Optimization of the Technological Parameters for Obtaining Zn-Ti Based Composites to Increase the Performance of H2S Removal from Syngas
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composites Synthesis
2.3. Composites Characterization
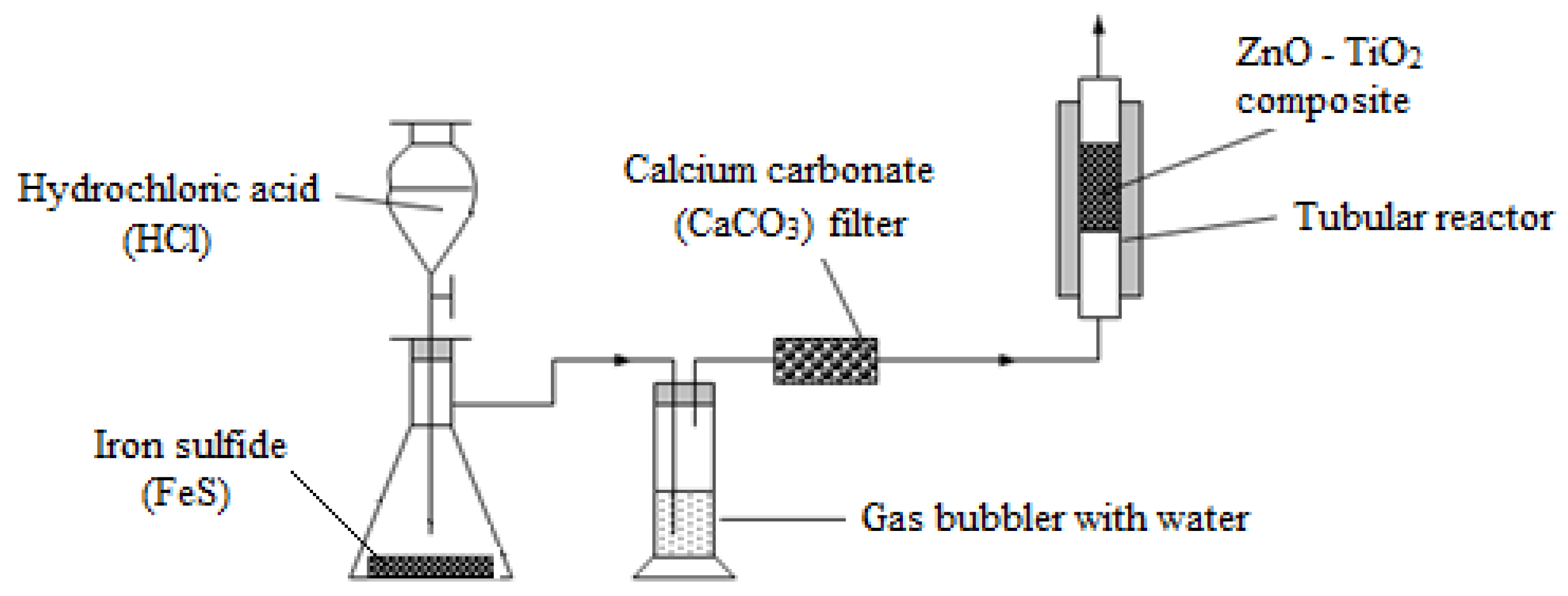
2.4. ZnO-TiO2 Composite Testing
2.4.1. ZnO-TiO2 Composite Sulfurization
2.4.2. ZnO-TiO2 Composite Regeneration
3. Results and Discussion
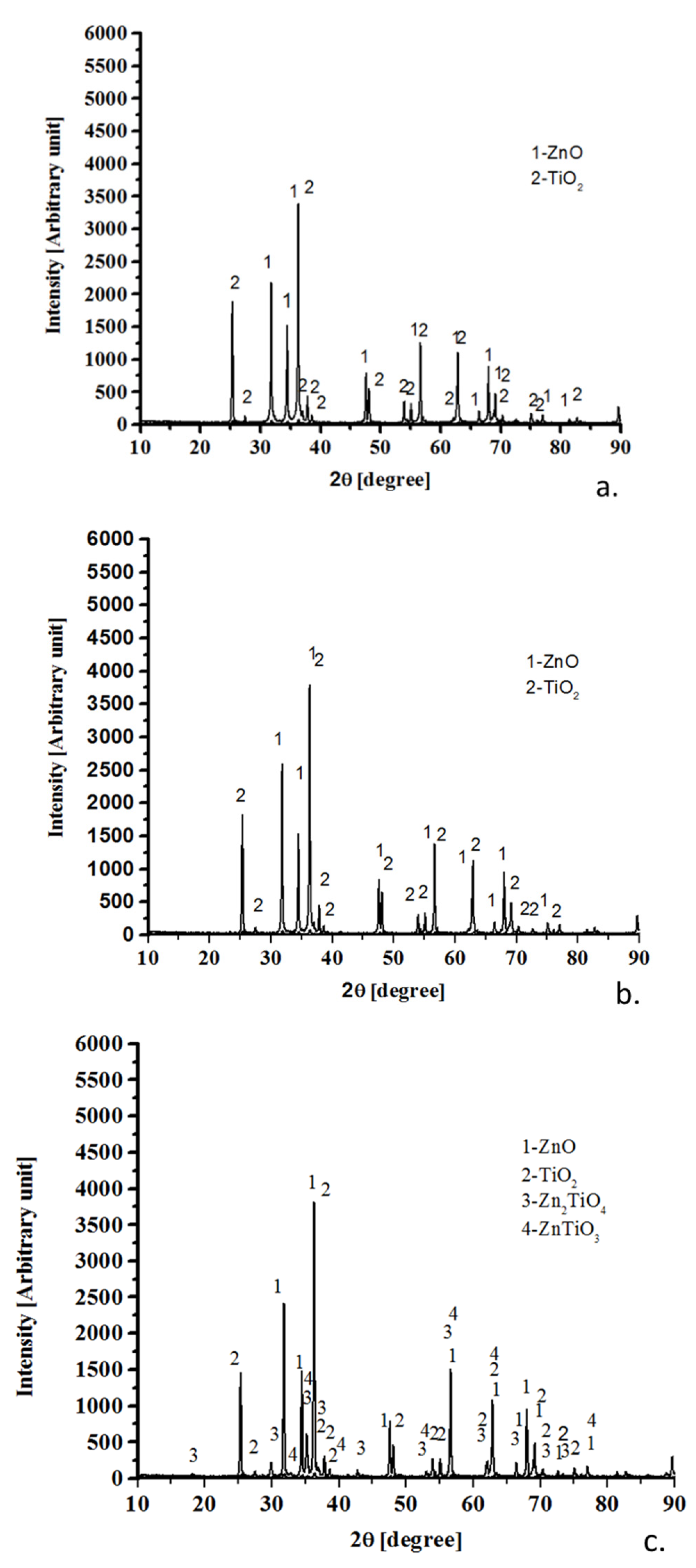
3.1. X-Ray Diffractometry
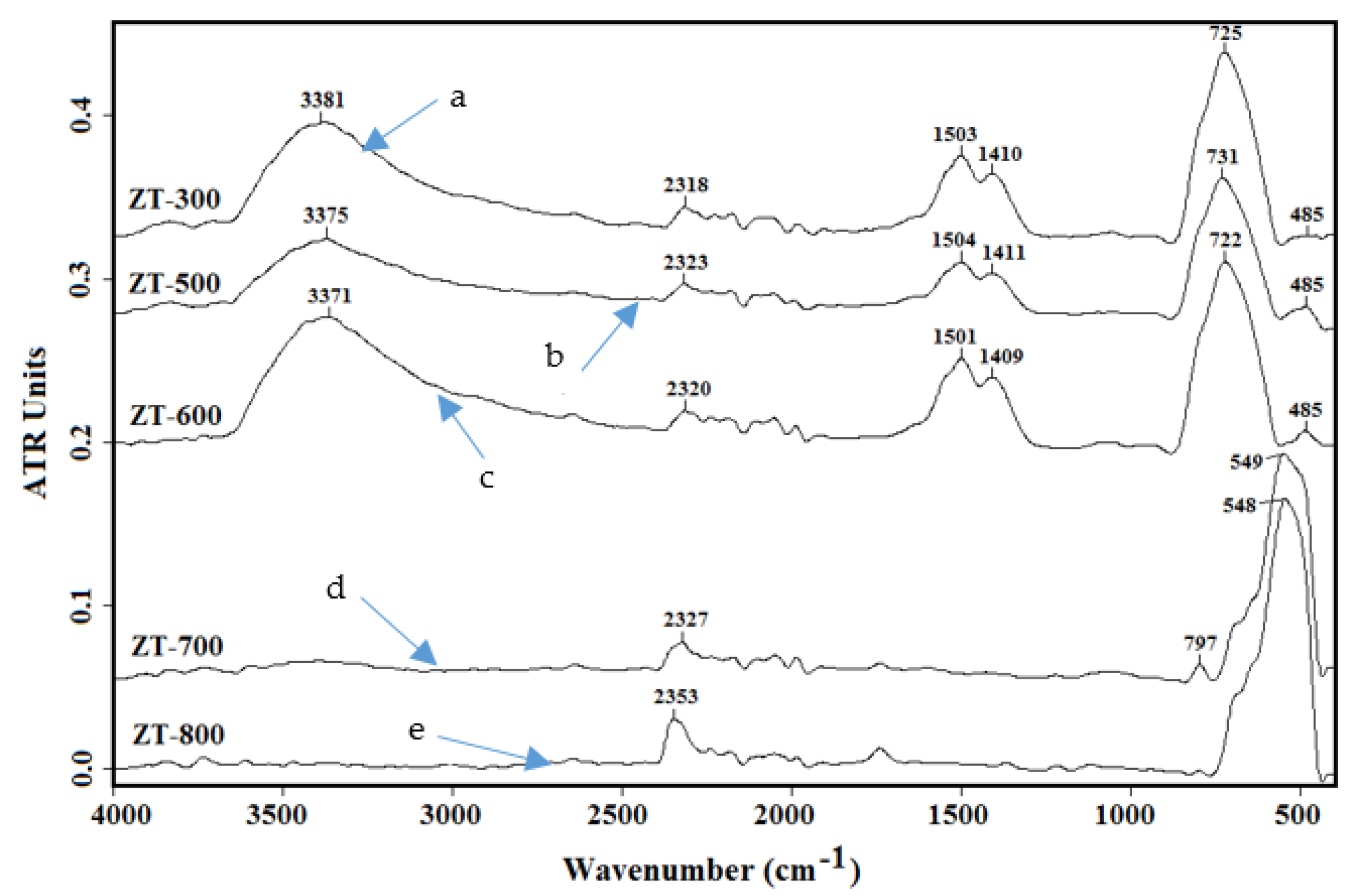
3.2. Infrared Spectroscopy
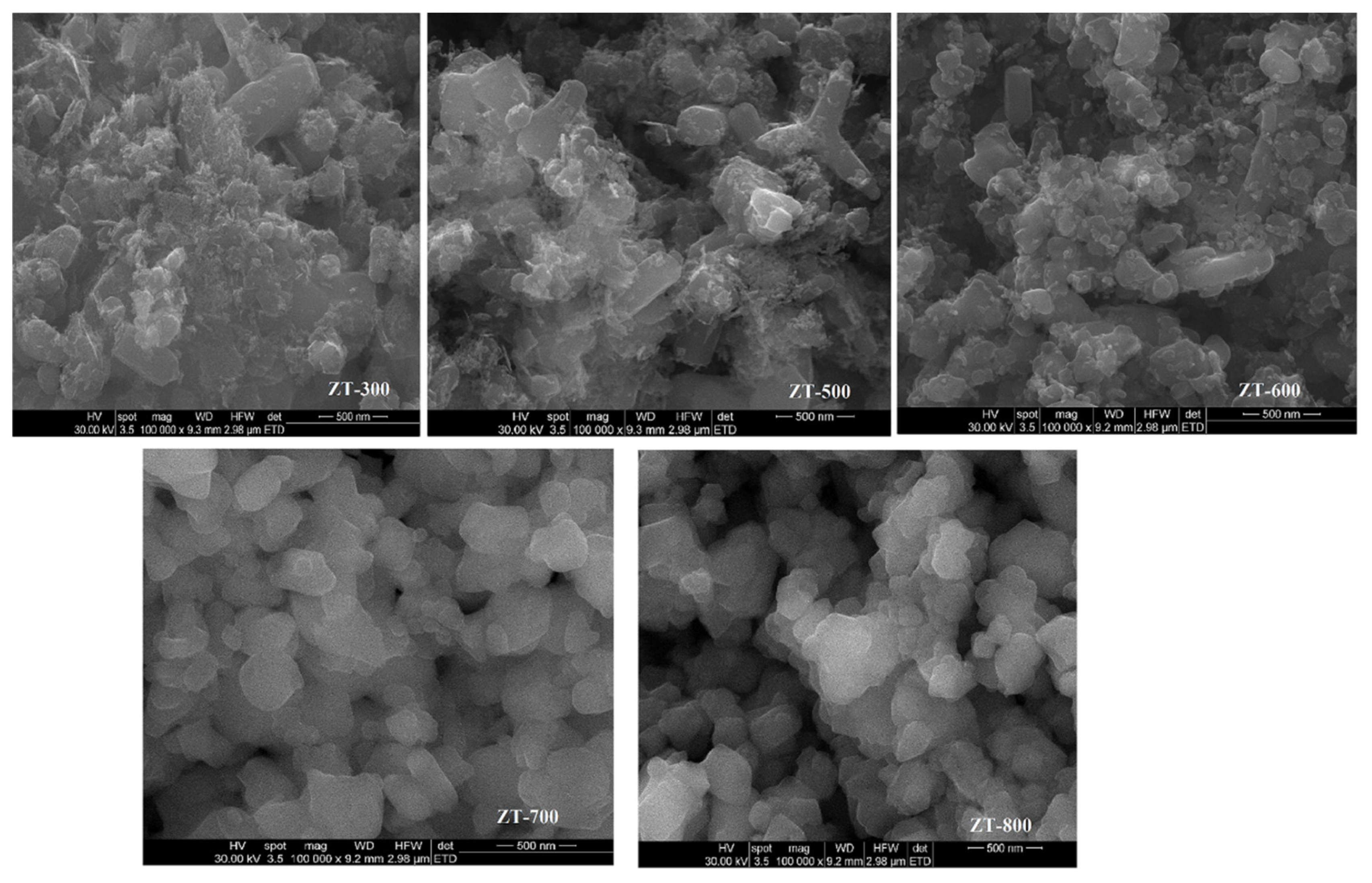
3.3. Scanning Electron Microscopy
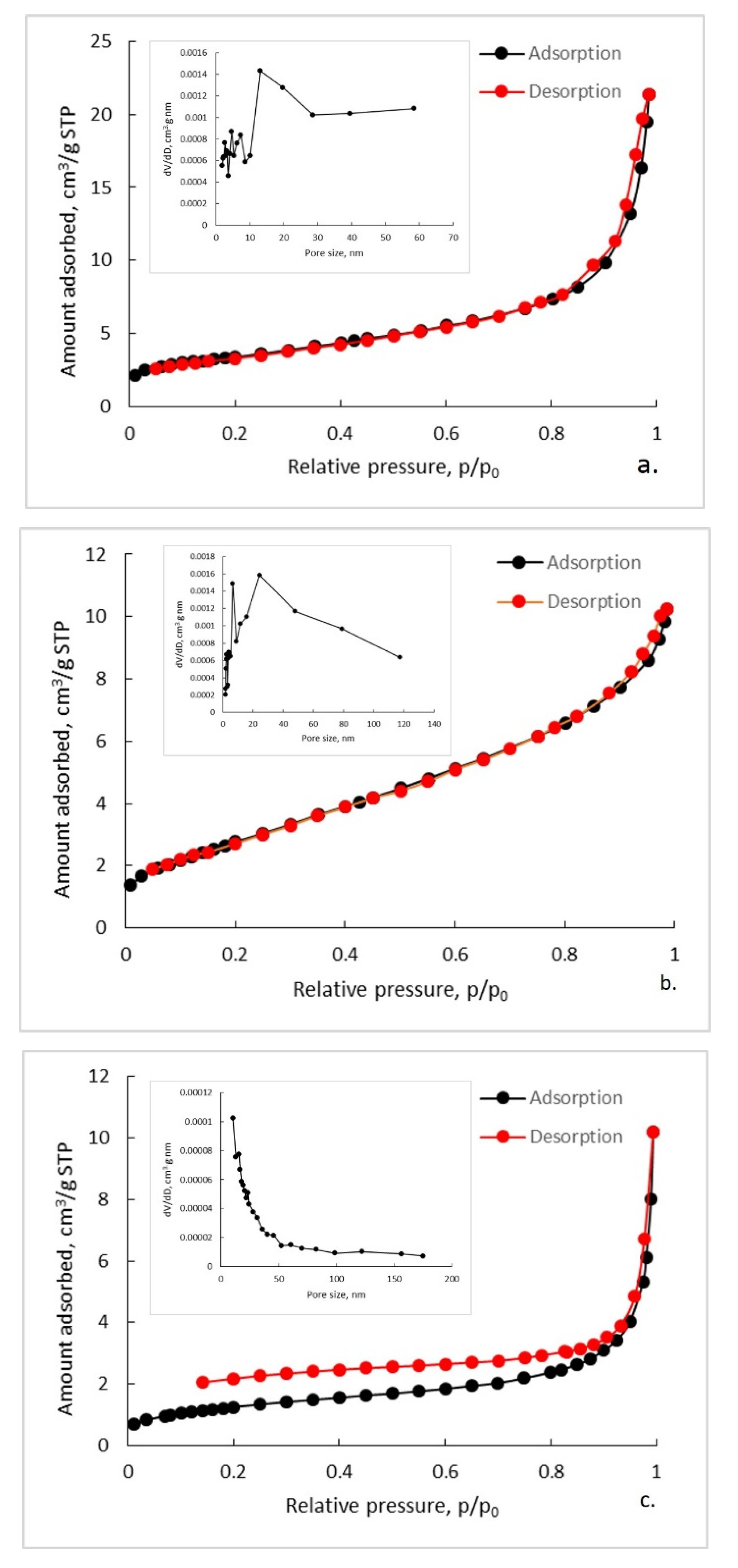
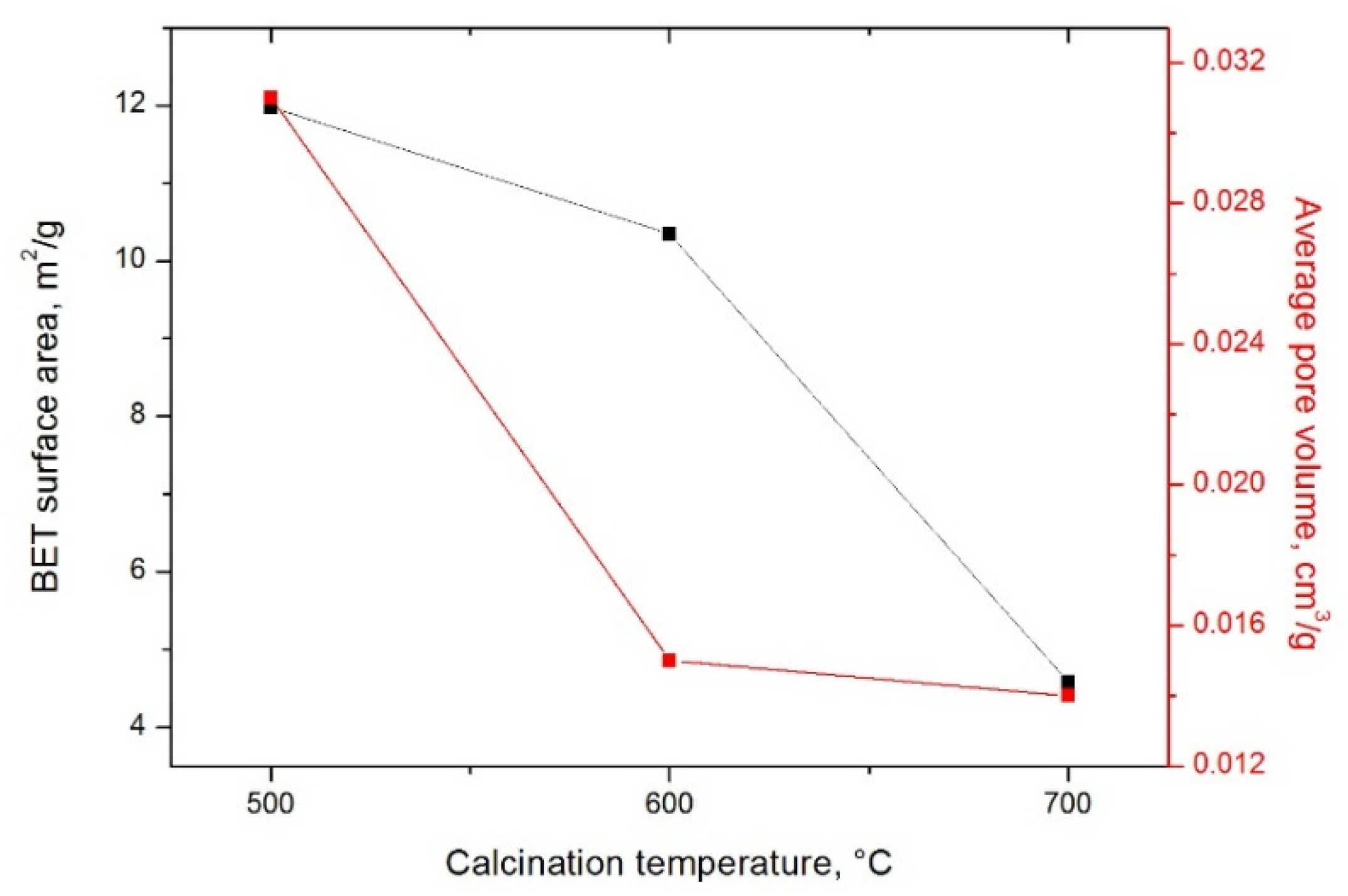
3.4. Textural Characterization of Synthetized Composites
3.5. ZnO-TiO2 Composite Testing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stolecka, K.; Rusin, A. Analysis of hazards related to syngas production and transport. Renew. Energy 2020, 146, 2535–2555. [Google Scholar] [CrossRef]
- Bassani, A.; Pirola, C.; Maggio, E.; Pettinau, A.; Frau, C.; Bozzano, G.; Pierucci, S.; Ranzi, E.; Manenti, F. Acid Gas to Syngas (AG2S™) technology applied to solid fuel gasification: Cutting H2S and CO2 emissions by improving syngas production. Appl. Energy 2016, 184, 1284–1291. [Google Scholar] [CrossRef]
