1. Introduction
Plastics are widely used materials due to their physical and chemical properties; [
1] additionally, they are cheap, light, long-lasting [
2], and relatively easy to produce [
3]. These outstanding properties are the ones that have led to their excessive production and irresponsible consumption, originating severe ecological problems that could cause irreversible damage to the environment [
4,
5,
6].
Essentially there are three traditional solutions for plastic waste management, each one presenting significant drawbacks: Landfill disposal, recycling, and combustion [
6]. In landfill disposal, none of the material resources used to produce plastics are recovered. With respect to the second alternative, the recycling capacity of polymers is inherently limited since after a determined number of recycling processes the materials exhibit reduced mechanical properties and the plastic ends up being thrown away. Regarding the third option, recovering energy from combustion is feasible, but it causes negative effects on the environment and human health.
Given the increasing social pressure to take care of the environment and the present trends towards circular economy, a better option to manage plastic waste would be to transform it into defined chemical compounds, especially the source monomer, with the required purity and quality to be used again as raw material in the same polymerization or in other processes. One such technique that permits the recovery of high-value compounds is pyrolysis, which can be finely tuned to favor some product distribution over others. The practical implementation of a chemical recycling strategy requires a concerted effort among different sectors of the society (the general population, municipal governments, polymer producers, and recycling companies), but there are already some successful examples of this model.
Pyrolysis of plastics can be carried out via thermal or catalytic routes, and the distribution of obtained products is influenced by a wide range of parameters, including the type of waste used, the type of reaction system, the residence time, the pressure range, the presence or absence of catalysts, the presence of hydrogen donor compounds, and several others that affect the composition and yield of the obtained products, which consist of complex mixtures that require further separation if individual products are desired.
Purely thermal pyrolysis processes temperatures can reach up to 900 °C which enhance the rate of secondary decomposition reactions and reduce the liquid yield, producing low-quality products with no predictable composition of a broad range of compounds [
7]. Catalytic pyrolysis, on the other hand, promotes the polymer decomposition at lower temperatures and shorter times, reducing the energy consumption and enhancing the selectivity of the obtained products, but implying extra costs of the catalyst and its regeneration. In the case of the present proposed process, despite the reactions taking place purely thermally (with temperatures above, but close to the ceiling point of polystyrene), the obtained distribution of products shows a selectivity similar to that of a catalytic process.
Submitting polymers to heat deeply alters their main chain structure, their substituent atoms, and their side chains, provoking random scission at weak links in the main chain, at a chain-end, or in labile structures and, depending on the site of reaction in the polymer structure and the conditions chosen for the particular system, the resulting radicals follow different paths of reaction, from depolymerization of the main chain to inter- or intramolecular transfer reactions [
8].
The pyrolysis of pyrolysis of polystyrene (PS) has been a topic of interest for many years, and it is not surprising that renewed interest is rising considering all of the above trends and given that PS is a thermoplastic used throughout the world in a variety of applications.
Thermal degradation of PS is considered to be a chain radical process [
9,
10,
11], but despite numerous studies and experiments performed to understand its mechanism, many aspects of the problem remain unresolved, making it still difficult to accurately predict the product distribution at a given set of conditions. Additionally, as the process system temperature increases, so does its complexity [
8].
The recovery of styrene monomer in the highest possible yield is a desired output of the pyrolysis process of PS, since this would contribute to the consolidation of a circular economy. Therefore, any effort directed towards this goal should be welcome. It is our hypothesis that modifying PS with a nitroxide moiety at a chain-end could enhance the generation of monomer when this material is subjected to thermal pyrolysis at a temperature higher than the ceiling temperature of PS. In this paper, empirical evidence that supports this hypothesis is presented and a mechanistic and mathematical model that is consistent with the experimental observations is also presented and discussed. Before that, background information that supports and motivates this hypothesis is introduced. In the next paragraphs, a brief literature review is made, including a description of the reaction mechanism and previous experimental work. Some of the studies are of academic nature, while many others are associated with patents.
Reaction Mechanism. It has been reported that the molecular weight of polystyrene decreases on heating in vacuum at 250 °C, and when heated above 300 °C volatile products are formed [
8,
10]. With regard to the initiation process, it is accepted that the radicals are formed by different mechanisms such as chain-end initiation, random mid-chain scission, and scission at irregular structures or “weak links” distributed along the polymer chain such as head-to-head linkages, chain branches, and unsaturated bonds [
9,
10,
12,
13]. Lehrle et al. [
13] concluded that between 450 to 480 °C initiation occurs both by random mid-chain scission and at chain-ends, and Madorsky [
14] pointed out that between 300 to 400 °C it degrades preferably at the ends and that simultaneously some random degradation takes place.
Propagation reactions include intramolecular transfer, which involves the transfer of a hydrogen atom within a single polymer chain and intermolecular hydrogen transfer, comprising the transfer of a hydrogen atom between polymer chains. Also included in this category is the depropagation reaction, where no hydrogen transfer occurs; this reaction is essentially the reverse of the polymerization propagation, also called unzipping or depolymerization, and is mainly responsible for the production of styrene monomer [
10,
15].
It has been claimed that the most frequent propagation reaction taking place in the 280–350 °C temperature range is β-scission [
9], either at a reaction end or at a mid-chain position, a reversible reaction that becomes faster as the ceiling temperature is approached (310–390 °C depending on the source [
16,
17]); below 300 °C the production of styrene is hardly observed. The second most important reaction is the hydrogen abstraction from the main chain by any radical, producing a saturated chain-end from the attacking radical, and a new radical that undergoes β-scission [
9]. At higher temperatures (600 and 700 °C), increased production of benzene, toluene, ethylbenzene, and α-methyl styrene and decreased production of styrene have been observed, probably due to the predominance of intramolecular hydrogen transfer reactions at this range of temperatures [
18]. Concerning termination, some authors propose a mechanism involving the recombination [
9,
11], or the disproportionation reaction [
19] between two radicals; while others suggest the occurrence of depropagation until the end of the polymer molecule [
13].
Published studies related to the thermal degradation and pyrolysis of polystyrene [
13,
20,
21,
22,
23,
24,
25] have been performed in a variety of reaction systems and sets of conditions, including reaction temperatures, reaction times, and molecular weights of the polystyrene sample, resulting in a broad collection of reaction yields and product distribution. The available reported experimental data fall into three types: chemical nature of the products (that help to elucidate the degradation mechanism), rate of evolution of products, and the change of molecular weight in the residue.
