Synthesis, Characterization of sym-2,4,6-trisubstituted-s-Triazine Derivatives and Their Effects on Flame Retardancy of Polypropylene Composites
Abstract
1. Introduction
2. Materials
3. Methods
3.1. General Method for the Synthesis of sym-2,4,6-trisubstituted-s-Triazine
3.1.1. N2,N4,N6-triphenyl-1,3,5-triazine-2,4,6-triamine (TAT); 3a
3.1.2. N2,N4,N6-tris(4-bromophenyl)-1,3,5-triazine-2,4,6-triamine (TBAT); 3b
3.1.3. N2,N4,N6-tris(4-chlorophenyl)-1,3,5-triazine-2,4,6-triamine (TCAT); 3c
3.1.4. 4,4′,4″-((1,3,5-triazine-2,4,6-triyl) tris(azanediyl)) triphenol (THAT) 3d
3.1.5. N2,N4,N6-tris(4-methoxyphenyl)-1,3,5-triazine-2,4,6-triamine (TMAT); 3e
3.2. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
3.3. Flammability Test UL94
4. Results and Discussion
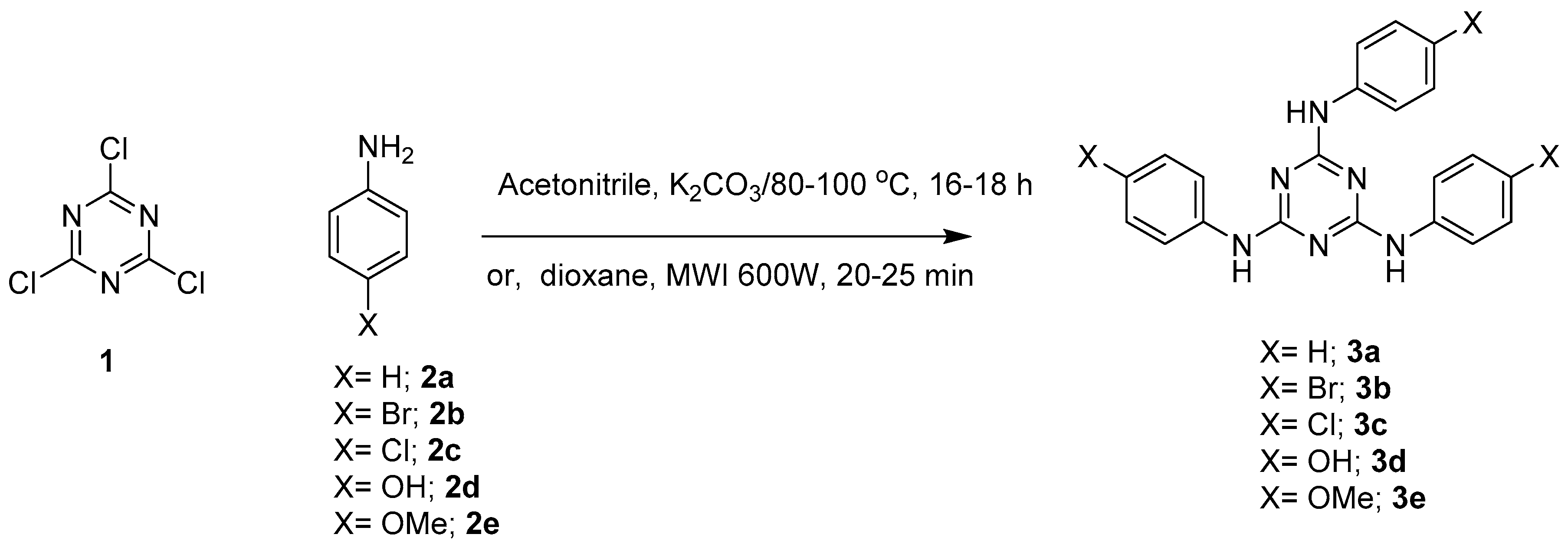
4.1. Synthesis of sym-2,4,6-trisubstituted-s-triazine Derivatives
4.2. Characterization of the Target Products
4.2.1. FTIR Spectroscopy
4.2.2. UV-Vis Spectra
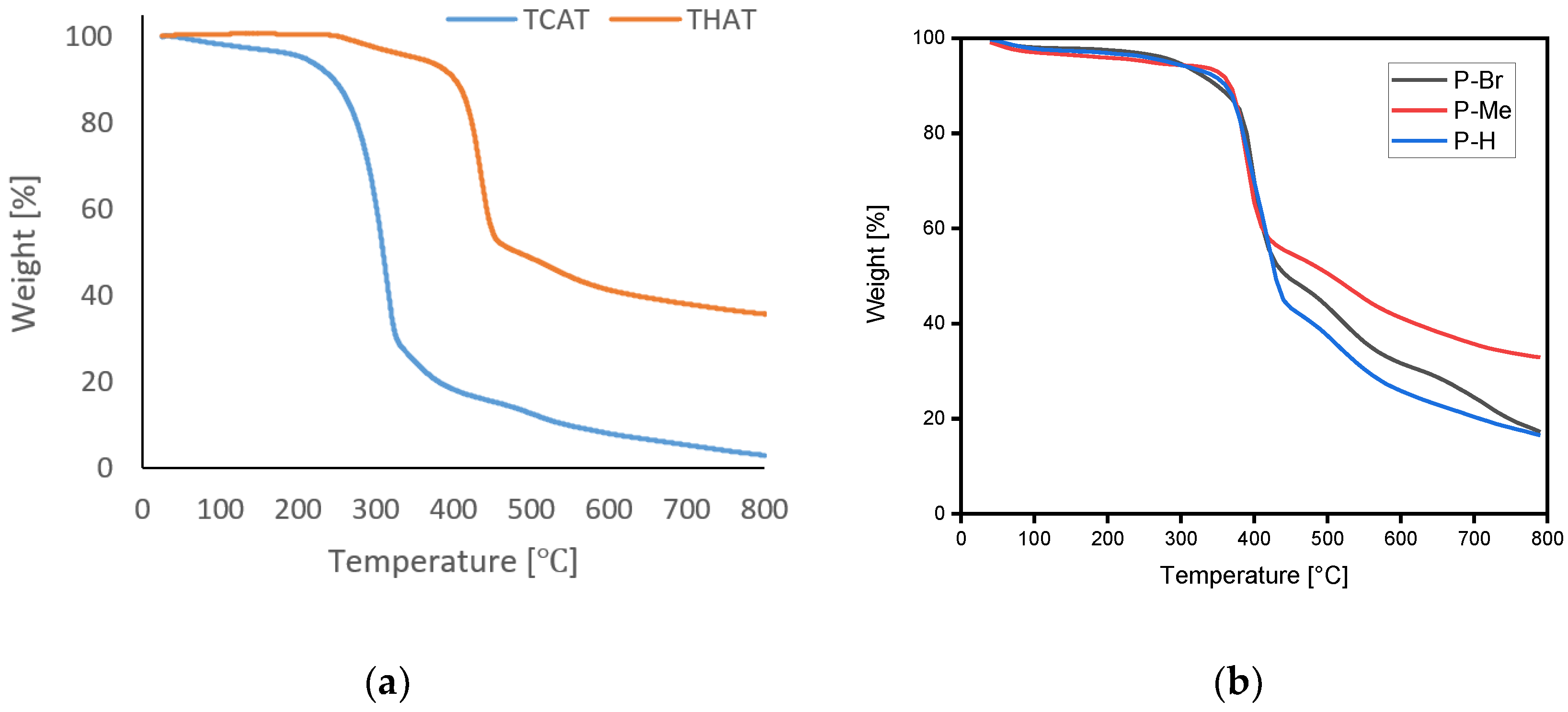
4.2.3. Thermogravimetric Analysis (TGA)
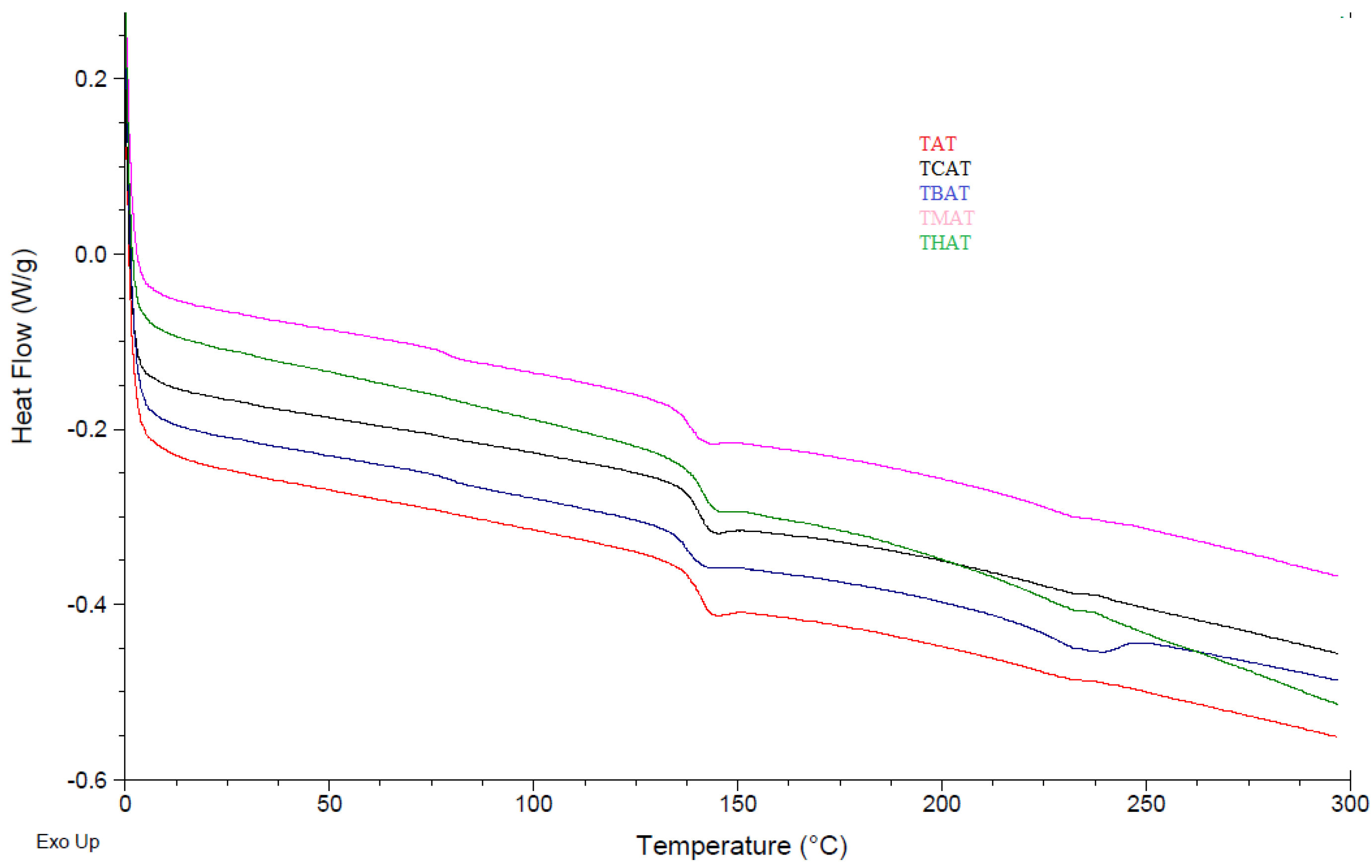
4.2.4. Differential Scanning Calorimetry (DSC)
4.2.5. Flammability of PP and Its Composites UL-94
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Duquesne, S.; Rochery, M. Recent advances for intumescent polymers. Macromol. Mater. Eng. 2004, 289, 499–511. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Jin, Y.; Zhang, J. Simulated effects of physical parameters on heat transfer of intumescent fire-retardant polypropylene during burning. J. Macromol. Sci. Phys. B 2011, 50, 1185–1195. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Deng, C.; Tan, Y.; Chen, M.-J.; Chen, L.; Wang, Y.-Z. Flame retardation of polypropylene via a novel intumescent flame retardant: Ethylenediamine-modified ammonium polyphosphate. Polym. Degrad. Stab. 2014, 106, 88–96. [Google Scholar] [CrossRef]
