prot. 2020, 134, 208–216. [Google Scholar] [CrossRef]
- Mishra, S.P.; Singh, V.K.; Tiwari, D. Radiotracer technique in adsorption study: Part XVII. Removal Behaviour of Alkali Metal (K- and Li-) Titanates for Cd (II). Appl. Radiat. Isot. 1998, 49, 1467–1475. [Google Scholar] [CrossRef]
- Nunes, L.M.; Cardoso, V.A.; Airoldi, C. Layered titanates in alkaline, acidic and intercalated with 1,8-octyldiamine forms as ion-exchangers with divalent cobalt, nickel and copper cations. Mater. Res. Bull. 2006, 41, 1089–1096. [Google Scholar] [CrossRef]
- Tretyachenko, E.V.; Gorokhovsky, A.V.; Yurkov, G.Y.; Fedorov, F.S.; Vikulova, M.A.; Kovaleva, D.S.; Orozaliev, E.E. Adsorption and photo-catalytic properties of layered lepidocrocite-like quasi-amorphous compounds based on modified potassium polytitanates. Particuology 2014, 17, 22–28. [Google Scholar] [CrossRef]
- Gorokhovsky, A.V.; Tret’yachenko, E.V.; Vikulova, M.A.; Kovaleva, D.S.; Yurkov, G.Y. Effect of Chemical Composition on the Photocatalytic Activity of Potassium Polytitanates Intercalated with Nickel Ions. Russ. J. Appl. Chem. 2013, 86, 343–350. [Google Scholar] [CrossRef]
- Ermolenko, A.; Shevelev, A.; Vikulova, M.; Blagova, T.; Altukhov, S.; Gorokhovsky, A.; Godymchuk, A.; Burmistrov, I.; Offor, P.O. Wastewater Treatment from Lead and Strontium by Potassium Polytitanates: Kinetic Analysis and Adsorption Mechanism. Processes 2020, 8, 217. [Google Scholar] [CrossRef]
- Mazov, I.; Burmistrov, I.; Il’Inykh, I.; Stepashkin, A.; Kuznetsov, D.; Issi, J.-P. Anisotropic thermal conductivity of polypropylene composites filled with carbon fibers and multiwall carbon nanotubes. Polym. Compos. 2015, 36, 1951–1957. [Google Scholar] [CrossRef]
