Efficiency Separation Process of H2/CO2/CH4 Mixtures by a Hollow Fiber Dual Membrane Separator
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Polyimide (PI) and Polydimethylsiloxane/Polyetherimide (PDMS/PEI) Hollow Fiber Membrane
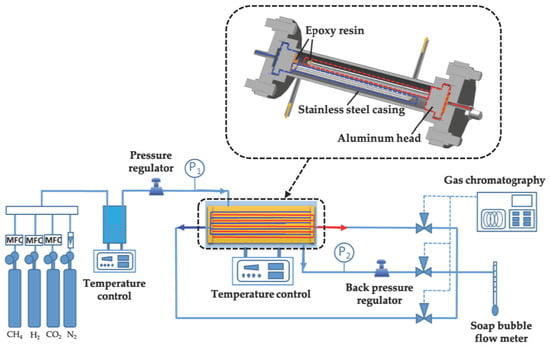
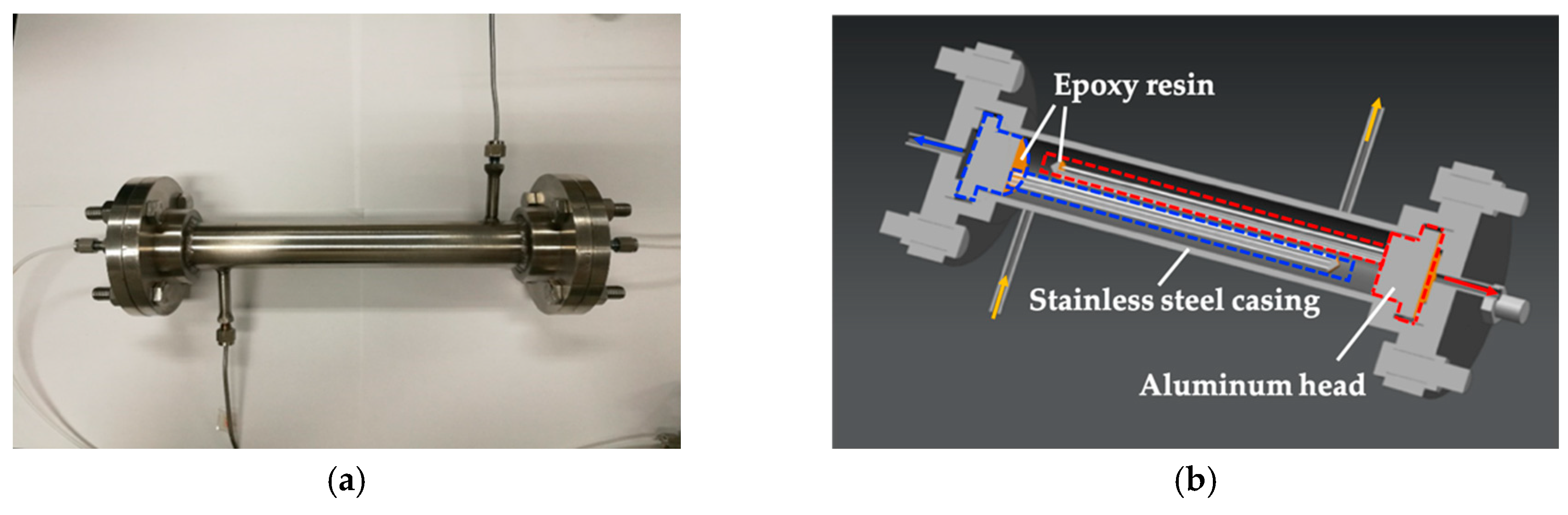
2.2. Design and Packaging of Dual Membrane Separator
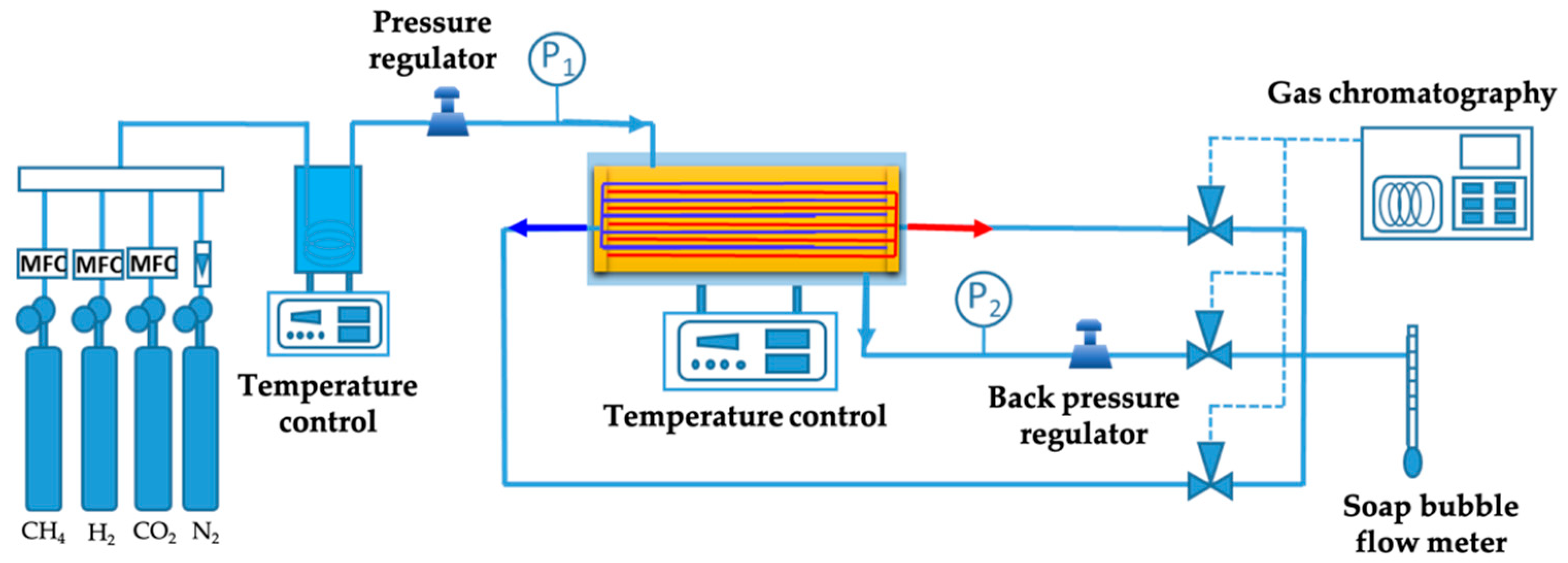
2.3. Experimental Set-Up and Analysis System
2.4. Theoretical Analysis
3. Results and Discussion
3.1. Basic Properties of the Two Hollow Fiber Membranes
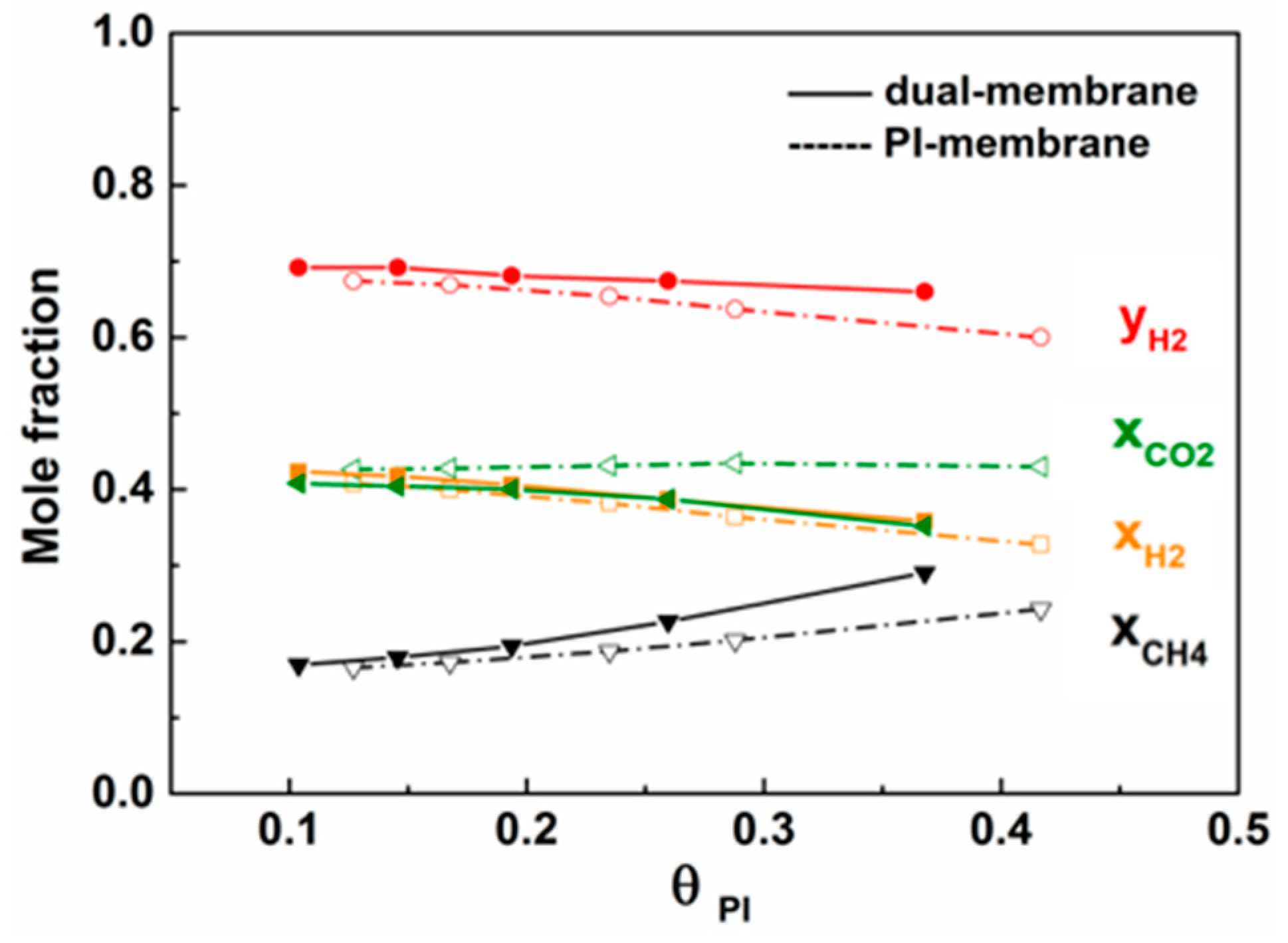
3.2. Parameter Optimization of PI/PDMS Dual Membrane Separator
3.2.1. Effect of Stage Cut
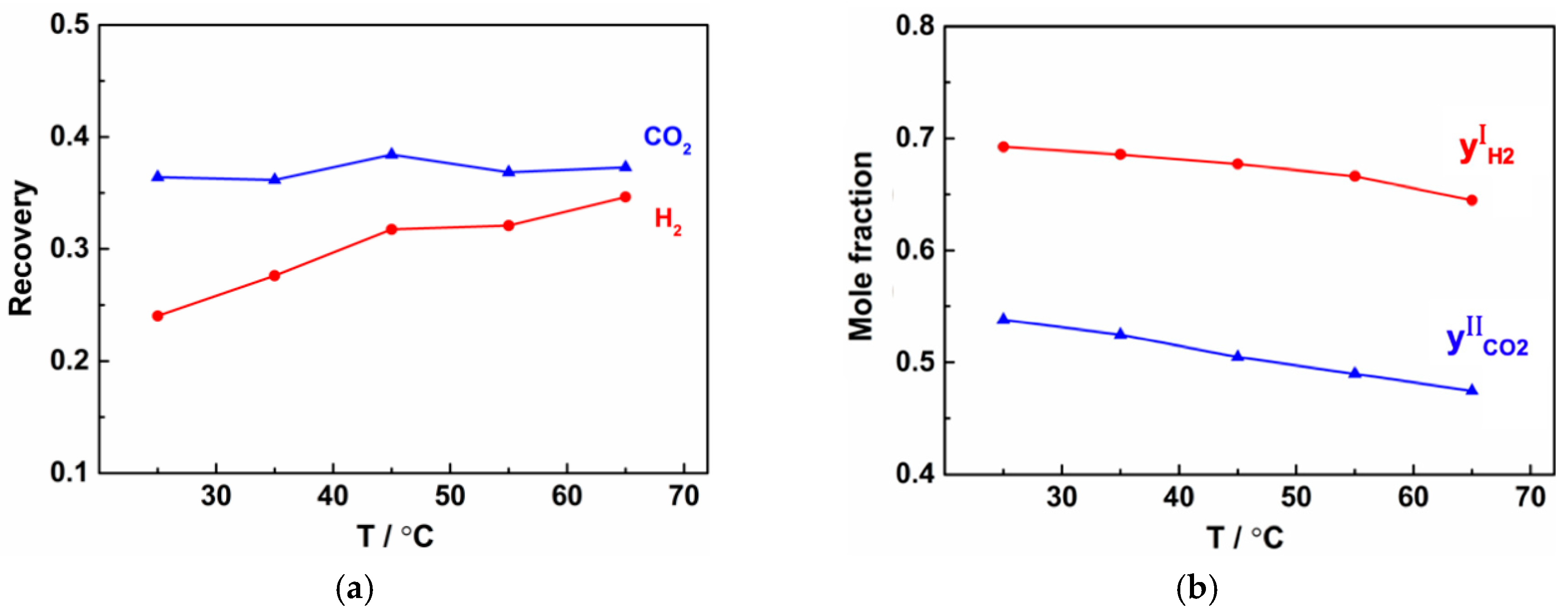
3.2.2. Effect of Operating Temperature
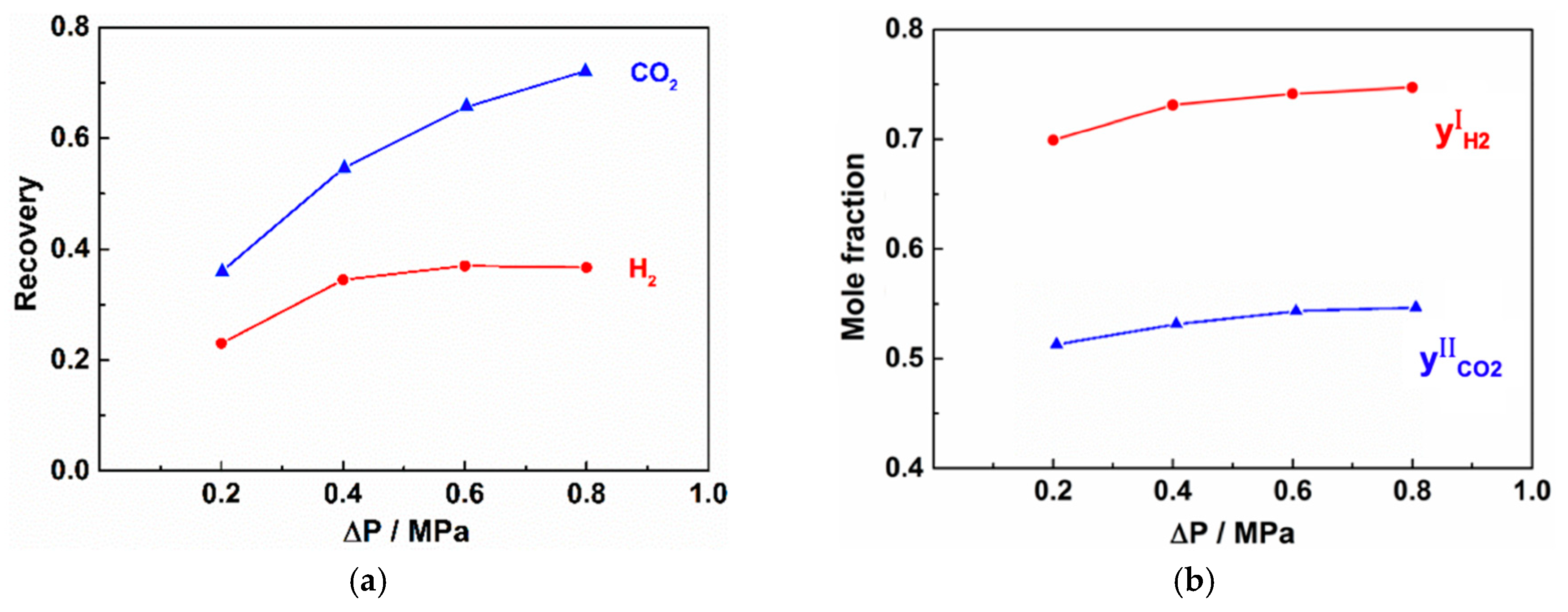
3.2.3. Effect of Operating Pressure
3.2.4. Effect of Membrane Area Ratio
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giordano, L.; Gubis, J.; Bierman, G.; Kapteijn, F. Conceptual design of membrane-based pre-combustion CO2 capture process: Role of permeance and selectivity on performance and costs. J. Membr. Sci. 2019, 575, 229–241. [Google Scholar] [CrossRef]
