Covalently Cross-Linked Nanoparticles Based on Ferulated Arabinoxylans Recovered from a Distiller’s Dried Grains Byproduct
Abstract
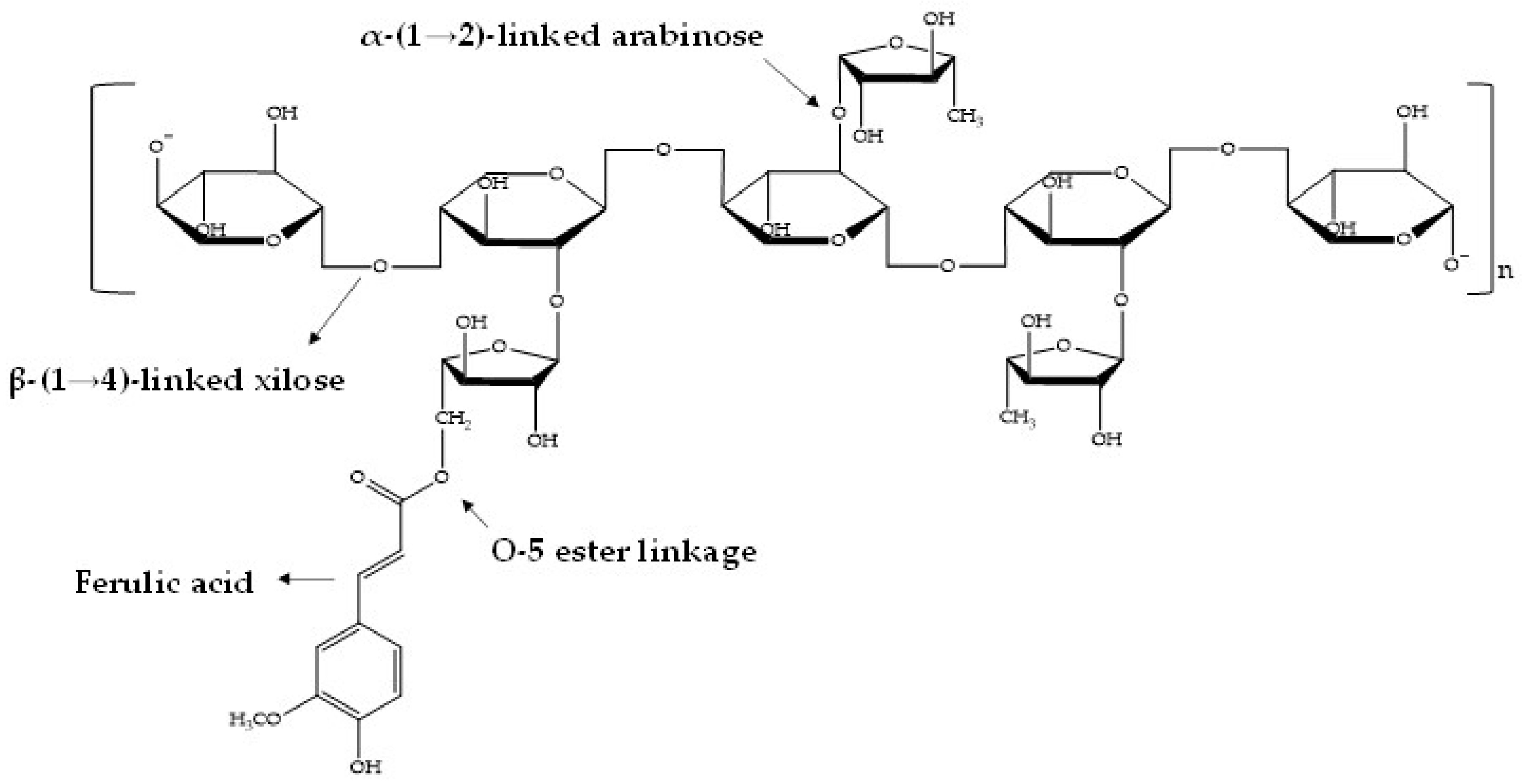
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. AX Recovery
2.3. AX Characterization
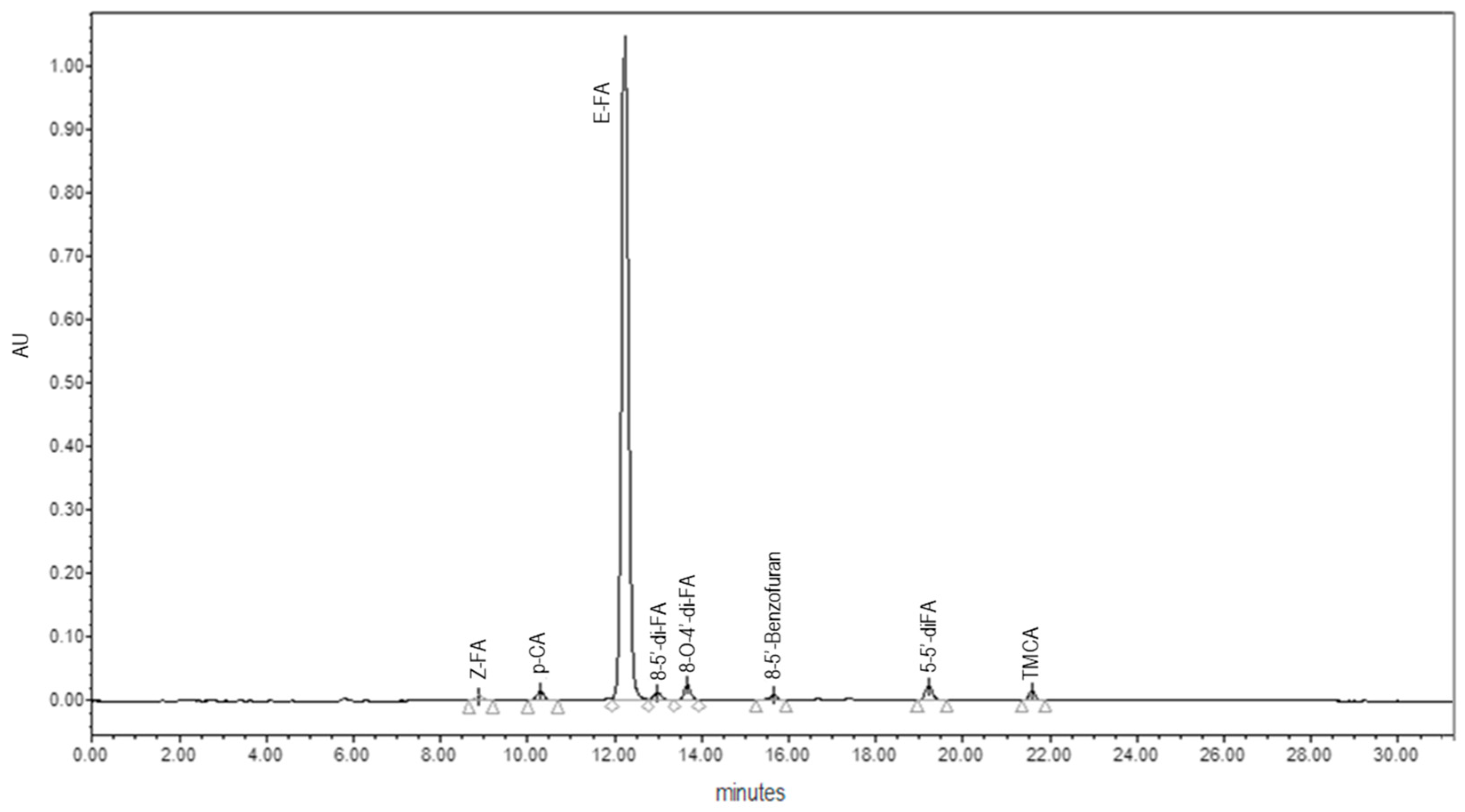
2.3.1. FA, di-FA and tri-FA Contents
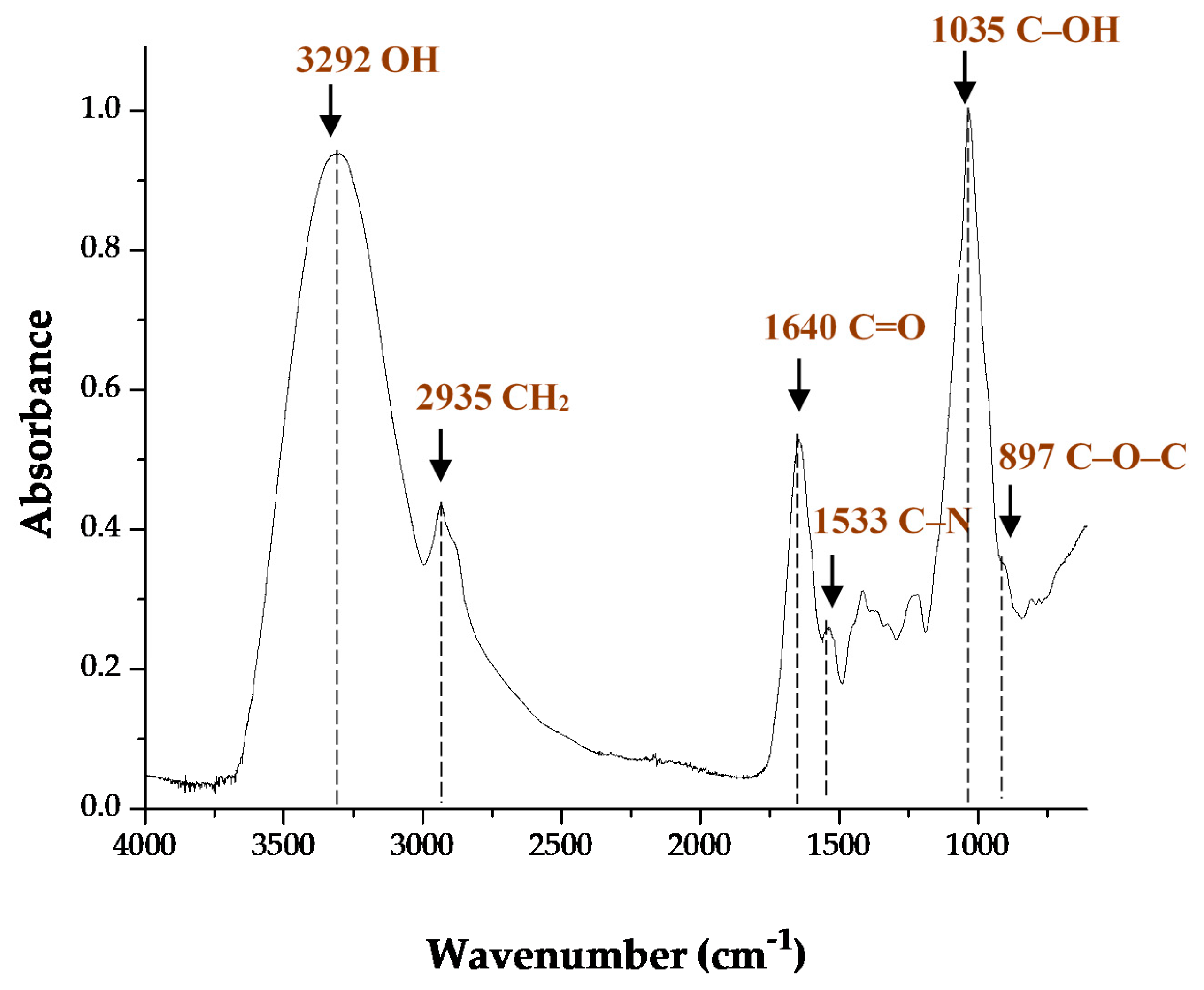
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.3. Macromolecular Characteristics
2.3.4. Gelation
2.3.5. Scanning Electron Microscopy (SEM) Analysis
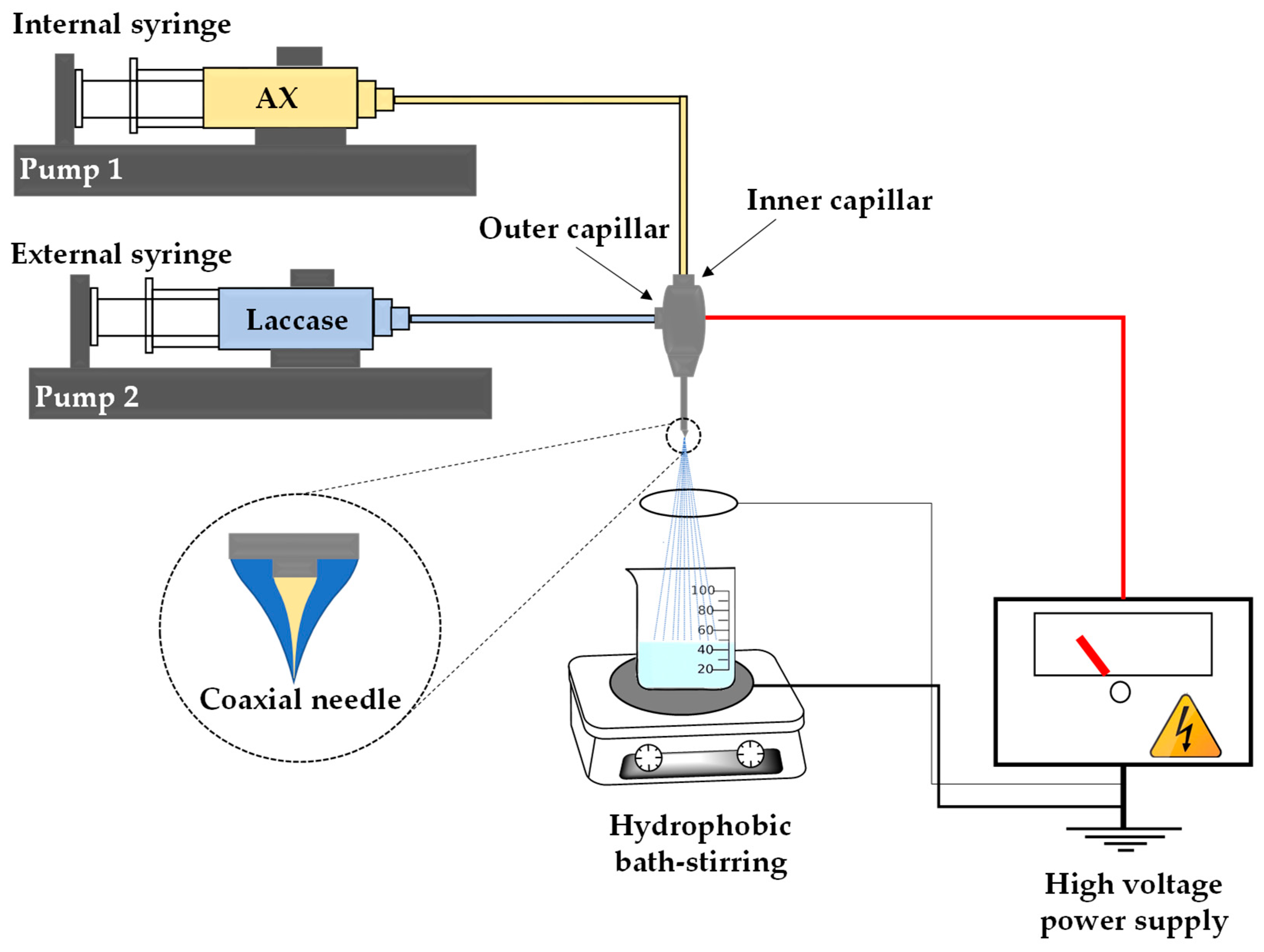
2.4. Arabinoxylan Nanoparticles (NAX) Preparation
2.5. NAX Characterization
2.5.1. Dynamic Light Scattering (DLS)
2.5.2. Transmission Electron Microscopy (TEM) Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. AX Recovery
3.2. AX Characterization
3.2.1. Phenolic Acids
3.2.2. FTIR Spectroscopy
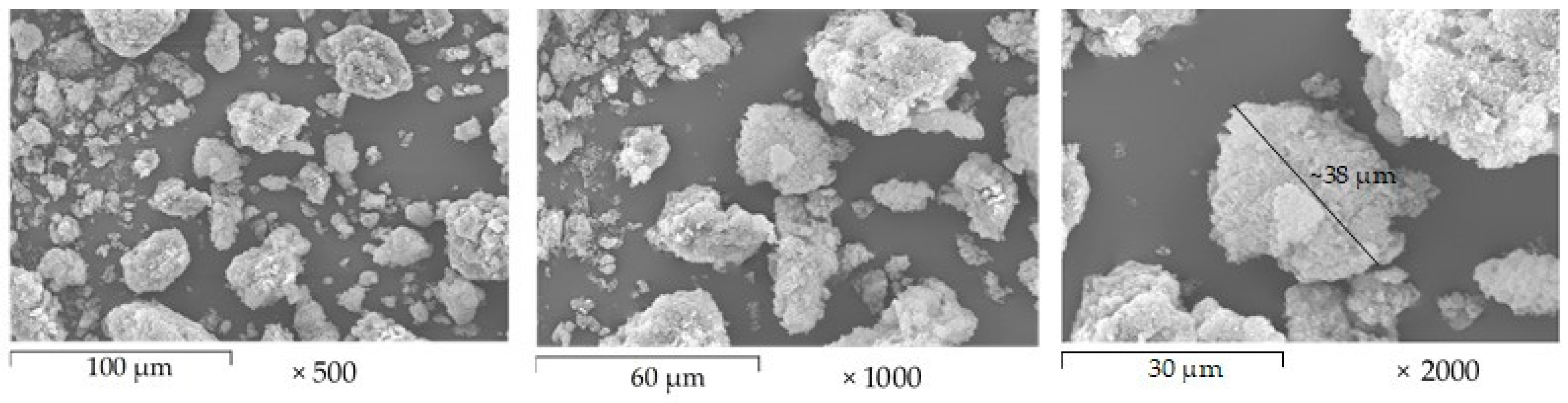
3.2.3. Microstructure of AX powder
3.2.4. Gelling of AX
3.3. AX Nanoparticles (NAX) Preparation and Characterization
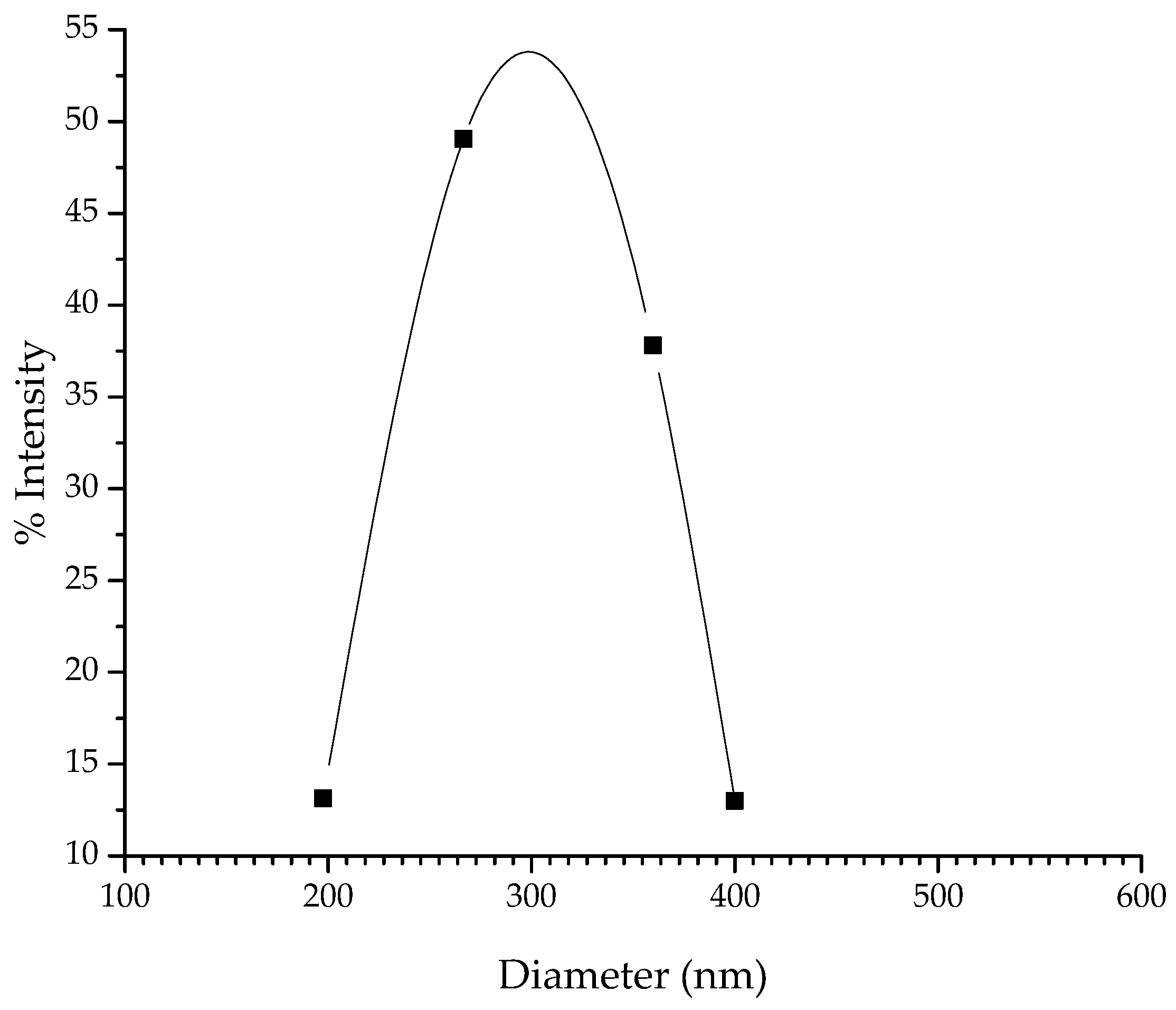
3.3.1. Size Distribution of NAX
3.3.2. Morphology of NAX
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Smith, C.; Li, W. Extraction and modification technology of arabinoxylans from cereal by-products: A critical review. Food Res. Int. 2014, 65, 423–436. [Google Scholar] [CrossRef]
- Saulnier, L.; Guillon, F.; Sado, P.-E.; Chateigner-Boutin, A.-L.; Rouau, X. Plant Cell Wall Polysaccharides in Storage Organs: Xylans (Food Applications). In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Kiszonas, A.; Fuerst, E.P.; Morris, C.F. Wheat Arabinoxylan Structure Provides Insight into Function. Cereal Chem. J. 2013, 90, 387–395. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Rascón-Chu, A.; Marquez-Escalante, J.; Micard, V.; De León, N.P.; Gardea, A. Maize bran gum: Extraction, characterization and functional properties. Carbohydr. Polym. 