- Sanchez-Monjaras, T.; Gorokhovsky, A.; Escalante-Garcia, J.I. Molten salt synthesis and characterization of potassium polytitanate ceramic precursors with varied TiO2/K2O molar ratios. J. Am. Ceram. Soc. 2008, 91, 3058–3065. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers Jr, L.S. The exchange adsorption of ions from aqueous solutions by organic zeolites. II. Kinetics1. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der Sogenannten Adsorption Gelöster Stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Intraparticle diffusion during the sorption of surfactants onto activated carbon. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 53–61. [Google Scholar]
- Checkol, F.; Elfwing, A.; Greczynski, G.; Mehretie, S.; Inganäs, O.; Admassie, S. Highly Stable and Efficient Lignin-PEDOT/PSS Composites for Removal of Toxic Metals. Adv. Sustain. Syst. 2018, 2, 1700114. [Google Scholar] [CrossRef]
- Karnib, M.; Kabbani, A.; Holail, H.; Olama, Z. Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energy Procedia 2014, 50, 113–120. [Google Scholar] [CrossRef]
- Nyairo, W.N.; Eker, Y.R.; Kowenje, C.; Akin, I.; Bingol, H.; Tor, A.; Ongeri, D.M. Efficient adsorption of lead (II) and copper (II) from aqueous phase using oxidized multiwalled carbon nanotubes/polypyrrole composite. Sep. Sci. Technol. 2018, 53, 1498–1510. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Shawon, Z.B.Z.; Tay, W.J.D.; Hidajat, K.; Uddin, M.S. Fe3O4/cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohydr. Polym. 