- Motta, I.L.; Miranda, N.T.; Filho, R.M.; Marciel, M.R.W. Biomass gasification in fluidized beds: A review of biomass moisture content and operating pressure effects. Renew. Sust. Energ. Rev. 2018, 94, 998–1023. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Xiong, X.; Tsang, D.C.W.; Zhang, S.; Clark, J.H.; Hu, C.; Ng, Y.H.; Shang, J.; Ok, Y.S. Biorenewable hydrogen production through biomass gasification: A review and future prospects. Environ. Res. 2020, 186, 109547. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.M.V.; Tezel, F.H.; Kennedy, D.A. Adsorbent screening for CO2/CO separation for applications in syngas production. Sep. Purif. Technol. 2019. [Google Scholar] [CrossRef]
- Minutillo, M.; Perna, A.; Jannelli, E.; Cigolotti, V.; Nam, S.W.; Yoon, S.P.; Kwon, B.W. Coupling of biomass gasification and SOFC—Gas Turbine Hybrid System for small scale cogeneration applications. Energy Procedia 2017, 105, 730–737. [Google Scholar] [CrossRef]
- Moradi, R.; Marcantonio, V.; Cioccolanti, L.; Bocci, E. Integrating biomass gasification with a steam-injected micro gas turbine and an Organic Rankine Cycle unit for combined heat and power production. Energy Convers. Manag. 2020, 205, 112464. [Google Scholar] [CrossRef]
- Pala, L.P.R.; Wang, Q.; Kolb, G.; Hessel, V. Steam gasification of biomass with subsequent syngas adjustment using shift reaction for syngas production: An Aspen Plus model. Renew. Energy 2017, 101, 484–492. [Google Scholar] [CrossRef]