2007, 69, 280–285. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Charalampopoulos, D. Distiller’s dried grains with solubles (DDGS) and intermediate products as starting materials in biorefinery strategies. In Sustainable Recovery and Reutilization of Cereal Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2018; pp. 63–86. [Google Scholar]
- Rodríguez, V.; Revilla, P.; Ordás, B. New Perspectives in Maize Breeding. In Maize Cultivation, Uses and Health Benefits; Jimenez-lopez, J.C., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 27–48. [Google Scholar]
- Reis, S.F.; Coelho, E.; Coimbra, M.A.; Abu-Ghannam, N. Influence of grain particle sizes on the structure of arabinoxylans from brewer’s spent grain. Carbohydr. Polym. 2015, 130, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Millan, E.; Landillon, V.; Morel, M.-H.; Rouau, X.; Doublier, J.-L.; Micard, V. Arabinoxylan Gels: Impact of the Feruloylation Degree on Their Structure and Properties. Biomacromolecules 2005, 6, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Espinoza, M.C.; Rouau, X. Oxidative Cross-Linking of Pentosans by a Fungal Laccase and Horseradish Peroxidase: Mechanism of Linkage Between Feruloylated Arabinoxylans. Cereal Chem. J. 1998, 75, 259–265. [Google Scholar] [CrossRef]
- Marquez-Escalante, J.; Carvajal-Millan, E.; Miki-Yoshida, M.; Álvarez-Contreras, L.; Toledo-Guillén, A.R.; Lizardi-Mendoza, J.; Rascón-Chu, A. Water Extractable Arabinoxylan Aerogels Prepared by Supercritical CO2 Drying. Molecules 2013, 18, 5531–5542. [Google Scholar] [CrossRef]
- González, R.; Calderón-Santoyo, M.; Carvajal-Millan, E.; Ascencio, F.; Ragazzo-Sánchez, J.A.; Brown, F.; Rascón-Chu, A. Covalently Cross-Linked Arabinoxylans Films for Debaryomyces hansenii Entrapment. Molecules 2015, 20, 11373–11386. [Google Scholar] [CrossRef]