- Roder, H.; Vogl, O. 17th international Herman F. Mark symposium: Polypropylene—A material of the future. Prog. Polym. Sci. 1999, 24, 1205–1215. [Google Scholar] [CrossRef]
- Hu, X.P.; Li, W.Y.; Wang, Y.Z. Synthesis and characterization of a novel nitrogen-containing flame retardant. Appl. Polym. Sci. 2004, 94, 1556–1561. [Google Scholar] [CrossRef]
- Wang, W.; Wen, P.; Zhan, J.; Hong, N.; Cai, W.; Gui, Z.; Hu, Y. Synthesis of a novel charring agent containing pentaerythritol and triazine structure and its intumescent flame retardant performance for polypropylene. Polym. Degrad. Stab. 2017, 144, 454–463. [Google Scholar] [CrossRef]
- Alongi, J.; Han, Z.D.; Bourbigot, S. Intumescence: Tradition versus novelty. A comprehensive review. Prog. Polym. Sci. 2015, 51, 28–73. [Google Scholar] [CrossRef]
- Li, B.; Xu, M. Effect of a novel charring foaming agent on flame retardancy and thermal degradation of intumescent flame retardant polypropylene. Polym. Degrad. Stab. 2006, 91, 1380–1386. [Google Scholar] [CrossRef]
- Huo, S.; Wang, J.; Yang, S.; Zhang, B.; Tang, Y. A phosphorus-containing phenolic derivative and its application in benzoxazine resins: Curing behavior, thermal, and flammability properties. J. Appl. Polym. Sci. 2016, 43403, 1–7. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, W.; He, X.; Yang, R. High-efficiency flame retardency of epoxy resin composites with perfect T8 caged phosphorus containing polyhedral oligomeric silsesquioxanes (P-POSSs). Compos. Sci. Technol. 2016, 127, 8–19. [Google Scholar] [CrossRef]
- Rothon, R.N.; Hornsby, P.R. Flame retardant effects of magnesium hydroxide. Polym. Degrad. Stab. 1996, 54, 382–387. [Google Scholar] [CrossRef]