- Merkel, T.C.; Zhou, M.; Baker, R.W. Carbon dioxide capture with membranes at an IGCC power plant. J. Membr. Sci. 2012, 389, 441–450. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, H.; Song, Y.; Zhao, L.; Jiang, B.; He, M.; Ruan, C.; Chen, H.; Xu, Y. Hydrogen production from the thermochemical conversion of biomass: Issues and challenges. Sustain. Energy Fuels 2019, 3, 314–342. [Google Scholar] [CrossRef]
- Chen, B.; Yang, T.; Xiao, W.; Nizamani, A.K. Conceptual design of pyrolytic oil upgrading process enhanced by membrane-integrated hydrogen production system. Processes 2019, 7, 284. [Google Scholar] [CrossRef]
- Wang, R.; Liu, S.; Wang, L.; Li, Q.; Zhang, S.; Chen, B.; Jiang, L.; Zhang, Y. Superior energy-saving splitter in monoethanolamine-based biphasic solvents for CO2 capture from coal-fired flue gas. Appl. Energy 2019, 242, 302–310. [Google Scholar] [CrossRef]
- Lin, H.; He, Z.; Sun, Z.; Kniep, J.; Ng, A.; Baker, R.W.; Merkel, T.C. CO2-selective membranes for hydrogen production and CO2 capture–Part II: Techno-economic analysis. J. Membr. Sci. 2015, 493, 794–806. [Google Scholar] [CrossRef]
- Chen, X.; Kaliaguine, S.; Rodrigue, D. Correlation between performances of hollow fibers and flat membranes for gas separation. Sep. Purif. Rev. 2018, 47, 66–87. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural gas processing with membranes: An overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas separation membrane materials: A perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Mores, P.L.; Arias, A.M.; Scenna, N.J.; Caballero, J.A.; Mussati, S.F.; Mussati, M.C. Membrane-based processes: Optimization of hydrogen separation by minimization of power, membrane area, and cost. Processes 2018, 6, 221. [Google Scholar] [CrossRef]
- Chung, T.S.; Shao, L.; Tin, P.S. Surface modification of polyimide membranes by diamines for H2 and CO2 separation. Macromol. Rapid Commun. 2006, 27, 998–1003. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Yu, R.; Li, J.; Hu, B.; Yang, L. Hollow fiber membrane separation process in the presence of gaseous and particle impurities for post-combustion CO2 capture. Int. J. Green Energy 2017, 14, 15–23. [Google Scholar] [CrossRef]
- Ramasubramanian, K.; Zhao, Y.N.; Ho, W.S.W. CO2 capture and H2 purification: Prospects for CO2-selective membrane processes. AICHE J. 2013, 59, 1033–1045. [Google Scholar] [CrossRef]
- Song, C.; Sun, Y.; Fan, Z.; Liu, Q.; Ji, N.; Kitamura, Y. Parametric study of a novel cryogenic-membrane hybrid system for efficient CO2 separation. Int. J. Greenh. Gas Control 2018, 72, 74–81. [Google Scholar] [CrossRef]