- Moon, J.; Jo, W.; Jeong, S.; Bang, B.; Choi, Y.; Hwang, J.; Lee, U. Gas cleaning with molten tin for hydrogen sulfide and tar in producer gas generated from biomass gasification. Fuel 2017, 130, 318–326. [Google Scholar] [CrossRef]
- Abdoulmoumine, N.; Adhikari, S.; Kulkarni, A.; Chattanathan, S. A review on biomass gasification syngas cleanup. Appl. Energy 2015, 155, 294–307. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Soriano, M.D.; Natoli, A.; Rodríguez-Castellón, E.; Nieto, J.M.L. Selective oxidation of hydrogen sulfide to sulfur using vanadium oxide supported on porous clay heterostructures (PCHs) formed by pillars silica, silica-zirconia or silica-titania. Materials 2018, 11, 1562. [Google Scholar] [CrossRef] [PubMed]
- Frilund, C.; Simell, P.; Kaisalo, N.; Kurkela, E.; Koskinen-Soivi, M.-L. Desulfurization of biomass syngas using ZnO-based adsorbents: Long-term hydrogen sulfide breakthrough experiments. Energ. Fuel 2020. [Google Scholar] [CrossRef] [PubMed]
- Marcantonio, V.; Bocci, E.; Ouweltjes, J.P.; Zotto, L.D.; Monarca, D. Evaluation of sorbents for high temperature removal of tars, hydrogen sulphide, hydrogen chloride and ammonia from biomass-derived syngas by using Aspen Plus. Int. J. Hydrogen Energy 2020, 45, 6651–6662. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Koduru, J.R.; Vikrant, K.; Tsang, Y.F.; Singhal, R.K.; Hussain, C.M.; Kim, K.-H. Recent progress on solution and materials chemistry for the removal of hydrogen sulfide from various gas plants. J. Mol. Liq. 2020, 297, 111886. [Google Scholar] [CrossRef]