2013, 91, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Z.; Gao, J.; Dai, J.; Han, J.; Wang, Y.; Xie, J.; Yan, Y. Selective adsorption behavior of Pb (II) by mesoporous silica SBA-15-supported Pb (II)-imprinted polymer based on surface molecularly imprinting technique. J. Hazard. Mater. 2011, 186, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Fallah, Z.; Isfahani, H.N.; Tajbakhsh, M.; Tashakkorian, H.; Amouei, A. TiO2-grafted cellulose via click reaction: An efficient heavy metal ions bioadsorbent from aqueous solutions. Cellulose 2018, 25, 639–660. [Google Scholar] [CrossRef]
- Khalek, M.D.; Mahmoud, G.A.; El-Kelesh, N.A. Synthesis and Characterization of Poly-Methacrylic Acid Grafted Chitosan-Bentonite Composite and Its Application for Heavy Metals Recovery. Chem. Mater. Res. 2012, 2, 1–12. [Google Scholar]
- Ghoul, M.; Bacquet, M.; Morcellet, M. Uptake of heavy metals from synthetic aqueous solutions using modified PEI—Silica gels. Water Res. 2003, 37, 729–734. [Google Scholar] [CrossRef]
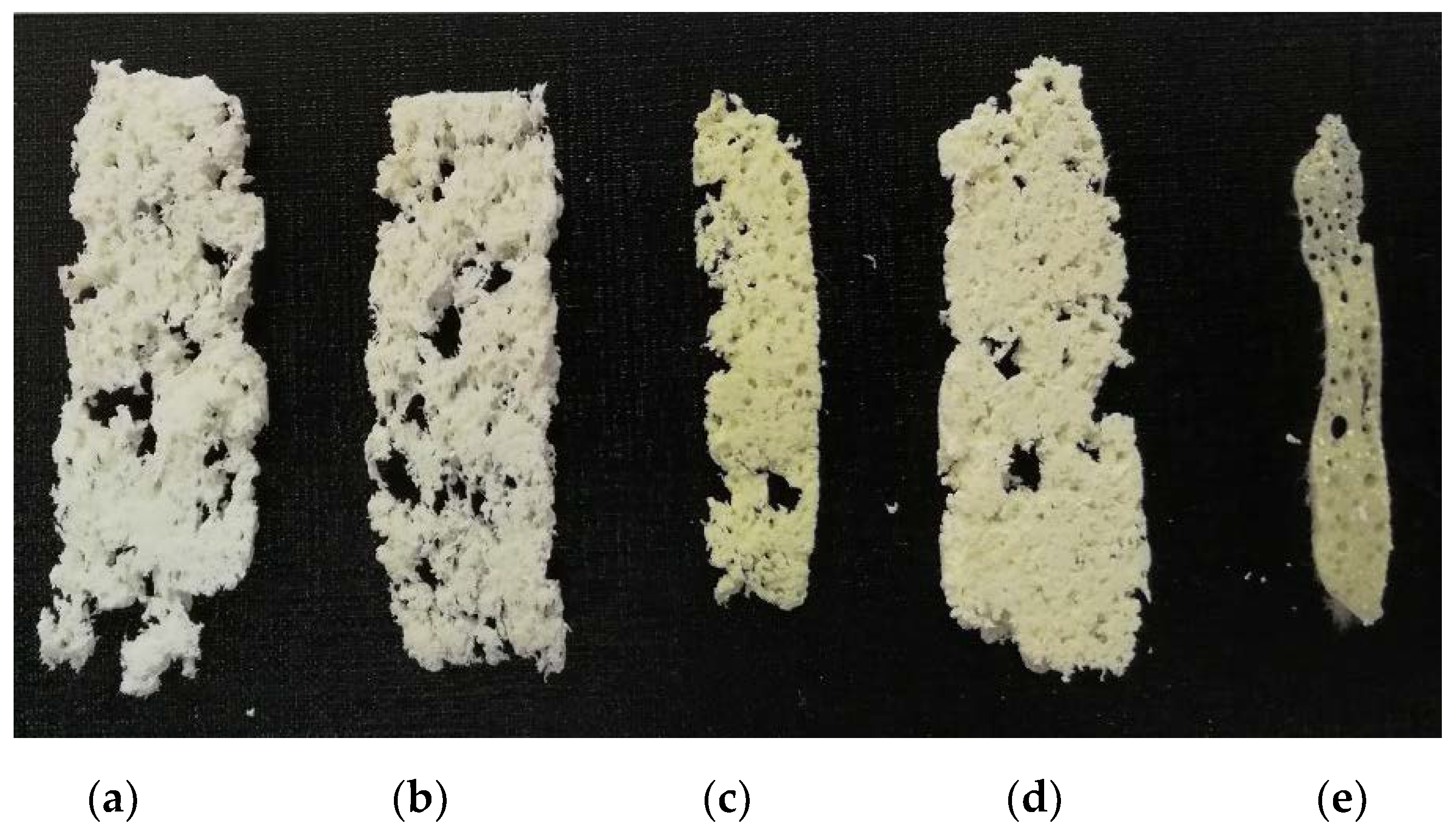
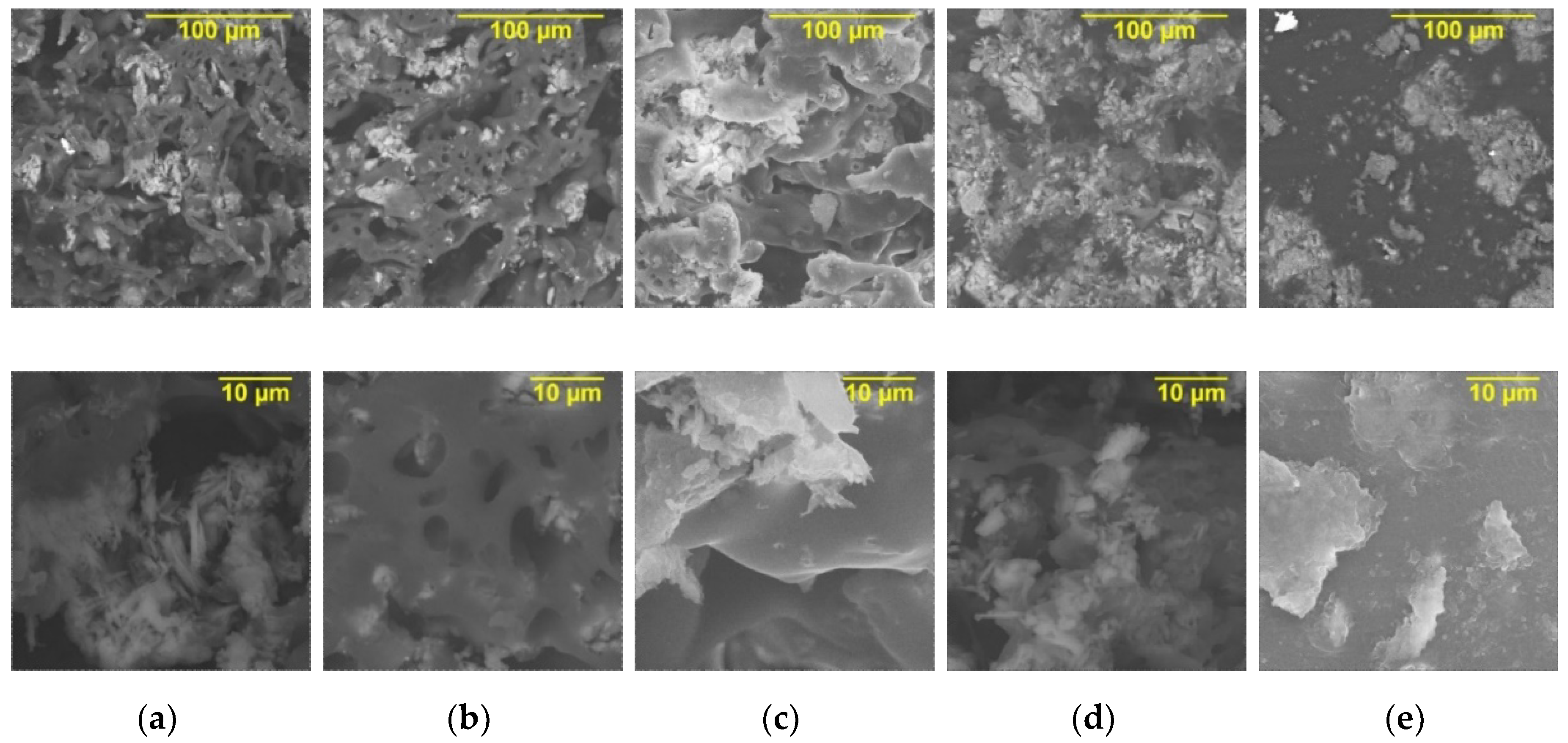
| N° | Sample Notation | PVB * Content in Composite, wt.% | PPT ** Content in Composite, wt.% | Ratio of Isopropyl Alcohol/Water |
|---|---|---|---|---|
| 1 | 1/6-70-30 | 70 | 30 | 1/6 |
| 2 | 1/4-70-30 | 70 | 30 | 1/4 |
| 3 | 1/2-70-30 | 70 | 30 | 1/2 |
| 4 | 1/4-50-50 | 50 | 50 | 1/4 |
| 5 | 1/4-90-10 | 90 | 10 | 1/4 |
| Polymer Composite | Sorption Capacity (mg/g) | Reference |
|---|---|---|
| Polyvinyl butyral/potassium polytitanate (1/6-70-30 sample) | 137.0 | - |
| Potassium polytitanate | 714.3 | [35] |
| Poly (3,4-ethylenedioxythiophene)/lignin | 452.8 | [42] |
| Polypyrrole/multi-wall carbon nanotube | 26.3 | [44] |