- Clarizia, G.; Algieri, C.; Drioli, E. Filler-polymer combination: A route to modify gas transport properties of a polymeric membrane. Polymer 2004, 45, 5671–5681. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.; Jiang, H.; Yaghi, O. Carbon capture and conversion using metal–organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef]
- Ohno, M.; Ozaki, O.; Sato, H. Radioactive rare-gas separation using a separation cell with two kinds of membrane differing in gas permeability tendency. J. Nucl. Sci. Technol. 1977, 14, 589–602. [Google Scholar] [CrossRef]
- Perrin, J.E.; Stern, S.A. Modeling of permeators with two different types of polymer membranes. AICHE J. 1985, 31, 1167–1177. [Google Scholar] [CrossRef]
- Chen, B.; Ruan, X.; Xiao, W.; Jiang, X.; He, G. Synergy of CO2 removal and light hydrocarbon recovery from oil-field associated gas by dual-membrane process. J. Nat. Gas Sci. Eng. 2015, 26, 1254–1263. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, X.; Xiao, W.; Dong, Y.; Hamouti, I.E.; He, G. Dual-membrane natural gas pretreatment process as CO2 source for enhanced gas recovery with synergy hydrocarbon recovery. J. Nat. Gas Sci. Eng. 2016, 34, 563–574. [Google Scholar] [CrossRef]
- Perrin, J.E.; Stern, S.A. Separation of a helium-methane mixture in permeators with two types of polymer membranes. AICHE J. 1986, 32, 1889–1901. [Google Scholar] [CrossRef]
- Sengupta, A.; Sirkar, K.K. Ternary gas mixture separation in two-membrane permeators. AICHE J. 1987, 33, 529–539. [Google Scholar] [CrossRef]