- Tuna, Ö.; Simsek, E.B.; Sarıoğlan, A.; DurakÇetin, Y. Influence of the process conditions on the kinetic behaviour of zinc orthotitanate for syngasclean-up. Biomass Bioenerg. 2019, 128, 105326. [Google Scholar] [CrossRef]
- Macedo, K.R.M.; Oliveira, G.A.C.; Pereira, K.A.B.; Mendes, L.C.; Araújo, A.S.; Cassella, R.J. Titanium-zinc polycitrate precursor: Influence of thermal treatment on structural, thermal, optical characteristics of zinc titanates. Mater. Chem. Phys. 2019, 236, 121768. [Google Scholar] [CrossRef]
- Budigi, L.; Nasina, M.R.; Shaik, K.; Amaravadi, S. Structural and optical properties of zinc titanates synthesized by precipitation method. J. Chem. Sci. 2015, 127, 509–518. [Google Scholar] [CrossRef]
- Untea, I.; Dancila, M.; Vasile, E.; Belcu, M. Structural, morphological and textural modifications of ZnO–TiO2 HTGD based sorbents induced by Al2O3 addition, thermal treatment and sulfurizing process. Powder Technol. 2009, 191, 27–33. [Google Scholar] [CrossRef]
- Kubiak, A.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Titania-based hybrid materials with ZnO, ZrO2 and MoS2: A review. Materials 2018, 11, 2295. [Google Scholar] [CrossRef]
- Ayed, S.; Abdelkefi, H.; Khemakhem, H.; Matoussi, A. Solid state synthesis and structural characterization of zinc titanates. J. Alloys Compd. 2016, 677, 185–189. [Google Scholar] [CrossRef]