Jellinek [
22] reported experiments carried out in long open-to-air tubes containing 0.1 to 0.5 g of material, to show the influence of oxygen on the thermal degradation of polystyrene. The reactions were described as too violent above 220 °C, many side reactions set in and the material quickly became yellow and brown. They assumed that the first stage of the oxidation consisted of the formation of hydro peroxide groups, which led to chain scission, and that at later stages, the reaction slowed down due to the formation of antioxidants such as benzaldehyde. For practical application, to avoid the presence of oxygen in the resulting products, pyrolysis processes are designed to occur in an inert atmosphere. Ebert et al. [
23] pyrolyzed samples of polydisperse polystyrene with molecular weights between 60,000 and 220,000 Da in ampoules free of oxygen at temperatures between 270 and 336 °C, assessing the sample weight loss as a function of degradation time. They also performed simulations of the thermal degradation assuming that scission and unzipping occur independently, and attributed scission as the main cause for the decrease in molecular weight, while unzipping as the sole cause of volatiles production. Additionally, in oxygen-free glass ampoules, Wegner and Patat [
26] analyzed n-butyl lithium initiated polystyrene samples with molecular weights between 110,000 to 900,000 Da, submitting them to temperatures between 200 to 325 °C, concluding that the polystyrene molecule does not contain thermolabile bonds corresponding to the weak-links theory.
Reactions conducted in a fluidized-bed reactor with operation temperatures in the range 450–700 °C, nitrogen atmosphere, heated by an external electric furnace, permitted liquid recovery above 90%, with the main components being styrene monomer, dimer and trimer, benzene, toluene, ethylbenzene, and α-methyl styrene [
20].
Simard et al. [
21] reported PS pyrolysis experiments using flat bottom vessels and temperatures between 370–420 °C, with a maximum reaction time of 45 min, observing that 85 to 95% of the obtained liquid fraction are styrene, dimer, and trimer. Anderson and Freeman [
25] assessed the weight loss of samples heated from 246 to 430 °C, observing decomposition at 320 °C and 99% of weight loss at 430 °C.
Small scale PS pyrolysis experiments have also been performed. Richards and Salter [
24] intentionally synthesized polystyrene containing weak links in its structure that differ from the usual head–tail links of the polymer backbone and should, therefore, have different thermal properties. The pyrolysis experiments were performed at 276–329 °C, and the main product from both polymers was styrene, although significant quantities of dimer and trimer were also formed, the main difference being that the molecular weight of the polymer with weak links decreased more rapidly.
Several patents regarding polystyrene pyrolysis have also been filed [
27,
28,
29,
30,
31,
32] reporting the use of temperatures varying in a wide range, 330–870 °C, styrene monomer recovery from 33 to 80%, and diverse processes, including the use of a fluidized bed reactor [
31] and the use of solvents like toluene [
32].
In general, pyrolysis temperature is a very important factor affecting the product distribution [
20]; in all the studies both higher temperatures and longer reaction times resulted in an increase in the amount of the liquid yield [
21] and a decrease in the molecular weight of the residue. Many of these experiments were done with very small samples (micrograms and milligrams) and none of them had an initial charge greater than 25 g. At increasing temperatures more monomer is formed reaching a maximum at 600 °C where secondary reactions take place.
As can be seen, the use of solvents and catalytic systems allows high yield of styrene recovery, although some drawbacks associated with these processes are evident, such as high operating temperatures and a wide range of obtained products by purely thermal pyrolysis.
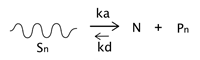
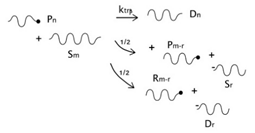
On the other hand, nitroxide-mediated polymerization (NMP) is among the most popular techniques in the category of RDRP (reversible deactivation radical polymerization) that provide living character to free radical polymerizations and control the structure of the resulting polymers, enabling the production of polymers with narrow molecular weight distributions and the formation of well-defined polymer architectures such as block copolymers [
33]. Roughly speaking, these techniques simultaneously provide some of the advantages of living polymerizations (structure control) and conventional radical polymerization (robustness to impurities and to protic media). NMP, which is especially suited for styrene polymerization, is based on a reversible termination mechanism between the propagating growing species and a stable free radical (nitroxide radical), that acts as a control agent to generate a macro alkoxyamine (dormant polymer) as predominant species. The dormant polymer eventually regenerates a propagating radical and a nitroxide radical through a homolytic breakage as the dormant and the radical species are in dynamic equilibrium resulting from the reversible deactivation-activation reactions just described (see
Figure 1). As a result of the NMP process, the final product is mainly constituted by dormant polymer end-functionalized with a nitroxide moiety. For most practical purposes this polymer can be prepared to be very similar to the product produced via conventional free-radical polymerization (except for its dispersity). However, its nitroxide-end functionality should make it more favorable to depolymerization in a certain range of temperatures (via unzipping) than the conventional PS, due to the increased possibility of chain-end initiation of the depolymerization reaction via rupture of the oxygen–carbon bond between the nitroxide moiety and the rest of the polymeric chain.
With respect to the thermal pyrolysis process, it is known that chain-end initiation in mild temperature conditions or moderately above the polystyrene ceiling temperature (310 °C) leads to unzipping and increased production of styrene monomer over other products of this process. Roland and Schmidt-Naake [
34] studied the polymerization of styrene with TEMPO and benzoyl peroxide, claiming that the reversible capping with TEMPO can introduce a weak link at the end of the polymer, such as the bond between polymer and nitroxide. They conclude that polymer degradation for this material occurs in the same temperature range as for non-nitroxide polystyrene (400 °C). However the PS-T thermal degradation curve shows an additional step of mass loss at temperatures below 300 °C, suggesting two reactions, one apparently being the cleavage of the polystyrene–nitroxide bond followed by depolymerization, and the other being the breakage of the N-O bond in the TEMPO moiety, the importance of both reactions changing with increasing temperature.
The lack of knowledge of the position and nature of the initial scission of the polystyrene thermal degradation has restricted its quantitative analysis.
Taking into account this background, the goals of this work are twofold: (i) First, to define a process to achieve the thermal decomposition of conventional (free-radical) polystyrene in one step, and (ii) to compare the developed process for the thermal pyrolysis of conventional PS with another in which PS possessing a nitroxide end-functionality is used. This functionality is introduced in the polymer previously polymerizing styrene in the presence of the TEMPO nitroxide (PS-T) (TEMPO is (2,2,6,6-tetramethylpiperidin-1-yl) oxyl). For the comparison, precise pyrolysis conditions to induce depolymerization reactions that generate styrene monomer as main product and chemicals of high energetic value in the absence of solvents and catalysts at relatively mild conditions of temperature and pressure are first determined. TEMPO is used to provide living character to the free radical polymerization producing polystyrene with an extreme ended in a nitroxide moiety (PS-T) that, from the origin, has characteristics that favor depolymerization. As mentioned above, the hypothesis behind this work is that PS-T will depolymerize in a certain range of temperatures (via unzipping) by chain-end initiation of the reaction at the nitroxide functionality, promoting depropagation reactions in relatively mild temperature conditions or moderately above the polystyrene ceiling temperature. It is believed that above the Tc of polystyrene the uncapping (activation) reaction of the nitroxide moiety at the end of the polystyrene chain will leave a polystyryl radical that will undergo unzipping. The experiments seem to validate this hypothesis to some extent. On the other hand, a mathematical model of the pyrolysis reaction is developed to explain major effects observed and especially the effect of the end-nitroxide group in the PS-T chain.