- Paz-Samaniego, R.; Rascón-Chu, A.; Brown, F.; Carvajal-Millan, E.; Pedroza-Montero, M.R.; Silva-Campa, E.; Sotelo-Cruz, N.; López-Franco, Y.; Lizardi-Mendoza, J. Electrospray-assisted fabrication of core-shell arabinoxylan gel particles for insulin and probiotics entrapment. J. Appl. Polym. Sci. 2018, 135, 46411. [Google Scholar] [CrossRef]
- Martínez-López, A.L.; Carvajal-Millan, E.; Miki-Yoshida, M.; Álvarez-Contreras, L.; Rascón-Chu, A.; Lizardi-Mendoza, J.; López-Franco, Y. Arabinoxylan Microspheres: Structural and Textural Characteristics. Molecules 2013, 18, 4640–4650. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Rascon-Chu, A.; Morales-Burgos, A.M.; Campa-Mada, A.C.; Martinez-Robison, K.G.; Marquez-Escalante J, T.-G.A.R. Method for the Obtaining Functionalized Arabinoxylans to Form Gelled Nanoparticles Covalent and to Enhance Beneficial Health Effects. Patent Application No. MX/a/2018/007070, 7 June 2018. [Google Scholar]
- Sonaje, K.; Lin, Y.-H.; Juang, J.-H.; Wey, S.-P.; Chen, C.-T.; Sung, H.-W. In vivo evaluation of safety and efficacy of self-assembled nanoparticles for oral insulin delivery. Biomaterials 2009, 30, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.; Tankhiwale, R. Investigation of dynamic release of vitamin B2 from calcium alginate/chitosan multilayered beads: Part II. React. Funct. Polym. 2006, 66, 1565–1574. [Google Scholar] [CrossRef]
- Seidi, F.; Jenjob, R.; Phakkeeree, T.; Crespy, D. Saccharides, oligosaccharides, and polysaccharides nanoparticles for biomedical applications. J. Control. Release 2018, 284, 188–212. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.K.; Reddy, P.R.; Lee, Y.-I.; Kim, C. Synthesis and characterization of chitosan–PEG–Ag nanocomposites for antimicrobial application. Carbohydr. Polym. 2012, 87, 920–925. [Google Scholar] [CrossRef]