- Chaves, A.C.; Lima, S.J.G.; Araújo, R.C.M.U.; Maurera, M.A.M.A.; Longo, E.; Pizani, P.S.; Simões, L.G.P.; Soledade, L.E.B.; Souza, A.G.; dos Santos, I.M.G. Photoluminescence in disordered Zn2TiO4. J. Solid State Chem. 2006, 179, 985–992. [Google Scholar] [CrossRef]
- Arin, J.; Thongtem, S.; Phuruangrat, A.; Thongtem, T. Template synthesis of Zn2TiO4 and Zn2Ti3O8 nanorods by hydrothermal calcination combined processes. Mater. Lett. 2017, 193, 270–273. [Google Scholar] [CrossRef]
- Fu, L.; Zhu, J.; Huang, W.; Fang, J.; Sun, X.; Wang, X.; Liao, K. Preparation of Nano-Porous Carbon-Silica Composites and Its Adsorption Capacity to Volatile Organic Compounds. Processes 2020, 8, 372. [Google Scholar] [CrossRef]
- Chai, Y.-L.; Chang, Y.-S.; Chen, G.-J.; Hsiao, Y.-J. The effects of heat-treatment on the structure evolution and crystallinity of ZnTiO3 nano-crystals prepared by Pechini process. Mater. Res. Bull. 2008, 43, 1066–1073. [Google Scholar] [CrossRef]
- Li, X.; Huang, W.; Liu, X.; Bian, H. Graphene oxide assisted ZIF-90 composites with enhanced n-hexane vapor adsorption capacity, efficiency and rate. J. Solid State Chem. 2019, 278, 120–890. [Google Scholar] [CrossRef]
- Brito, L.; Almenglo, F.; Martín Ramírez, M.; Cantero, D. Feedback and Feedforward Control of a Biotrickling Filter for H2S Desulfurization with Nitrite as Electron Acceptor. Appl. Sci. 2019, 9, 2669. [Google Scholar] [CrossRef]