- Hornsby, P.R. The Application of Magnesium Hydroxide as a Fire Retardant and Smoke-suppressing Additive for Polymers. Fire Mater. 1994, 18, 267–269. [Google Scholar] [CrossRef]
- Yang, R.; Ma, B.; Zhao, H.; Li, J. Preparation, thermal degradation and fire behaviors of intumescent flame retardant polypropylene with a charring agent containing pentaerythritol and triazine. Ind. Eng. Chem. Res. 2016, 55, 5298–5305. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, X.; Liu, G.S. Synthesis of an integrated intumescent flame retardant and its flame retardancy properties for polypropylene. Polym. Degrad. Stab. 2017, 138, 106–114. [Google Scholar] [CrossRef]
- Johanna, P.; Ren, Y.L.; Boris, M.; Huo, T.G. Phosphorylated sodium alginate/APP/DPER intumescent flame retardant used for polypropylene. J. Appl. Polym. Sci. 2019, 136, 47794. [Google Scholar] [CrossRef]
- Huang, H.; Shi, Y.; Lv, G.P.; Liu, Y.; Wang, Q. Flame resistance and aging mechanism of flame retardant polycarbonate sheet containing linear phenolic resin charring agent. Polym. Degrad. Stab. 2015, 122, 139–145. [Google Scholar] [CrossRef]
- Chen, M.; Tang, M.; Ma, Y.; Chen, X.; Qin, J.; He, W.; Zhang, Z. Influence of polyamide 6 as a charring agent on the flame retardancy, thermal, and mechanical properties of polypropylene composites. Polym. Eng. Sci. 2015, 55, 1355–1360. [Google Scholar] [CrossRef]
- Dong, L.P.; Deng, C.; Wang, Y.Z. Influence of small difference in structure of polyamide charring agents on their flame-retardant efficiency in EVA. Polym. Degrad. Stab. 2017, 135, 130–139. [Google Scholar] [CrossRef]
- Bai, G.; Guo, C.G.; Li, L.P. Synergistic effect of intumescent flame retardant and expandable graphite on mechanical and flame-retardant properties of wood flour-polypropylene composites. Constr. Build. Mater. 2014, 50, 148–153. [Google Scholar] [CrossRef]