- Yang, J.; Han, S.; Zheng, H.; Dong, H.; Liu, J. Preparation and application of micro/nanoparticles based on natural polysaccharides. Carbohydr. Polym. 2015, 123, 53–66. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Torres-Chávez, P.I.; Ramírez-Wong, B.; Rascón-Chu, A.; Plascencia-Jatomea, M.; Barreras-Urbina, C.G.; Rangel-Vázquez, N.A.; Rodríguez-Félix, F. Micro- and Nanoparticles by Electrospray: Advances and Applications in Foods. J. Agric. Food Chem. 2015, 63, 4699–4707. [Google Scholar] [CrossRef] [PubMed]
- Jaworek, A.; Sobczyk, A.T.; Krupa, A. Electrospray application to powder production and surface coating. J. Aerosol Sci. 2018, 125, 57–92. [Google Scholar] [CrossRef]
- Lenggoro, W.; Xia, B.; Okuyama, K.; De La Mora, J.F. Sizing of Colloidal Nanoparticles by Electrospray and Differential Mobility Analyzer Methods. Langmuir 2002, 18, 4584–4591. [Google Scholar] [CrossRef]
- Vansteenkiste, E.; Babot, C.; Rouau, X.; Micard, V. Oxidative gelation of feruloylated arabinoxylan as affected by protein. Influence on protein enzymatic hydrolysis. Food Hydrocoll. 2004, 18, 557–564. [Google Scholar] [CrossRef]
- Marquez-Escalante, J.; Carvajal-Millan, E. Feruloylated Arabinoxylans from Maize Distiller’s Dried Grains with Solubles: Effect of Feruloyl Esterase on their Macromolecular Characteristics, Gelling, and Antioxidant Properties. Sustainability 2019, 11, 6449. [Google Scholar] [CrossRef]
- Dervilly-Pinel, G.; Thibault, J.-F.; Saulnier, L. Experimental evidence for a semi-flexible conformation for arabinoxylans. Carbohydr. Res. 2001, 330, 365–372. [Google Scholar] [CrossRef]