3. Results
3.1. Product Analysis
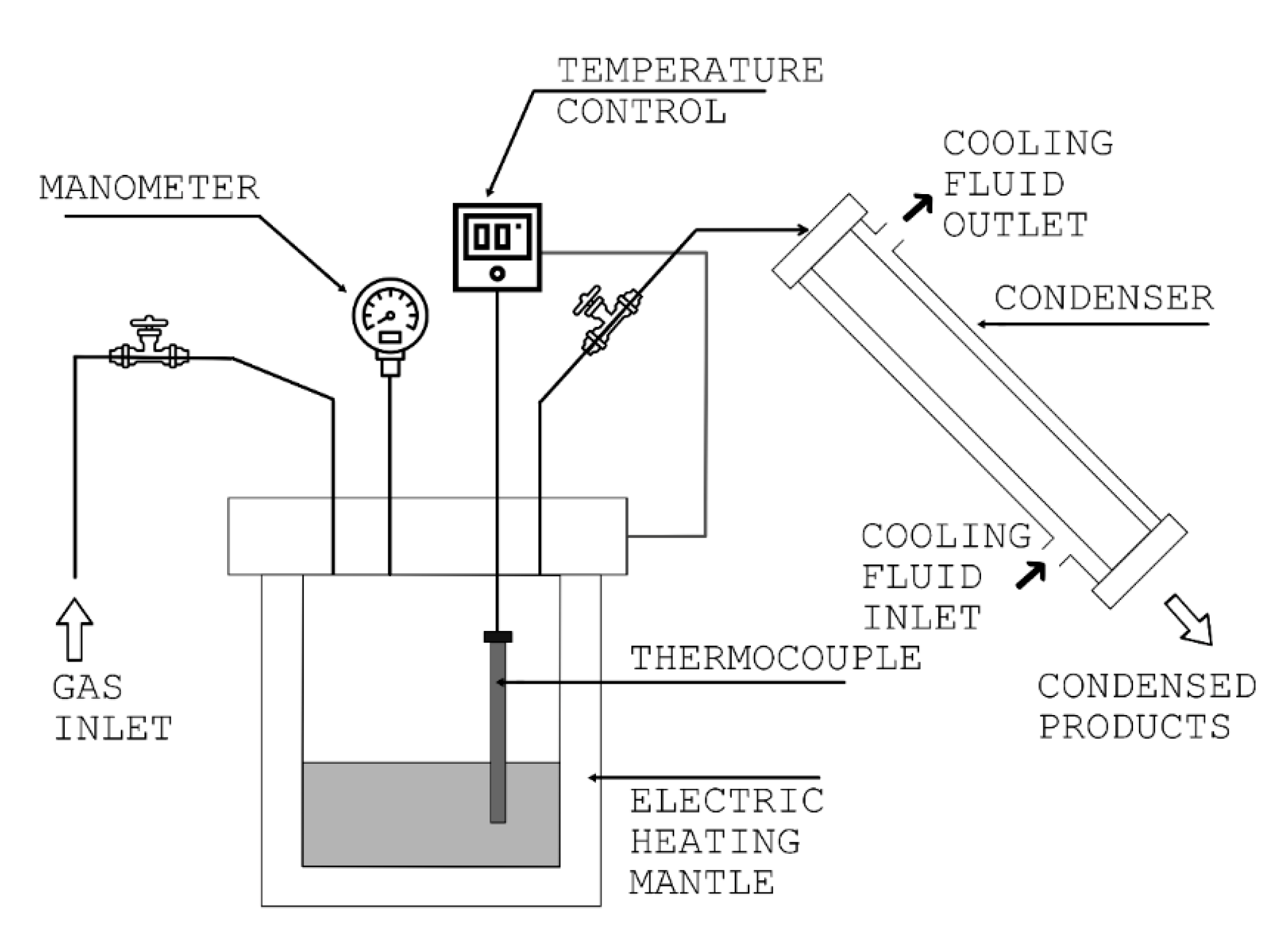
The obtained products from the polystyrene pyrolysis experiments were analyzed to determine the most abundant components present in the liquid mixture. A gas chromatograph (Thermo Finnigan by Thermo Fisher Scientific Waltham, MA, USA) equipped with a selective mass detector (DSQ Trace 2000) (Thermo Electron Corporation, Austin, TX, USA) and a Thermo TG-5ms column were used. All the samples were analyzed under the same conditions with helium as the carrier gas. The temperature program was as follows: the temperature was held at 60 °C for 3 min, then programmed to reach 300 °C at a heating rate of 10 °C min−1 and held at that temperature for 10 min. The injector temperature was set at 250 °C. The transfer temperature line of the mass detector was set at 280 °C and the mass range was from 32 to 650 amu. The products were identified according to their fragmentation patterns using a library included in the software of the chromatograph.
3.2. Kinetic Model
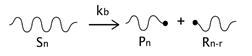
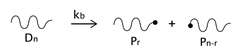
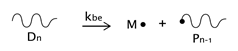
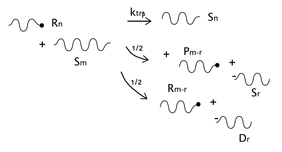
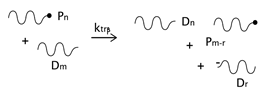
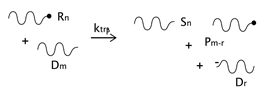
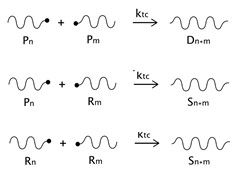
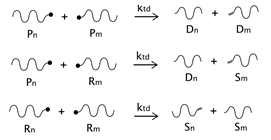
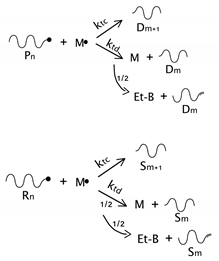
As described before, the radical process of conventional polystyrene pyrolysis comprises the typical steps of initiation, propagation, transfer to polymer, and termination: Although initiation can occur due to several causes, there seems to be general agreement that consists of the formation of free radicals after bond breakage by the action of heat mainly either at a chain-end (which results in the production of monomer) or at a mid-chain position along the polymer backbone. Propagation covers the competition of three different reaction mechanisms: unzipping, intramolecular, and intermolecular hydrogen transfer, the first one is sometimes also called depolymerization and is taken to be the reverse of chain growth, while transfer to polymer consists in the abstraction of a hydrogen from the same or another molecule. The termination step can occur via recombination and disproportionation reactions.