- Wang, G.; Bai, S.B. Synergistic effect of expandable graphite and melamine phosphate on flame-retardant polystyrene. J. Appl. Polym. Sci. 2017, 134, 45474. [Google Scholar] [CrossRef]
- Zhu, C.J.; He, M.S.; Cui, J.G.; Tai, Q.L.; Song, L.; Hu, Y. Synthesis of a novel hyperbranched and phosphorus-containing charring-foaming agent and its application in polypropylene. Polym. Adv. Technol. 2018, 29, 2449–2456. [Google Scholar] [CrossRef]
- Xu, B.; Ma, W.; Wu, X.; Qian, L.J.; Jiang, S. Flame retardancy and thermal behavior of intumescent flame-retardant EVA composites with an efficient triazine based charring agent. Mater. Res. Express 2018, 5, 045309. [Google Scholar] [CrossRef]
- Wang, X.J.; Wang, Z.B.; Li, J. Effects of a semi-bio-based triazine derivation on intumescent flame-reardant polypropylene. Polym. Adv. Technol. 2019, 30, 1259–1268. [Google Scholar] [CrossRef]
- Zhu, C.J.; He, M.S.; Liu, Y.; Cui, J.G.; Tai, Q.L.; Song, L.; Hu, Y. Synthesis and application of a mono-component intumescent flame retardant for polypropylene. Polym. Degrad. Stab. 2018, 151, 144–151. [Google Scholar] [CrossRef]
- Yang, K.; Xu, M.J.; Li, B. Synthesis of N-ethyl triazine-piperazine copolymer and flame retardancy and water resistance of intumescent flame retardant polypropylene. Polym. Degrad. Stab. 2013, 98, 1397–1406. [Google Scholar] [CrossRef]
- Zheng, Z.H.; Liu, S.F.; Wang, B.N.; Yang, T.; Cui, X.J.; Wang, H.Y. Preparation of a novel phosphorus- and nitrogen-containing flame retardant and its synergistic effect in the intumescent flame-retarding polypropylene system. Polym. Compos. 2015, 36, 1606–1619. [Google Scholar] [CrossRef]