- Rascón-Chu, A.; Baca, J.A.D.; Carvajal-Millan, E.; Pérez, E.; Hotchkiss, A.; González-Ríos, H.; Balandrán-Quintana, R.R.; Campa-Mada, A.C. Electrosprayed Core-Shell Composite Microbeads Based on Pectin-Arabinoxylans for Insulin Carrying: Aggregation and Size Dispersion Control. Polymers 2018, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Rochín-Wong, S.; Rosas-Durazo, A.; Zavala-Rivera, P.; Maldonado, A.; Mertinez-Barbosa, M.E.; Vélaz, I.; Córdova, J.T. Drug Release Properties of Diflunisal from Layer-By-Layer Self-Assembled κ-Carrageenan/Chitosan Nanocapsules: Effect of Deposited Layers. Polymers 2018, 10, 760. [Google Scholar] [CrossRef]
- Mendez-Encinas, M.A.; Carvajal-Millan, E.; Yadav, M.P.; López-Franco, Y.; Rascón-Chu, A.; Lizardi-Mendoza, J.; Brown, F.; Silva-Campa, E.; Pedroza-Montero, M.R. Partial removal of protein associated with arabinoxylans: Impact on the viscoelasticity, crosslinking content, and microstructure of the gels formed. J. Appl. Polym. Sci. 2018, 136, 136. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Guigliarelli, B.; Belle, V.; Rouau, X.; Micard, V. Storage stability of laccase induced arabinoxylan gels. Carbohydr. Polym. 2005, 59, 181–188. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Biliaderis, C.G. Cereal arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Kale, M.S.; Hamaker, B.R.; Campanella, O. Alkaline extraction conditions determine gelling properties of corn bran arabinoxylans. Food Hydrocoll. 2013, 31, 121–126. [Google Scholar] [CrossRef]
- Bunzel, M.; Ralph, J.; Marita, J.M.; Hatfield, R.D.; Steinhart, H. Diferulates as structural components in soluble and insoluble cereal dietary fibre. J. Sci. Food Agric. 2001, 81, 653–660. [Google Scholar] [CrossRef]
- Morales-Ortega, A.; Carvajal-Millan, E.; López-Franco, Y.; Rascón-Chu, A.; Lizardi-Mendoza, J.; Torres-Chávez, P.; Campa-Mada, A.C. Characterization of Water Extractable Arabinoxylans from a Spring Wheat Flour: Rheological Properties and Microstructure. Molecules 2013, 18, 8417–8428. [Google Scholar] [CrossRef]