- Cai, J.J.; Hawboldt, K.; Abdi, M.A. Improving gas absorption efficiency using a novel dual membrane contactor. J. Membr. Sci. 2016, 510, 249–258. [Google Scholar] [CrossRef]
- Chen, B.; Ruan, X.; Jiang, X.; Xiao, W.; He, G. Dual-membrane module and its optimal flow pattern for H2/CO2 separation. Ind. Eng. Chem. Res. 2016, 55, 1064–1075. [Google Scholar] [CrossRef]
- Dai, Y.; Ruan, X.; Bai, F.; Yu, M.; Li, H.; Zhao, Z.; He, G. High solvent resistance PTFPMS/PEI hollow fiber composite membrane for gas separation. Appl. Surf. Sci. 2016, 360, 164–173. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Koros, W.J.; Fleming, G.K. Membrane-based gas separation. J. Membr. Sci. 1993, 83, 1–80. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, N.; Burns, C.M.; Feng, X.S. Substrate resistance in composite membranes for organic vapour/gas separations. J. Membr. Sci. 2009, 338, 153–160. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, J.H.; Lee, S.B. Sorption and permeation behaviors of a series of olefins and nitrogen through PDMS membranes. J. Membr. Sci. 2007, 299, 54–62. [Google Scholar] [CrossRef]




| 25 °C, 0.2 MPa | Outer Diameter/μm | Internal Diameter/μm | J/GPU (Gas Permeation Unit) | α | |||
|---|---|---|---|---|---|---|---|