- Wen, P.Y.; Feng, X.M.; Kan, Y.C.; Hu, Y.; Yuen, R.K.K. Synthesis of a novel triazine based polymeric flame retardant and its application in polypropylene. Polym. Degrad. Stab. 2016, 134, 202–210. [Google Scholar] [CrossRef]
- Xu, L.F.; Tan, X.W.; Xu, R.J.; Xie, J.Y.; Lei, C.H. Influence of functionalized molybdenum disulfide (MoS2) with triazine derivatives on the thermal stability and flame retardancy of intumescent poly(lactic acid) system. Polym. Compos. 2019, 40, 2244–2257. [Google Scholar] [CrossRef]
- Tang, S.; Qian, L.J.; Qiu, Y.; Sun, N. The effect of morphology on the flameretardant behaviors of melamine cyanurate in PA6 composites. J. Appl. Polym. Sci. 2014, 131, 40558. [Google Scholar] [CrossRef]
- Tang, W.; Qian, L.; Chen, Y.; Qiu, Y.; Xu, B. Intumescent flame retardant behavior of charring agents with different aggregation of piperazine/triazine groups in polypropylene. Polym. Degrad. Stab. 2019, 169, 108982. [Google Scholar] [CrossRef]
- Feng, C.M.; Zhang, Y.; Liu, S.W.; Chi, Z.G.; Xu, J.R. Synthesis of novel triazine charring agent and its effect in intumescent flame-retardant polypropylene. J. Appl. Polym. Sci. 2012, 123, 3208–3216. [Google Scholar] [CrossRef]
- Sibdas, S.M.; Niranjan, K. s-Triazine containing flame retardant hyperbranched polyamines: Synthesis, characterization and properties evaluation. Polym. Degrad. Stab. 2007, 92, 947–955. [Google Scholar]
- Xie, H.L.; Lai, X.J.; Li, H.Q.; Zeng, X.R. Synthesis of a novel macromolecular charring agent with free-radical quenching capability and its synergism in flame retardant polypropylene. Polym. Degrad. Stab. 2016, 130, 68–77. [Google Scholar] [CrossRef]
- Su, X.Q.; Yi, Y.W.; Tao, J.; Qi, H.Q.; Li, D.Y. Synergistic effect between a novel triazine charring agent and ammonium polyphosphate on flame retardancy and thermal behavior of polypropylene. Polym.Degrad. Stab. 2014, 105, 12–20. [Google Scholar] [CrossRef]