Our proposed model, which aims to describe both conventional and nitroxide-modified polystyrene pyrolysis behavior, contains the following reactions: mid-chain random scission, end chain scission or activation, transfer to polymer, β-scission, depropagation, and termination by combination and by disproportionation, as can be seen in
Table 3.
denotes living polymer with length
, while
,
,
,
, and
denote live polymer of length
n with nitroxide in one end, dormant polymer of length
n, dead polymer of length n, monomer and monomer radical, respectively. The kinetic constants are denoted as:
(mid-chain random scission),
(end-chain scission),
(transfer to polymer + β-scission, simplified mechanism),
(depropagation),
(termination by combination), and
(termination by disproportionation). To simplify the description, some assumptions and approximations are made:
Dormant polymer with one or two nitroxide-functionalized ends will be lumped together into a single quantity. Upon mid-chain random scission of these species to form two radicals, it will be assumed that only one radical contains a nitroxide-functionalized end. Notice that as the number of chain ends increases in the system due to the pyrolysis process, the probability of a chain ending in a nitroxide moiety decreases. This introduces some error, but it will be neglected for simplicity since it is assumed that on the average the error introduced is small.
Initiation includes the possible formation of free radicals either in the terminal or in a mid-chain random position. The corresponding kinetic coefficients could be different depending on the position (end-chain or mid-chain) since the radicals produced in each case are of different nature, mid-chain scission forms one primary radical and one secondary benzyl radical, while end-chain scission forms a secondary benzyl radical; both reactions can occur on dormant or dead polymer (
,
) as described in
Table 3. Transfer to polymer followed by β-scission can occur between any of two types of living chains (normal,
, or with a nitroxide-end functionality,
) and one of two types of inactive chains (dormant,
, or dead,
). In the case of dormant polymer, the initiation can also occur at the nitroxide end via the activation reaction. The rest of the reactions, depropagation and termination, are the same as those appearing in the standard mechanism of free-radical polymerization, with subtle variations depending on the possible presence of a nitroxide-end functionality in one or both reacting chains.
3.3. Mathematical Model
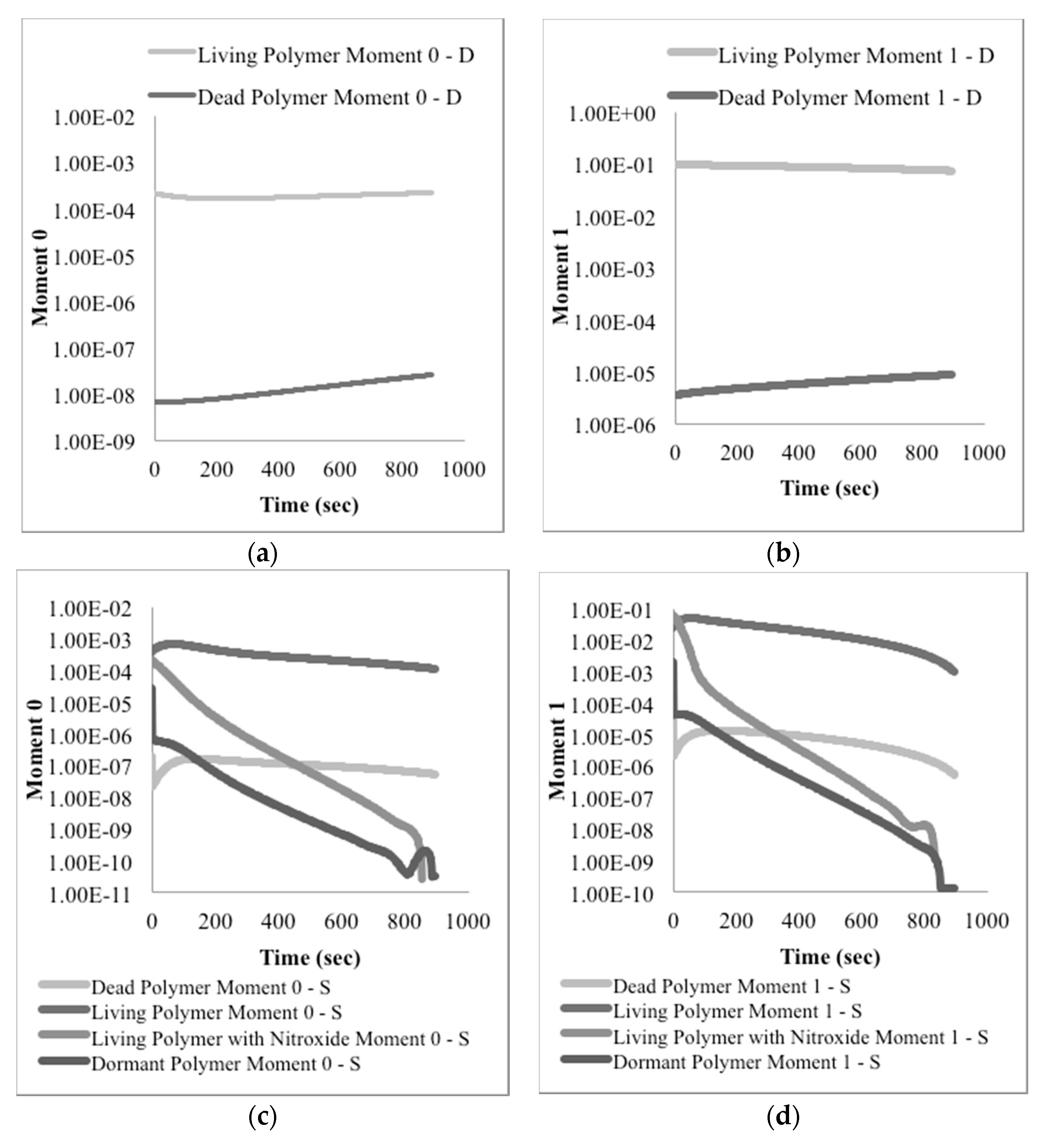
Based on the described reaction scheme, the corresponding mathematical model was formulated writing a mass balance equation for each species. Then, the method of moments was used to keep track of changes of the average molecular weights as a function of time.