- Kubiak, A.; Siwińska-Ciesielczyk, K.; Bielan, Z.; Zielińska-Jurek, A.; Jesionowski, T. Synthesis of highly crystalline photocatalysts based on TiO2 and ZnO for the degradation of organic impurities under visible-light irradiation. Adsorption 2019, 25, 309–325. [Google Scholar] [CrossRef]
- Nethi, S.K.; Anand, P.N.A.; Rico-Oller, B.; Rodríguez-Diéguez, A.; Gómez-Ruiz, S.; Patra, C.R. Design, synthesis and characterization of doped-titanium oxide nanomaterials with environmental and angiogenic applications. Sci. Total Environ. 2017, 599–600, 1263–1274. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.N.; Franco, C.; Lopes, H.; Carolino, C.; Costa, R.; Gulyurtlu, I. Co-gasification of coal and wastes in a pilot-scale installation. 2: Effect of catalysts in syngas treatment to achieve sulphur and nitrogen compounds abatement. Fuel 2010, 89, 3340–3351. [Google Scholar] [CrossRef]
- Bu, X.; Ying, Y.; Ji, X.; Zhang, C.; Peng, W. New development of zinc-based sorbents for hot gas desulfurization. Fuel Process. Technol. 2007, 88, 143–147. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Mu, X.; Li, Y.; Fang, K. Highly selective conversion of H2S–CO2 to syngas by combination of nonthermal plasma and MoS2/Al2O3. J. CO2 Util. 2020, 37, 45–54. [Google Scholar]
- Chomiak, M.; Trawczyński, J.; Blok, Z.; Babiński, P. Monolithic Zn–Co–Ti based sorbents for hot syngas desulfurization. Fuel Process. Technol. 2016, 144, 64–70. [Google Scholar] [CrossRef]



| Sample Code | ZnO:TiO2 Molar Ratio | Preparation Conditions | Calcination Temperature, °C |