- Schendel, R.R.; Meyer, M.R.; Bunzel, M. Quantitative Profiling of Feruloylated Arabinoxylan Side-Chains from Graminaceous Cell Walls. Front. Plant Sci. 2016, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, A.L.; Carvajal-Millan, E.; Lizardi-Mendoza, J.; lópez-Franco, Y.L.; Rascon-Chu, A.; Salas-Muñoz, E.; Ramírez-Wong, B. Ferulated Arabinoxylans as by-Product from Maize Wet-Milling Process: Characterization and Gelling Capability. In Maize Cultivation, Uses and Health Benefits; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 65–74. [Google Scholar]
- Xiang, Z.; Anthony, R.; Tobimatsu, Y.; Runge, T. Emulsifying properties of an arabinoxylan–protein gum from distillers’s grains and the co-production of animal feed. Cellulose 2014, 21, 3623–3635. [Google Scholar] [CrossRef]
- Iravani, S.; Fitchett, C.S.; Georget, D.M. Physical characterization of arabinoxylan powder and its hydrogel containing a methyl xanthine. Carbohydr. Polym. 2011, 85, 201–207. [Google Scholar] [CrossRef]
- Kacuráková, M.; Ebringerová, A.; Hirsch, J.; Hromádková, Z. Infrared study of arabinoxylans. J. Sci. Food Agric. 1994, 66, 423–427. [Google Scholar] [CrossRef]
- Sene, C.; McCann, M.C.; Wilson, R.H.; Grinter, R. Fourier-Transform Raman and Fourier-Transform Infrared Spectroscopy (An Investigation of Five Higher Plant Cell Walls and Their Components). Plant Physiol. 1994, 106, 1623–1631. [Google Scholar] [CrossRef]
- Robert, P.; Marquis, M.; Barron, C.; Guillon, F.; Saulnier, L. FT-IR Investigation of Cell Wall Polysaccharides from Cereal Grains. Arabinoxylan Infrared Assignment. J. Agric. Food Chem. 2005, 53, 7014–7018. [Google Scholar] [CrossRef]
- Martínez-López, A.; Carvajal-Millan, E.; Rascón-Chu, A.; Márquez-Escalante, J.; Martínez-Robinson, K. Gels of ferulated arabinoxylans extracted from nixtamalized and non-nixtamalized maize bran: Rheological and structural characteristics. CyTA J. Food 2013, 11, 22–28. [Google Scholar] [CrossRef]