- Kien-Sin, L.; Soo-Tueen, B.; Lee, T.S.; Tiam-Ting, T.; Ratnam, C.T.; David, H.; Rahmat, A.R. A review of application of ammonium polyphosphate as intumescent flame retardant in thermoplastic composites. Compos. B Eng. 2016, 84, 155–174. [Google Scholar]
- Chen, J.L.; Wang, J.H.; Ni, A.Q.; Chen, H.D.; Shen, P.L. Synthesis of a novel phosphorous-nitrogen based charring agent and its application in flameretardant HDPE/IFR composites. Polymers 2019, 11, 1062. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wang, W.; Liu, Z.Q.; Yao, Y.Y.; Qian, L.J. Synthesis of a novel flame retardant containing phosphazene and trizine groups and its enhanced charring effect in poly(lactic acid) resin. J. Appl. Polym. Sci. 2017, 34, 44660. [Google Scholar] [CrossRef]
- Sharma, A.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Exploring the Orthogonal Chemoselectivity of 2,4,6-Trichloro-1,3,5-triazine (TCT) as a Trifunctional Linker with Different Nucleophiles: Rules of the Game. Front. Chem. 2018, 6, 516. [Google Scholar] [CrossRef]
- Huo, S.; Wang, J.; Yang, S.; Cai, H.; Zhang, B.; Chen, X.; Wu, Q.; Yang, L. Synergistic effect between a novel triazine-based flame retardant and DOPO/HPCP on epoxy resin. Polym. Adv. Technol. 2018, 29, 2774–2783. [Google Scholar] [CrossRef]
- Kolmakov, K.A. An Efficient, “Green” Approach to Aryl Amination of Cyanuric Chloride Using Acetic Acid as Solvent. J. Heterocycl. Chem. 2008, 45, 533–539. [Google Scholar] [CrossRef]