|---|---|---|---|
| ZT-300 | 2:1 | Dry mixing of ZnO and TiO2 for 30 min | - |
| ZT-500 | Semi-wet mixing of the ZnO and TiO2 mixture with ammonium bicarbonate (NH4HCO3) solution (12.5% wt.) for 60 min | 500 | |
| ZT-600 | Drying at 105 °C to the constant mass | 600 | |
| ZT-700 | Pre-calcination at 300 °C for 4 h | 700 | |
| ZT-800 | Calcination at different temperatures for 4 h | 800 |
| Sample Code | ZnO:TiO2 Molar Ratio | Theoretical Sulfur Content * (St), % (wt.) | Experimental Sulfur Content (Se), % (wt.) | Sulfur Removal Degree, % |
|---|---|---|---|---|
| ZT 700 | 2:1 | 23.32 | 22.63 | 97.04 |
| Sorbent/Catalyst | Operational Conditions | Removal Efficiency, % | Reference |
|---|---|---|---|
| Dolomite and Ni-based catalyst (Ni 11%, CaO 6–9%, Al2O3 76–82%) | Temperature: 850 °C, atmospheric pressure, saturated conditions | over 97 | [29] |
| G-201 and G-202 sorbents (Zn/Ti molar ratio of 1.5 and 1.0 respectively) | Temperature: 550–650 °C Pressure: 0.8 mpa | over 99 | [30] |
| Non-thermal plasma combined with 5% MoS2/Al2O3 | Low temperature (120 °C) and atmospheric pressure | 98–100 | [31] |
| Zinc oxide | Around 2% humidity and temperature 460 °C | near the 100 | [13] |
| Monolithic sorbent ZTC (Zn:Ti:Co = 1:1:0.25) 60%, natural clay 27%, silica gel 3%, colloidal dispersion of graphite 10% | Temperature: 540 °C | 99.6–99.9 | [32] |