| Poly (ethylene imine)/silica gels | 82.6 | [49] |
| Ion-imprinted polymer Pb(II)/SBA-15 | 42.6 | [46] |
| Poly-methacrylic acid grafted chitosan-bentonite nanocomposite | 111.0 | [48] |
| Cellulose/TiO2 | 120.5 | [47] |
| Carboxymethyl-β-cyclodextrin/Fe3O4 | 64.5 | [45] |
| Poly (N-vinylcarbazole)/graphene oxide | 982.9 | [43] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermolenko, A.; Vikulova, M.; Shevelev, A.; Mastalygina, E.; Ogbuna Offor, P.; Konyukhov, Y.; Razinov, A.; Gorokhovsky, A.; Burmistrov, I. Sorbent Based on Polyvinyl Butyral and Potassium Polytitanate for Purifying Wastewater from Heavy Metal Ions. Processes 2020, 8, 690. https://doi.org/10.3390/pr8060690
Ermolenko A, Vikulova M, Shevelev A, Mastalygina E, Ogbuna Offor P, Konyukhov Y, Razinov A, Gorokhovsky A, Burmistrov I. Sorbent Based on Polyvinyl Butyral and Potassium Polytitanate for Purifying Wastewater from Heavy Metal Ions. Processes. 2020; 8(6):690. https://doi.org/10.3390/pr8060690
Chicago/Turabian StyleErmolenko, Anna, Maria Vikulova, Alexey Shevelev, Elena Mastalygina, Peter Ogbuna Offor, Yuri Konyukhov, Anton Razinov, Alexander Gorokhovsky, and Igor Burmistrov. 2020. "Sorbent Based on Polyvinyl Butyral and Potassium Polytitanate for Purifying Wastewater from Heavy Metal Ions" Processes 8, no. 6: 690. https://doi.org/10.3390/pr8060690
APA StyleErmolenko, A., Vikulova, M., Shevelev, A., Mastalygina, E., Ogbuna Offor, P., Konyukhov, Y., Razinov, A., Gorokhovsky, A., & Burmistrov, I. (2020). Sorbent Based on Polyvinyl Butyral and Potassium Polytitanate for Purifying Wastewater from Heavy Metal Ions. Processes, 8(6), 690. https://doi.org/10.3390/pr8060690