- Hatfield, R.D.; Ralph, J. Modelling the feasibility of intramolecular dehydrodiferulate formation in grass walls. J. Sci. Food Agric. 1999, 79, 425–427. [Google Scholar] [CrossRef]
- Mendez-Encinas, M.A.; Carvajal-Millan, E.; Rascón-Chu, A.; Astiazarán-García, H.; Valencia-Rivera, D.E.; Brown, F.; Alday, E.; Velazquez, C. Arabinoxylan-Based Particles: In Vitro Antioxidant Capacity and Cytotoxicity on a Human Colon Cell Line. Medicina 2019, 55, 349. [Google Scholar] [CrossRef]
- Anderson, C.; Simsek, S. What Are the Characteristics of arabinoxylan gels? Food Nutr. Sci. 2018, 9, 818–833. [Google Scholar] [CrossRef]
- Ghayempour, S.; Mortazavi, S.M. Fabrication of micro–nanocapsules by a new electrospraying method using coaxial jets and examination of effective parameters on their production. J. Electrost. 2013, 71, 717–727. [Google Scholar] [CrossRef]
- Kim, J.; Moon, M.J.; Kim, D.Y.; Heo, S.H.; Jeong, Y.Y. Hyaluronic Acid-Based Nanomaterials for Cancer Therapy. Polymers 2018, 10, 1133. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Sarmentocde, B.; Ribeiro, A.J.; Veiga, F.; Sampaio, P.; Neufeld, R.; Ferreira, D. Alginate/Chitosan Nanoparticles are Effective for Oral Insulin Delivery. Pharm. Res. 2007, 24, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Ahuja, M. Psyllium arabinoxylan: Carboxymethylation, characterization and evaluation for nanoparticulate drug delivery. Int. J. Biol. Macromol. 2015, 72, 495–501. [Google Scholar] [CrossRef]
- Huang, X.; Dai, Y.; Cai, J.; Zhong, N.; Xiao, H.; McClements, D.J.; Hu, K. Resveratrol encapsulation in core-shell biopolymer nanoparticles: Impact on antioxidant and anticancer activities. Food Hydrocoll. 2017, 64, 157–165. [Google Scholar] [CrossRef]


| Component | Value |