| ZT-700 (ZnO:TiO2 molar ratio of 2:1; calcinated at 700 °C) | Temperature: 600–700 °C | 97 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dăncilă, A.M.; Căprărescu, S.; Bobiricǎ, C.; Purcar, V.; Gârleanu, G.; Vasile, E.; Modrogan, C.; Borda, C.; Dobrotǎ, D. Optimization of the Technological Parameters for Obtaining Zn-Ti Based Composites to Increase the Performance of H2S Removal from Syngas. Processes 2020, 8, 562. https://doi.org/10.3390/pr8050562
Dăncilă AM, Căprărescu S, Bobiricǎ C, Purcar V, Gârleanu G, Vasile E, Modrogan C, Borda C, Dobrotǎ D. Optimization of the Technological Parameters for Obtaining Zn-Ti Based Composites to Increase the Performance of H2S Removal from Syngas. Processes. 2020; 8(5):562. https://doi.org/10.3390/pr8050562
Chicago/Turabian StyleDăncilă, Annette Madelene, Simona Căprărescu, Constantin Bobiricǎ, Violeta Purcar, Gabriel Gârleanu, Eugeniu Vasile, Cristina Modrogan, Claudia Borda, and Dan Dobrotǎ. 2020. "Optimization of the Technological Parameters for Obtaining Zn-Ti Based Composites to Increase the Performance of H2S Removal from Syngas" Processes 8, no. 5: 562. https://doi.org/10.3390/pr8050562
APA StyleDăncilă, A. M., Căprărescu, S., Bobiricǎ, C., Purcar, V., Gârleanu, G., Vasile, E., Modrogan, C., Borda, C., & Dobrotǎ, D. (2020). Optimization of the Technological Parameters for Obtaining Zn-Ti Based Composites to Increase the Performance of H2S Removal from Syngas. Processes, 8(5), 562. https://doi.org/10.3390/pr8050562