- Stagi, L.; Chiriu, D.; Scholz, M.; Carbonaro, C.; Corpino, R.; Porcheddu, A.; Rajamaki, S.; Cappellini, G.; Cardia, R.; Ricci, P. Vibrational and optical characterization of s-triazine derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 183, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.; Jin, K.; Sun, J.; Fang, Q. s-Triazine-based functional monomers with thermocrosslinkable propargyl units: Synthesis and conversion to the heat-resistant polymers. Polymer 2016, 102, 301–307. [Google Scholar] [CrossRef]
- de Hoog, P.; Gamez, P.; Driessen, W.; Reedijk, L.J. New polydentate and polynucleating N-donor ligands from amines and 2,4,6-trichloro-1,3,5-triazine. Tetrahedron Lett. 2002, 43, 6783–6786. [Google Scholar] [CrossRef]
- Cao, C.; Fang, Z. Substituent effects on the UV spectra of extended benzylidene anilines p-X-PhCH=NPhCH=CHPh-p-Y. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 111, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Chiu, Y.C.; Chen, T.Y. Phosphorus-containing polyaryloxy diphenylsilanes with high flame retardance arising from a phosphorus–silicon synergistic effect. Polym. Int. 2003, 52, 1256–1261. [Google Scholar] [CrossRef]
- van Krevelen, D.W. Some basic aspects of flame resistance of polymeric materials. Polymer 1975, 16, 615–620. [Google Scholar] [CrossRef]
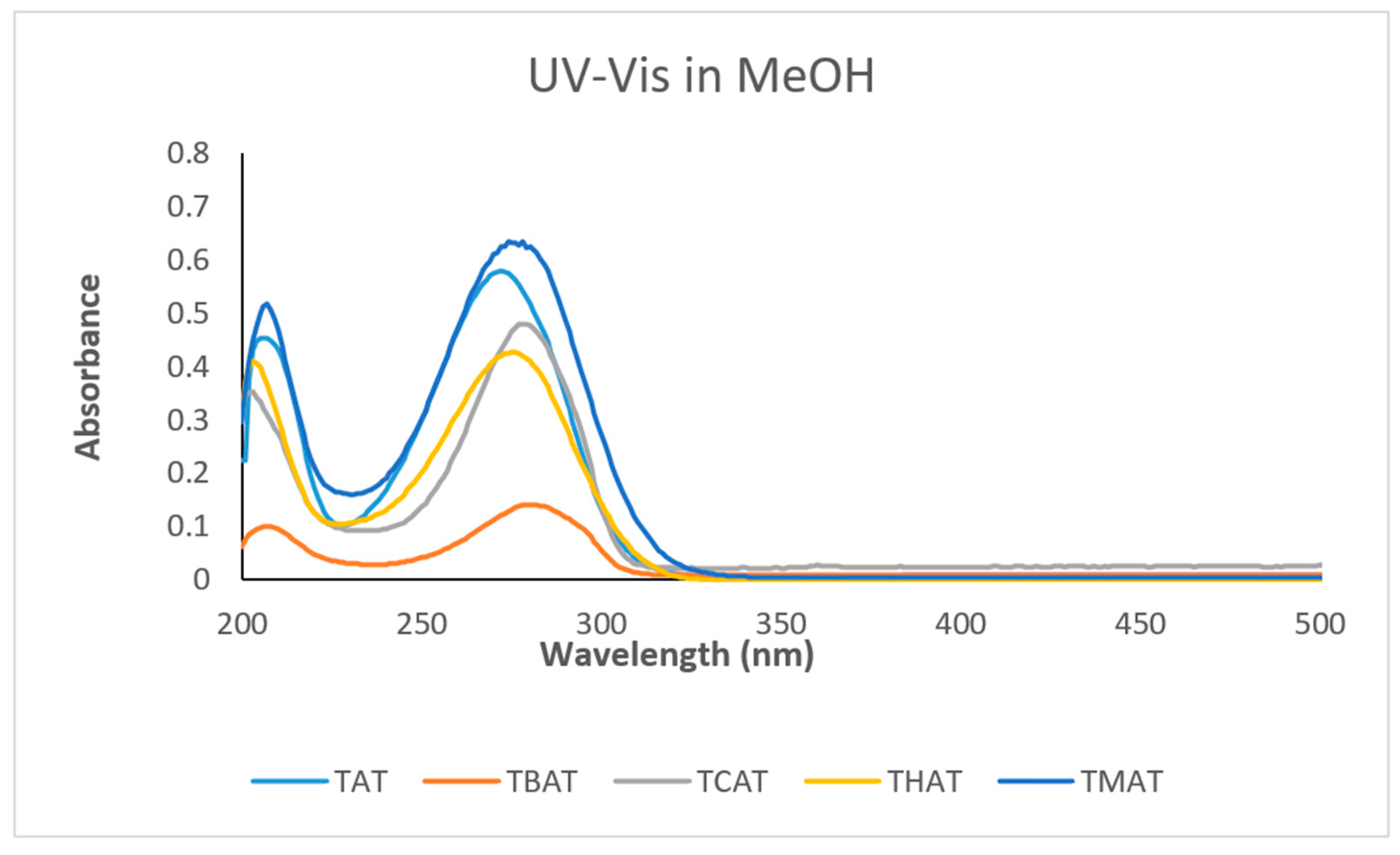

| Compound | FT-IR (cm−1) | λmax (nm) |
|---|---|---|
| TAT; 3a | 3370 (NH), 1500 (C=N), 1620, 1495 (C=C), 1260 (C-N) | 275 |
| TBAT; 3b | 3380 (NH), 1550 (C=N), 1600, 1440 (C=C), 1250 (C-N) | 283 |
| TCAT; 3c | 3390 (NH), 1550 (C=N), 1630, 1420 (C=C), 1240 (C-N) | 280 |
| THAT; 3d | 3400 (NH), 1500 (C=N), 1630, 1430 (C=C), 1260 (C-N) | 275 |
| TMAT; 3e | 3400 (NH), 1500 (C=N), 1640, 1450 (C=C), 1260 (C-N) | 278 |
| Sample ID | Temperature Range (°C) | Mass Loss (%) | Tg (°C) | CR at 600 °C | LOI at 600 °C | CR at 800 °C | LOI at 800 °C |
|---|---|---|---|---|---|---|---|
| TAT | 320–440 | 50 | 145 | 29 | 29.1 | 20 | 25.5 |
