1. Introduction
The Moldavian dragonhead (
Dracocephalum moldavica) is an annual plant growing up to about 80 cm. There are two varieties of this plant, with white and blue flowers, and it belongs to the Lamiaceae family. Under natural conditions, the plant is found in China and Mongolia, central Asia and central and eastern Europe [
1,
2].
The dried leaves of the Moldavian dragonhead have a lemon aroma and are used in natural medicine to prepare infusions due to their antiseptic, antibacterial, antioxidant, calming and appetite-stimulating properties [
3]. The herb of the Moldavian dragonhead contains mainly terpenoids: neral, geraniol, geranyl acetate and flavonoids, iridoids as well as tannins [
4,
5]. The composition of the essential oil depends mainly on the climate in which the Moldavian dragonhead was grown. The oil obtained from cultivation in temperate climates contains higher amounts of alcoholic monoterpene derivatives, and from cultivation in warmer climates it contains higher amounts of aldehyde and ester monoterpene derivatives [
6]. The oil and extracts from the dried dragonhead leaves are used in the food industry [
7,
8]. Dried leaves of Moldavian dragonhead can be used as tea [
9], as well as a functional additive for bread [
10] and snacks production [
11]. A high moisture content in the leaves dramatically reduces it shelf life and decreases its quality. Thus, the fast and proper preservation of bioactive compounds through the processing of dragonhead leaves is a very important issue.One of the main methods of food and medicinal plants preservation is drying, which allows for long-term storage of dried food by reducing water activity and the related reduction of microbial development, as well as mass reduction and elimination of seasonal availability of raw materials. However, drying of food is associated with a number of adverse changes occurring during the process. These changes are mainly due to the use of increased drying temperatures required to achievethe optimum water content [
12,
13].
For most plant raw materials, due to their health-promoting properties, the aim is to limit the adverse physicochemical changes occurring during drying. A compromise is needed between the drying kinetics, the intensification of which makes it possible to obtain more dried material, the energy intensity of the process, which makes it possible to reduce the company’s costs, and the change in the physicochemical properties of the dried product, which is the main indicator of consumer preferences. In order to reconcile these contradictory relations, it is necessary to select the optimal method and parameters of drying for a given raw material. Attempts are also made to apply variable drying conditions (during the process), the use of intermittent drying and to combine different drying methods during one technological process [
14,
15]. Plants containing essential oils were added to food to improve taste and were also valued for their aromatic properties. Nowadays, plant essential oils are increasingly used in medicine as they have antibacterial, antiviral, anticancer, antifungal, spasmolytic andhepatotropic properties [
16,
17,
18]. Essential oils and other bioactive compounds contained in the plant raw materials are impermanent and, as a result of drying, their amount is reduced; there are also reactions consisting of the formation of new components from the existing oil components [
19,
20]. Guo et al. [
21] studied the drying process of lotus leaves and they found that an air-drying temperature between 55 and 65 °C is the most adequate to keep the higher antioxidant activity in leaves. Yap et al. [
22] showed that an air-drying temperature higher than 60 °C decreased the total phenolics content in papaya leaves. Moreover, they proved that freeze-drying is the best method to preserve bioactive compounds in papaya leaves. A similar tendency was found by Choo et al. [
23] during drying of
Murraya koenigii leaves. They also found that an air-drying temperature higher than 40 °C significantly decreases antioxidant activity. De Suza et al. [
24] showed that an air-drying temperature higher than 50 °C causes significant decreases in the content of the essential oil of clove basil leaves. However, other authors found that the highest essential oil content in dry
Piper umbellatum L. leaves is obtained when the air-drying temperature does not exceed 40 °C [
25]. On the basis of the presented data it can be concluded that the quantity and quality of oils and other bioactive compounds are most affected by the drying method and drying temperature.