| H2 | CO2 | CH4 | H2/CO2 | CO2/H2 | |||
| PI | 333 | 186 | 148 | 32 | 1 | 4.6 | - |
| PDMS/PEI | 939 | 592 | 50 | 163 | 45 | - | 3.3 |
| R | J/GPU | α | ||
|---|---|---|---|---|
| H2 | CO2 | CH4 | H2/CO2 | |
| 0.87 | 123 | 28 | 1 | 4.4 |
| 1.85 | 128 | 38 | 3 | 3.3 |
| 2.94 | 100 | 35 | 7 | 2.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, W.; Gao, P.; Dai, Y.; Ruan, X.; Jiang, X.; Wu, X.; Fang, Y.; He, G. Efficiency Separation Process of H2/CO2/CH4 Mixtures by a Hollow Fiber Dual Membrane Separator. Processes 2020, 8, 560. https://doi.org/10.3390/pr8050560
Xiao W, Gao P, Dai Y, Ruan X, Jiang X, Wu X, Fang Y, He G. Efficiency Separation Process of H2/CO2/CH4 Mixtures by a Hollow Fiber Dual Membrane Separator. Processes. 2020; 8(5):560. https://doi.org/10.3390/pr8050560
Chicago/Turabian StyleXiao, Wu, Pei Gao, Yan Dai, Xuehua Ruan, Xiaobin Jiang, Xuemei Wu, Yuanxin Fang, and Gaohong He. 2020. "Efficiency Separation Process of H2/CO2/CH4 Mixtures by a Hollow Fiber Dual Membrane Separator" Processes 8, no. 5: 560. https://doi.org/10.3390/pr8050560
APA StyleXiao, W., Gao, P., Dai, Y., Ruan, X., Jiang, X., Wu, X., Fang, Y., & He, G. (2020). Efficiency Separation Process of H2/CO2/CH4 Mixtures by a Hollow Fiber Dual Membrane Separator. Processes, 8(5), 560. https://doi.org/10.3390/pr8050560