| TBAT | 330–440 | 60 | 140 | 35 | 31.5 | 22 | 26.3 |
| TCAT | 240–340 | 80 | 143 | 15 | 23.5 | 6 | 19.9 |
| THAT | 330–450 | 50 | 143 | 45 | 35.5 | 35 | 31.5 |
| TMAT | 350–450 | 50 | 141 | 45 | 35.5 | 36 | 31.9 |
| Sample ID | PP (wt%) | APP (wt%) | FR (wt%) | UL94 | Drip |
|---|---|---|---|---|---|
| PP neat | 100 | 0 | 0 | N/R | Yes |
| PPz | 75 | 0 | 25 | N/R | Yes |
| PPx | 75 | 0 | 25 | N/R | Yes |
| PPy | 75 | 0 | 25 | N/R | Yes |
| PPc | 75 | 12.5 | 12.5 | V-2 a | Yes |
| PPa | 75 | 12.5 | 12.5 | V-2 a | Yes |
| PPb | 75 | 12.5 | 12.5 | V-2 a | Yes |
| PP3 | 75 | 16.66 | 8.33 | V-0 b | No |
| PP1 | 75 | 16.66 | 8.33 | V-1 c | No |
| PP2 | 75 | 16.66 | 8.33 | V-1 c | No |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldalbahi, A.; Alotaibi, B.; El-Faham, A. Synthesis, Characterization of sym-2,4,6-trisubstituted-s-Triazine Derivatives and Their Effects on Flame Retardancy of Polypropylene Composites. Processes 2020, 8, 581. https://doi.org/10.3390/pr8050581
Aldalbahi A, Alotaibi B, El-Faham A. Synthesis, Characterization of sym-2,4,6-trisubstituted-s-Triazine Derivatives and Their Effects on Flame Retardancy of Polypropylene Composites. Processes. 2020; 8(5):581. https://doi.org/10.3390/pr8050581
Chicago/Turabian StyleAldalbahi, Ali, Bander Alotaibi, and Ayman El-Faham. 2020. "Synthesis, Characterization of sym-2,4,6-trisubstituted-s-Triazine Derivatives and Their Effects on Flame Retardancy of Polypropylene Composites" Processes 8, no. 5: 581. https://doi.org/10.3390/pr8050581
APA StyleAldalbahi, A., Alotaibi, B., & El-Faham, A. (2020). Synthesis, Characterization of sym-2,4,6-trisubstituted-s-Triazine Derivatives and Their Effects on Flame Retardancy of Polypropylene Composites. Processes, 8(5), 581. https://doi.org/10.3390/pr8050581