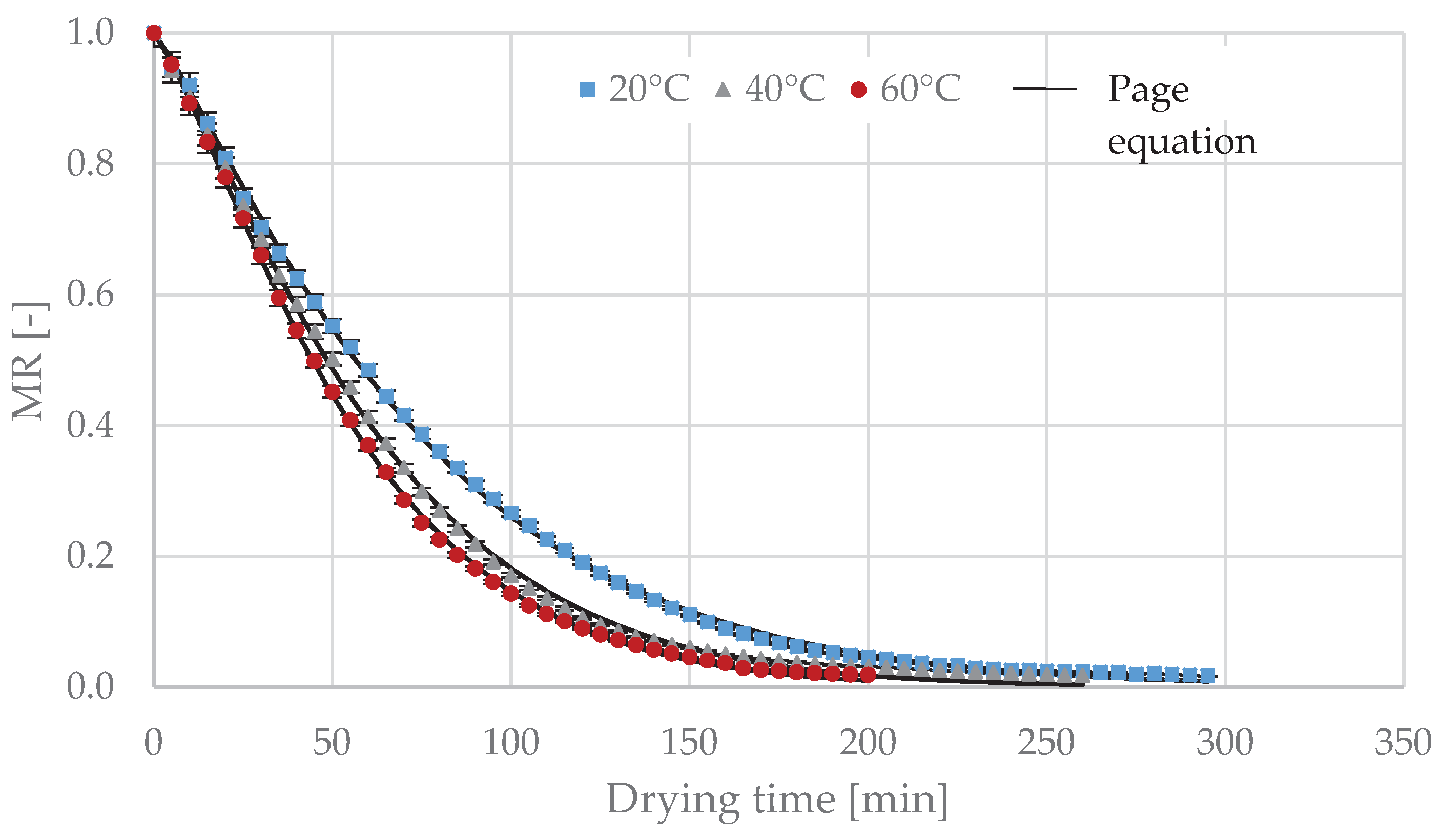
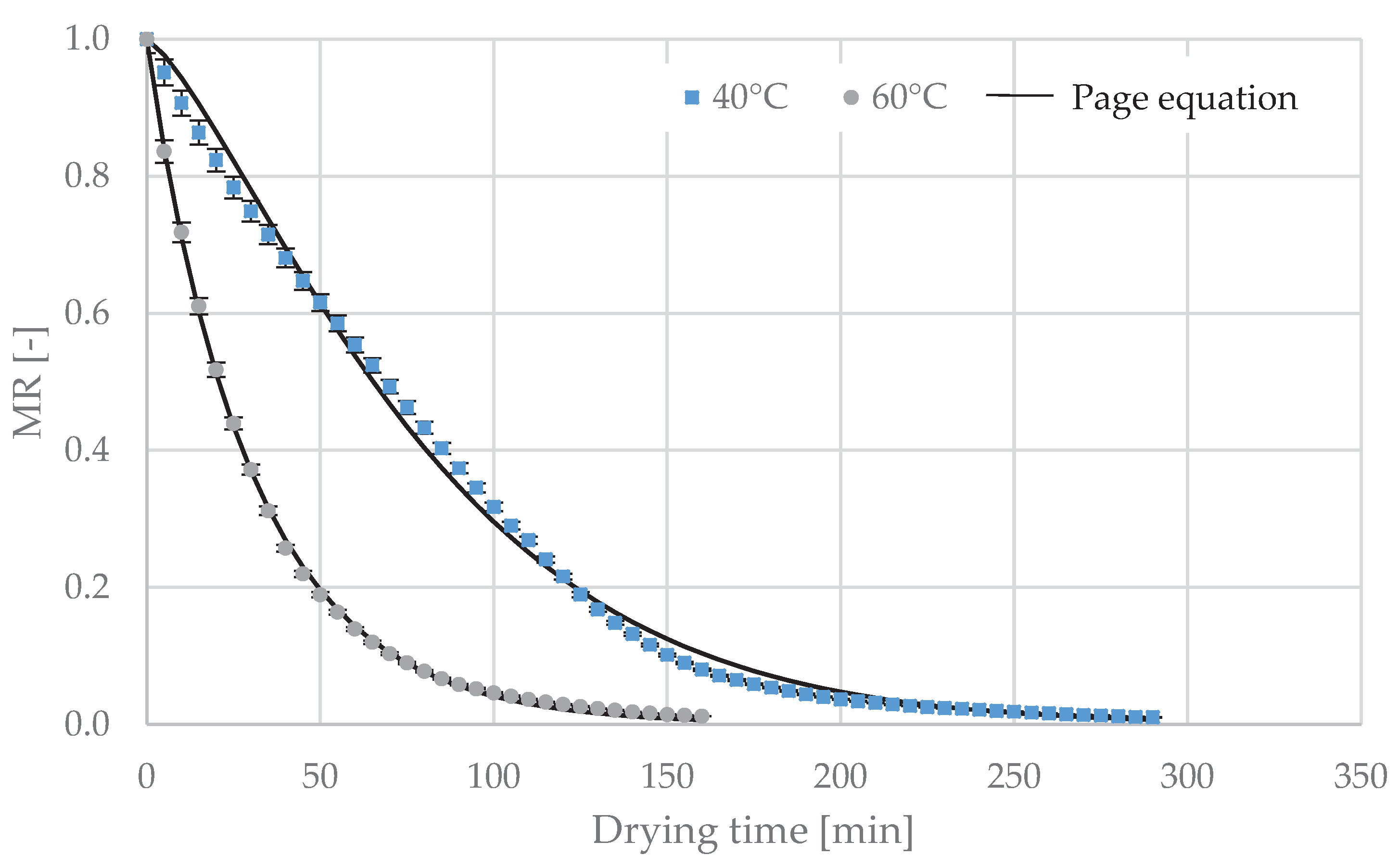
Therefore, this study aimed to determine the effect of the temperature of the sublimation, vacuum and convective drying process on the kinetics of drying of the Moldavian dragonhead leaves, as well as onthe selected physical and chemical properties of the dried material (color change, polyphenols content, antioxidant capacity, total monomeric anthocyanins content, total flavonoids content and essential oil content).
2. Materials and Methods
2.1. Material
The Moldavian dragonhead (
Dracocephalum moldavica) with white flowers came from the experimental station of the University of Life Sciences in Lublin (51°34′ N, 23°02′ E), Poland. The dragonhead leaves were collected from three plots (50 m
2 each). The tillage system for this plant was described by Wójtowicz et al. [
11]. Plants of the Moldavian dragonhead were cut off from three plots between 12 and 14 July 2018 (at the beginning of the flowering phase).After harvesting, leaves weremanually collected, placed in paper bags and stored at 10 °C for 3 h before convection drying and vacuum drying.The leaves were dried whole, and before sublimation drying they were frozen at −30 °C for 24 h. The moisture content of the fresh leaves of the Moldavian dragonhead was determined by weight method, drying them under vacuum at 95 °C ± 0.5 °C to obtain a constant weight. The moisture content of the leaves of the Moldavian dragonhead was 83.24% ± 0.35% (wet basis). Each method of drying was performed individually for leaves from each plot. In order to standardize the sample, dried leaves from each plot were mixed.