Dormant polymer,
n = 1,…,
Live polymer with a nitroxide at the chain-end,
n = 1,…,
Four polymer populations exist in this system: Dead polymer, dormant polymer, growing polymeric radical and polymer radical with a nitroxide end functionality; moments for these species are defined in Equations (9)–(12) respectively:
The method of moments applied to the previously described balance equations generates Equations (13)–(15) for the zero-th, first, and second moments of dead polymer, respectively, Equations (16)–(18) for the moments of dormant polymer, Equations (19)–(21) for polymeric radical moments, and Equations (22)–(24) for the moments of polymeric radicals with a nitroxide end functionality.
From the previous equations, the number average molecular weight can be calculated using Equation (25)
Notice that the moment equations are not closed since the second moment depends on the third moment. To solve this problem, the well-known expression of Saidel and Katz [
35] is used to estimate the third moment in terms of the lower moments.
The mathematical model formulation yielded a set of ordinary differential equations (ODE’s) that were numerically solved using the FORTRAN programming language with the DDASSL routine for the integration of the equations. Kinetic rate constant values were estimated to match the recorded experimental data as explained below.
3.4. Experimental Results
Since the experiments at the extreme levels of N/I (0 and 1.3) were run by triplicate, it was possible to estimate a pooled variance (σ2) for the relevant responses (one for each temperature), and therefore error bars of 1σ are included in most of the plots.
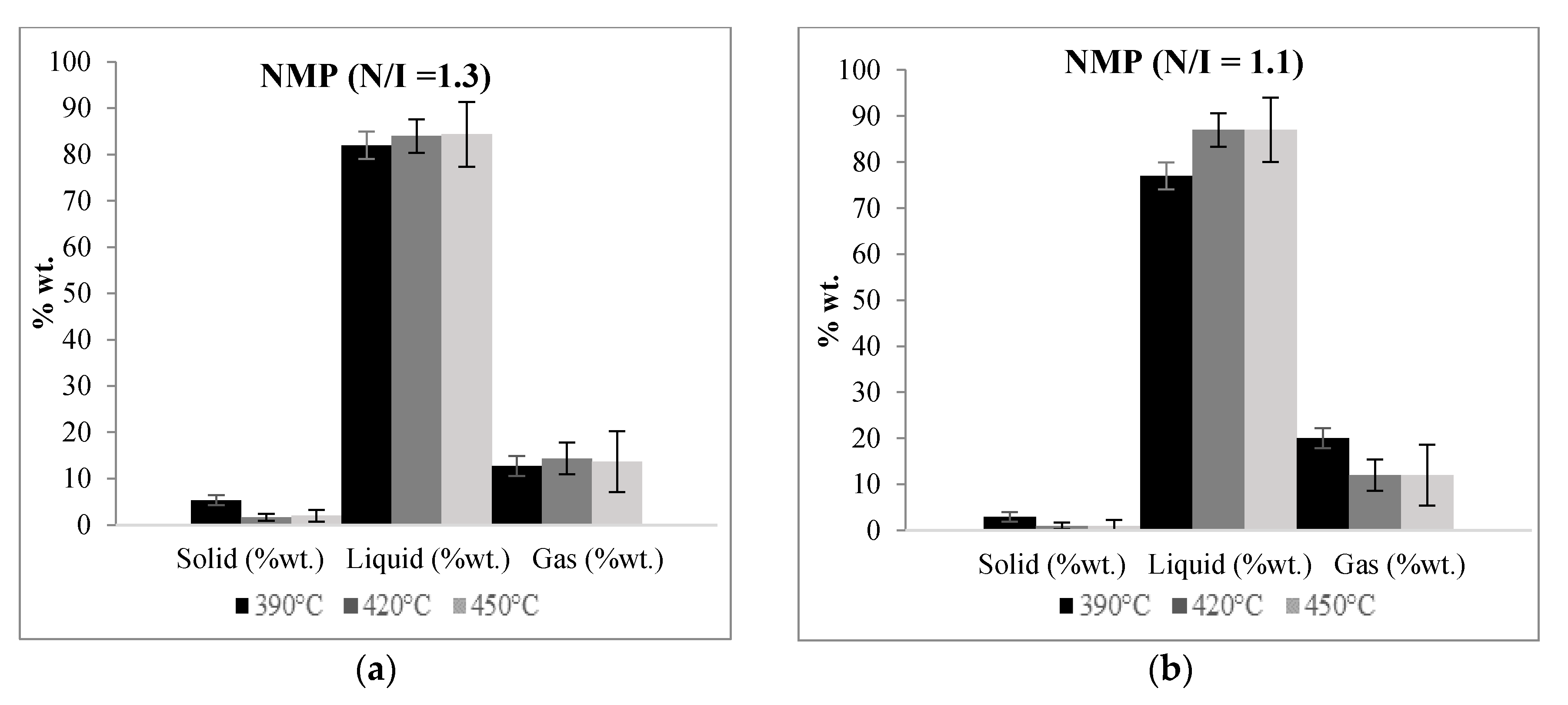
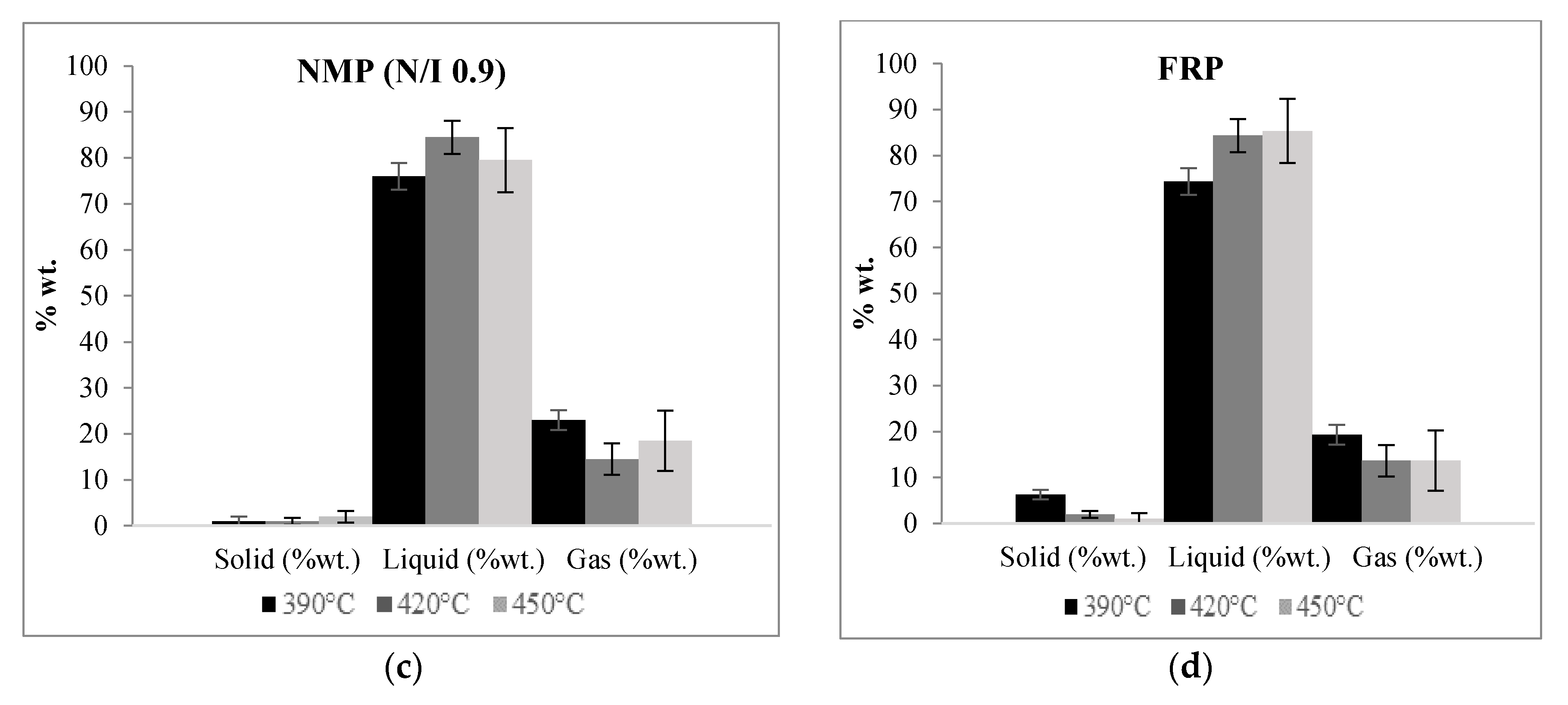
Figure 3 shows the relative yields of the solid, liquid, and gaseous fractions obtained in the pyrolysis experiments using a given N/I ratio or by FRP carried at different temperatures. It can be noted that at higher pyrolysis temperatures the liquid fraction has a tendency to increase while the gas and solid fractions diminish. Comparing all the samples, the ones corresponding to the N/I ratio of 1.3 exhibit the higher values of recovered liquid fraction, the maximum value reached being 88% at 420 °C, while the lowest values correspond to the FRP samples. These results point out to a favorable effect of the presence of the nitroxide moiety at the end of the polymer chains on the pyrolysis process; more will be discussed below. All the percentages are referred to the total amount of polymer charged at the beginning of the experiments.