|---|---|
| FA (µg/mg AX) 1 | 7.30 ± 0.20 |
| Total, di-FA (µg/mg AX) 1 | 0.212 ± 0.009 |
| 5,5′ | 0.100 ± 0.005 |
| 8–5′ | 0.090 ± 0.002 |
| 8-O-4′ | 0.021 ± 0.006 |
| tri-FA (µg/mg AX) | traces |
| A/X ratio 1 | 1.2 |
| Molecular weight (Mw) kDa 2 | 661 |
| Polydispersity index I = (Mw/Mn) 2 | 2.4 |
| Intrinsic viscosity [η] (mL/g) 2 | 149 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Anda-Flores, Y.; Carvajal-Millan, E.; Lizardi-Mendoza, J.; Rascon-Chu, A.; Martínez-López, A.L.; Marquez-Escalante, J.; Brown-Bojorquez, F.; Tanori-Cordova, J. Covalently Cross-Linked Nanoparticles Based on Ferulated Arabinoxylans Recovered from a Distiller’s Dried Grains Byproduct. Processes 2020, 8, 691. https://doi.org/10.3390/pr8060691
De Anda-Flores Y, Carvajal-Millan E, Lizardi-Mendoza J, Rascon-Chu A, Martínez-López AL, Marquez-Escalante J, Brown-Bojorquez F, Tanori-Cordova J. Covalently Cross-Linked Nanoparticles Based on Ferulated Arabinoxylans Recovered from a Distiller’s Dried Grains Byproduct. Processes. 2020; 8(6):691. https://doi.org/10.3390/pr8060691
Chicago/Turabian StyleDe Anda-Flores, Yubia, Elizabeth Carvajal-Millan, Jaime Lizardi-Mendoza, Agustin Rascon-Chu, Ana Luisa Martínez-López, Jorge Marquez-Escalante, Francisco Brown-Bojorquez, and Judith Tanori-Cordova. 2020. "Covalently Cross-Linked Nanoparticles Based on Ferulated Arabinoxylans Recovered from a Distiller’s Dried Grains Byproduct" Processes 8, no. 6: 691. https://doi.org/10.3390/pr8060691
APA StyleDe Anda-Flores, Y., Carvajal-Millan, E., Lizardi-Mendoza, J., Rascon-Chu, A., Martínez-López, A. L., Marquez-Escalante, J., Brown-Bojorquez, F., & Tanori-Cordova, J. (2020). Covalently Cross-Linked Nanoparticles Based on Ferulated Arabinoxylans Recovered from a Distiller’s Dried Grains Byproduct. Processes, 8(6), 691. https://doi.org/10.3390/pr8060691