2.2. Drying Method
The convection drying process was carried out in a laboratory convection dryer (Promis Tech, Wrocław, Poland). Drying was carried out at a drying air temperature of 40 °C and 60 °C and a constant air flow velocity of 0.5 m·s−1. Registration of changes in the mass of the material during drying was provided by a laboratory electronic scale, type B3 (AXIS, Gdańsk, Poland) with an accuracy of ±0.1 g, connected to a computer recording its indications. The drying process led to a final material moisture content of 10%.
The sublimation and vacuum drying processes were carried out in an ALPHA 1–4 (Martin Christ Company, Osterode am Harz, Germany) laboratory vacuum dryer. The device consists of a drying chamber, vacuum pump, ice capacitor, heating system consisting of 5 shelves placed on a rack and a control and measurement system. The device is equipped with a weighing system that records, in a continuous manner, changes in weight during the process (accuracy of weight ±0.1 g). The heat required for the phase transition is delivered to the material by contact. The heating panel temperature control is performed by means of a temperature sensor placed inside one of the lyophilizer panels [
26]. The sublimation drying process was carried out for heating panel temperatures of 20 °C, 40 °C and 60 °C with a constant pressure value of 63 Pa. Vacuum drying was carried out for heating panel temperatures of 20 °C, 40 °C and 60 °C with a constant pressure value of 2000 Pa (in case of vacuum drying, the material was not previously frozen). Sublimation and vacuum drying were carried out to obtain a constant dried material humidity of 10%.We did not carry out convection drying at 20 °C, because this kind of drying at such low temperature is ineffective, takes a long time, can lead to the development of microorganisms and often does not allow to achieve an adequate level of moisture content after drying. The drying temperature was selected on the basis of the literature data mentioned in the introduction [
21,
22,
23,
24,
25].
2.3. Modeling of Drying Curves
The drying kinetics is presented as a change in reduced water content (
MR) as a function of drying time:
where
ut is the water content in the course of drying (kg H
2O/kg DM),
u0 is the initial water content (kg H
2O·kg DM
−1)and
ur is the equilibrium water content (kg H
2O·kg DM
−1). The equilibrium water content after sublimation and vacuum drying is very low, so the equilibrium water content (
ur) is assumed to be 0 over the entire measuring range. Such simplification has little effect on drying kinetics [
27,
28,
29].
The most frequent models in the literature were used to describe the curves of sublimation, vacuum and convective drying. The model equations are presented in
Table 1.
2.4. Measurement of Color Coordinates
Color measurement was performed using the reflection method with an X-Rite 8200 spherical spectrophotometer with a measuring hole of 12.7 mm in diameter. A D65 light source and a standard colorimetric observer with a 10° field of view were used. Before each measurement, the instrument was calibrated using a white standard.
The color coordinates were determined in the CIEL*a*b* system. The measurement of color in this system is based on the numerical determination of the three coordinates L*, a*, b*, where L* stands for color brightness and ranges from 0 for a perfectly black body to 100 for a perfectly white body. The coordinate a* indicates the color change from green (−a*) to red (+a*), and b* indicates the color change from blue (−b*) to yellow (+b*).
On the basis of the designated color coordinates, the total change in color (Δ
E) in relation to the raw material was determined, as recorded in the cylindrical coordinates for the color saturation
(C) and color shade
(HU) of the dried material [
35].
where
L0*,
a0* and
b0* are the color parameters of the fresh sample.
2.5. Total Phenolic Compounds Content
The content of total phenolic compounds (
TPC) was determined by the Folin–Ciocalteu method [
36]. A 0.5 mL extract sample was mixed with 0.5 mL H
2O, 2 mL Folin reagent (1:5 H
2O) and, after 3 min, with 10 mL 10% Na
2CO
3. After 30 min, the absorbance of the mixed samples was measured at 725 nm. The measurement was carried out on a UV spectrophotometer Mini 1240 (Shimadzu, Japan). The content of the total phenolic compounds was expressed as gallic acid equivalent per gram of dry matter.