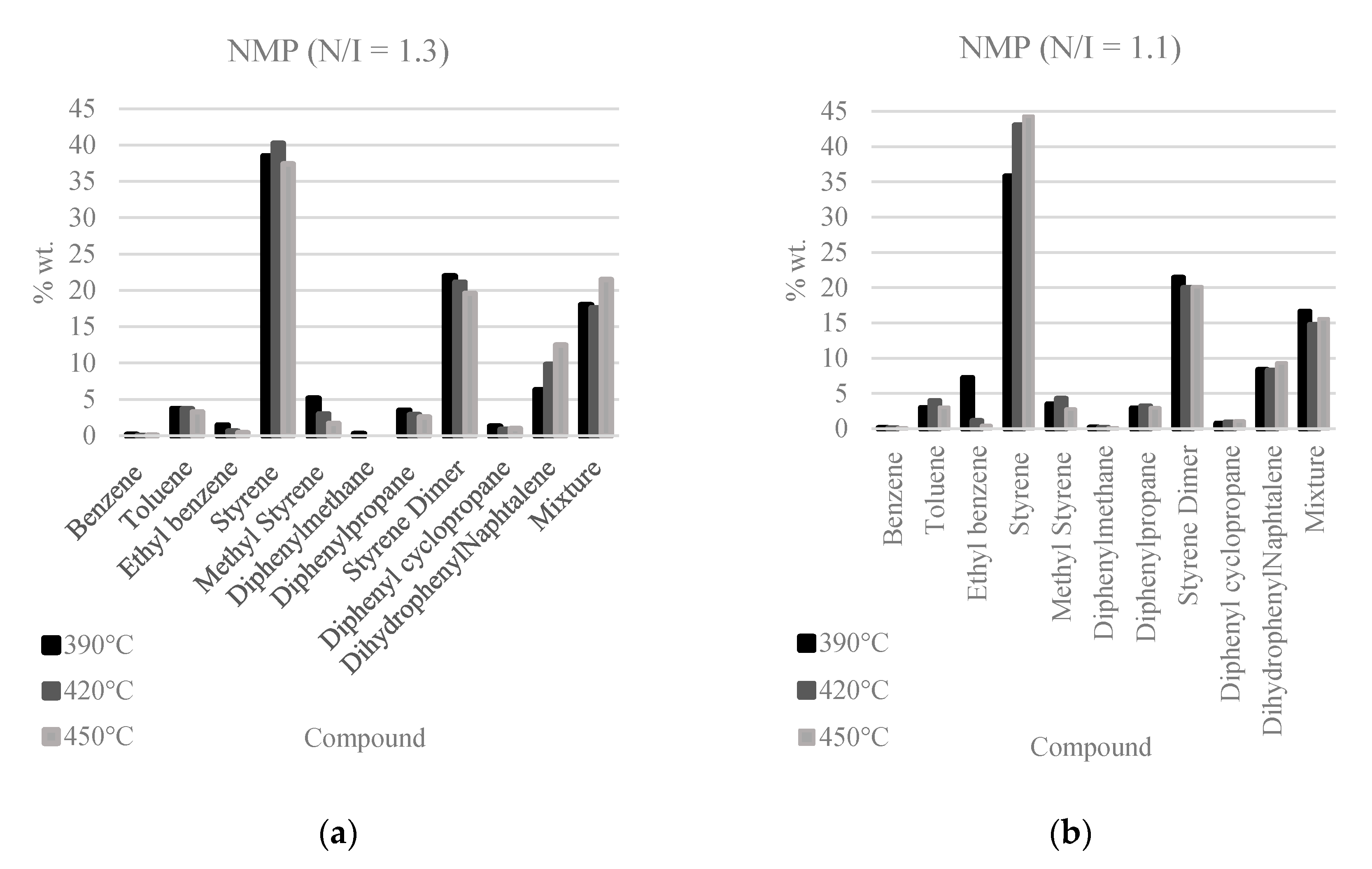
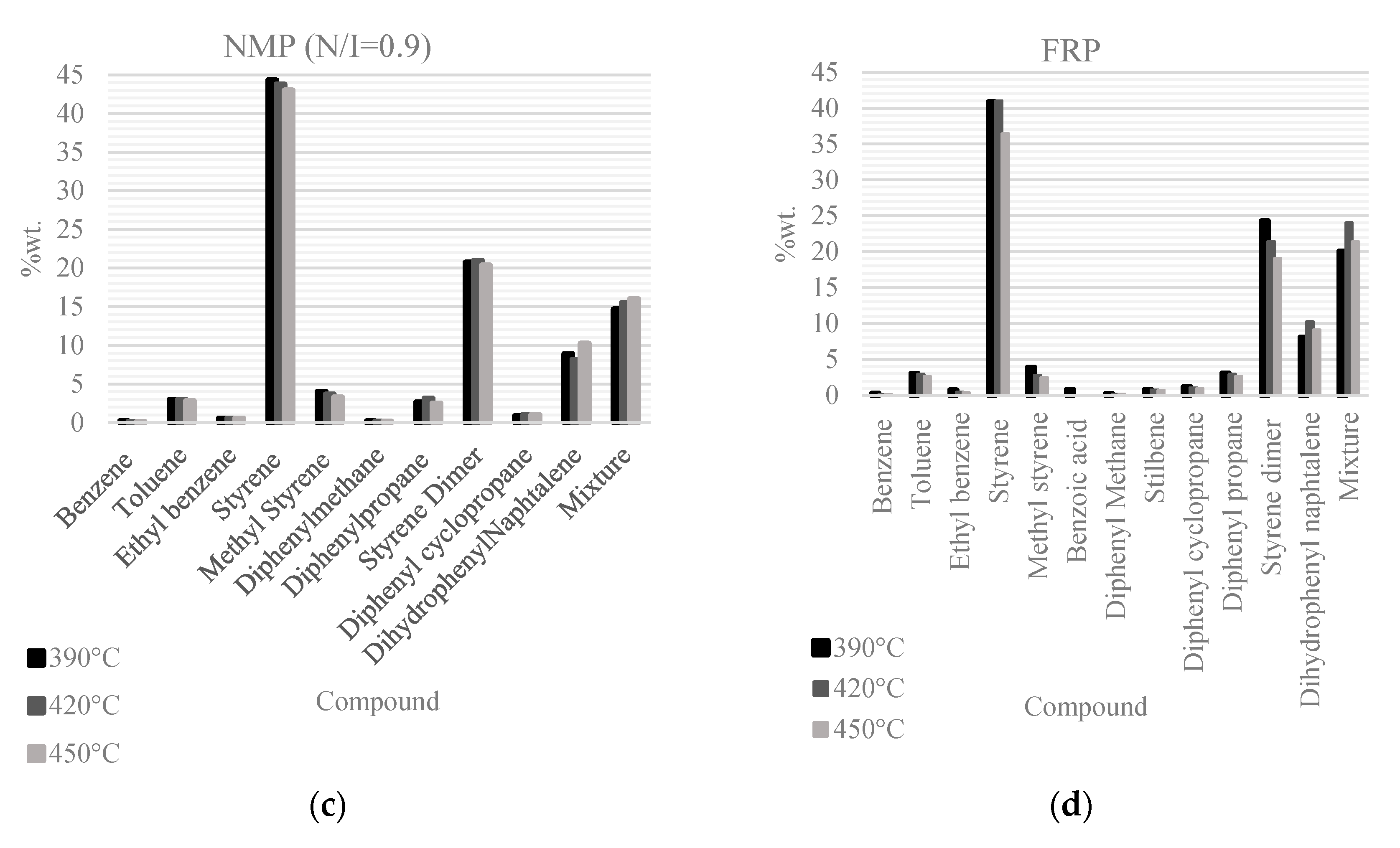
Figure 4 shows the identified products of the PS pyrolysis in the liquid fraction; their relative amounts are plotted for each PS pyrolysis experiment at three reaction temperatures (390, 420, and 450 °C). The main product in all cases is styrene followed by styrene dimer in second place; an extra category is included denoted as “mixture”, which corresponds to the sum of all the components that could not be identified, but individually amount to less than 1 wt.%. In the case of the FRP samples, stilbene and benzoic acid are also generated and the compounds in the non-identified mixture reach the highest values of all the samples.
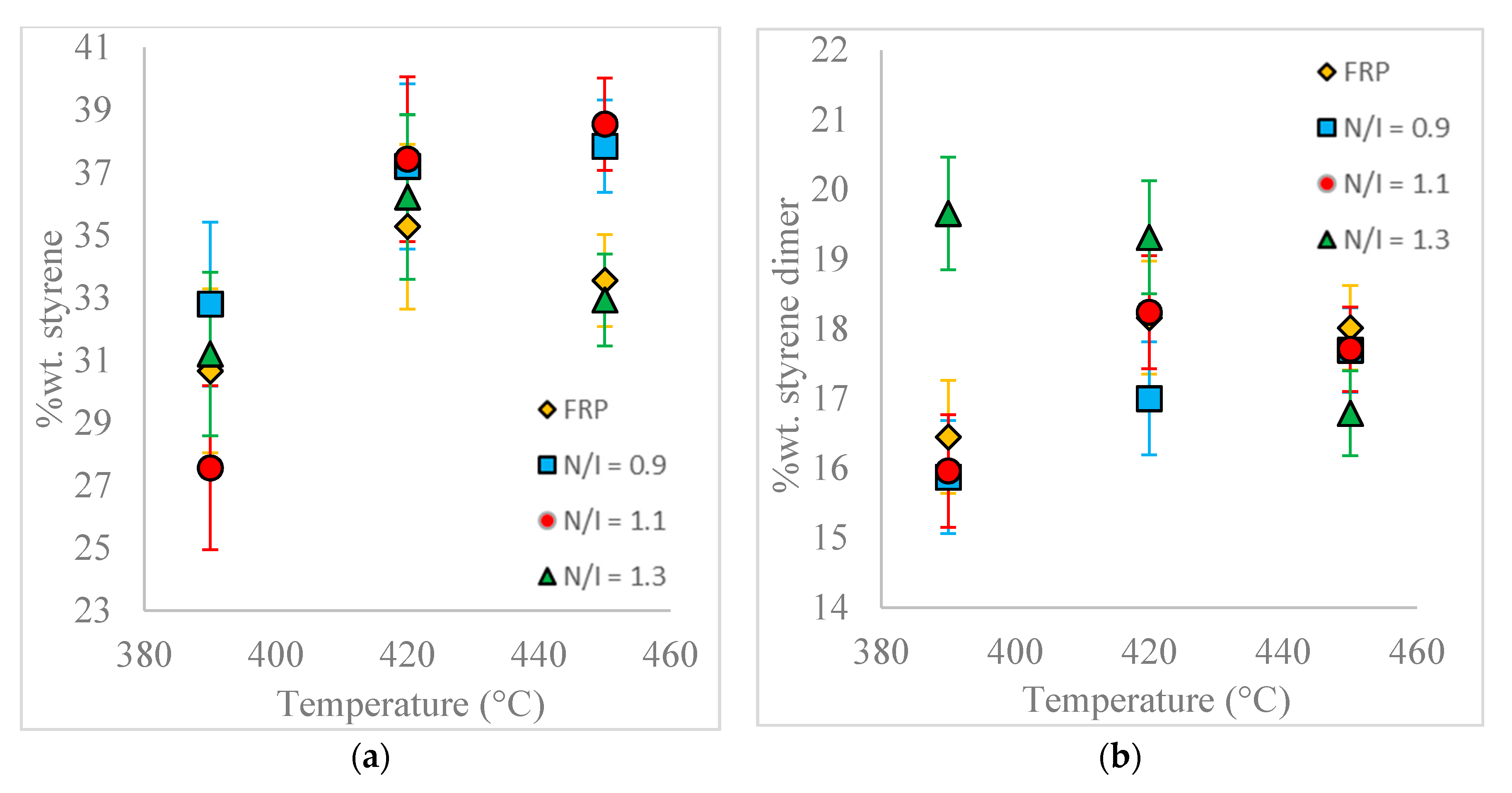
Figure 5 shows the absolute yields of styrene monomer and styrene dimer of the different samples (with respect to the total load of PS, not only with respect to the liquid fraction recovered), indicating that at higher reaction temperatures the styrene dimer concentration tends to lower for the reaction with N/I = 1.3, but it is the opposite for the reaction with N/I = 0.9. The monomer recovery yield exhibits a more complex behavior with