2.6. Antiradical Activity
The ability to neutralize free radicals against ABTS (2,2’-azinobis (3-ethylbenzothiazoline-6-sulfonate)) was carried out by the method developed by Re et al. [
37]. The ability to neutralize free radicals against DPPH (2,2-diphenyl-1picyclohydrazil) was carried out according to Brand-Williams et al. [
38]. The decrease in absorbance was measured quantitatively on the spectrophotometer at 734 nm for ABTS and 517 nm for DPPH.
The ability to neutralize free radicals of ABTS and DPPH was expressed as the EC50 index—the dry matter concentration (mg·mL−1) causing a 50% decrease in the initial ABTS or DPPH radical concentration.
2.7. Total Flavonoids Content
Total flavonoids were estimated according to the method described by Bahorun et al. [
39]. One milliliter of sample was mixed with 1 mL 2% AlCl
3·6H
2O. After 10 min, absorbance at 430 nm was measured. The total flavonoids content was expresses as quercetin equivalent (QE) in milligrams per dry weight (DW).
2.8. Total Monomeric Anthocyanins
Total monomeric anthocyanins (
TAC) were quantified by the pH-differential method according to Giusti and Wrolstad [
40]. Extracts of cranberry powders were diluted with two buffer solutions at pH 1 and 4.5. Cy-3-G (MW = 449.2 g∙mol
−1) was used as a standard with a molar absorptivity coefficient of 26900. Results were expressed as mg of cyanidin-3-galactoside equivalent as mg of Cy-3-G per g DM.
2.9. Quantity and Composition of Essential Oils
The volume of essential oil was determined by steam distillation in the Dering apparatus. The 20 g of crushed material was placed in a distillation flask containing 400 mL of distilled water. The distillation process was carried out for 3 h from the start of boiling. The resulting volume of oil was converted into 100 g of dry matter. The qualitative composition and percentage content of the essential oil components was determined by GC/MS method, using the Varian Chrompack CP-3800 gas chromatograph with Varian 4000 MS/MS mass detector and flame ionization detector (FID). The VF-5 ms column (equivalent to DB-5) was used. The carrier gas was helium with a constant flow through the column of 0.5 mL·min−1. The column temperature program was as follows: 50 °C for 1 min, increased to 250 °C at 4 °Cmin−1, and then 250 °C for 10 min. Doser: 250 °C, split 1:50. A total of 1 µL of solution was dosed (10 µL of sample in 1000 µL of hexane). The recorded range was 40–1000 m·z−1, and the scanning rate 0.8 sec·skan−1. Kovats retention indices (non-isothermal Kovats retention index) were determined on the basis of the C10–C40 alkanes series, using the NIST Library and HP Chemstation spectral libraries.
2.10. Statistical Analysis of the Results
One- and two-factor analysis of variance was performed. Tukey’s test was used to determine the significance of differences between the means. Each drying test was performed in triplicate and all analyses were performed in 5 repetitions. The analysis of drying kinetics regression was performed by non-linear estimation using the least squares method, determining the determination factor and the root mean square error (
RMSE) as well as chi-squared test values (
χ2). The mean root square error (
RMSE) and chi-squared test values (
χ2) were determined from the relationship
where
MRi,p—predicted value of reduced water content;
MRi,e—experimental value of reduced water content;
N—number of measurements; and
n—number of parameters in the model equation.
Statistica 13 from StatSoft was used for statistical analysis. All calculations were performed with the significance level α = 0.05. This level of α is used in many biological studies.
4. Conclusions
The best match of the analyzed models describing the change in reduced water content as a function of drying time was obtained using a logarithmic model in case of vacuum drying, and a Page model for sublimation and vacuum drying. At a given temperature level, sublimation and vacuum drying were characterized by comparable process times. As the temperature increased, the determinants of the color of dried dragonhead leaves, the content of the phenolic compounds, the antioxidant capacity and the composition of essential oils differed to a greater extent from the values of these parameters for the raw material before drying. The differences were small for the dried material obtained after sublimation and vacuum drying, especially at 20 °C and 40 °C, and quite significant after convective drying. If the main emphasis is placed on the quality characteristics of the dried material, and the drying time plays a smaller role, it is recommended to carry out the process of drying Moldavian dragonhead leaves by sublimation or vacuum at a heating-panel temperature not exceeding 40 °C. If the drying time plays a major role, the drying heating process should be carried out by convection at 60 °C.