temperature. For samples N/I = 1.1 and N/I = 0.9, the monomer recovery yield increases with higher temperatures, while for the N/I = 1.3 and the FRP samples maxima for monomer yield are exhibited. Clearly, not all the effects observed are significant, but in some cases the differences are evident, although generally moderate.
Comparing all the samples at 450 °C, the highest styrene yield (38.5%) is obtained using N/I = 1.1, and the lowest using N/I = 1.3 (around 33%) or, almost identical, FRP. Interestingly, at 420 °C all the samples with nitroxide show higher styrene yield than the FRP sample.
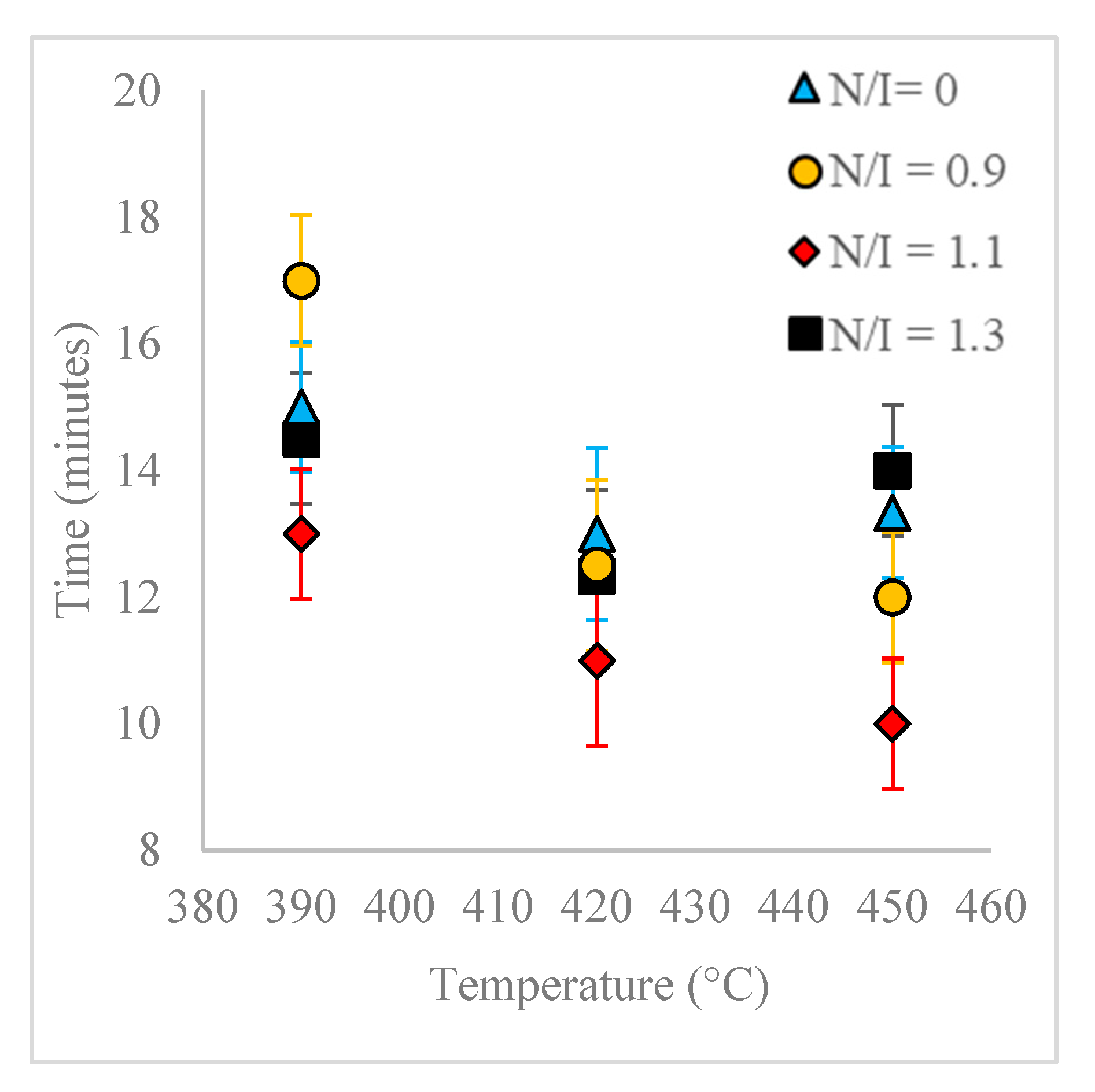
Pyrolysis reaction time is also affected by the use of PS samples containing nitroxide. Compared with FRP PS, the pyrolysis of these samples results in shorter reaction times at comparable temperatures, except in the case of FRP PS at 450 °C (
Figure 6).
During the experimentation, special emphasis was put in obtaining reproducible results, and therefore the experimental design included replicate runs. Additionally, by performing replicate experiments, we were able to observe clear qualitative differences in the presence and absence of nitroxide in the samples. For example, for the blank reactions, the pressure increase was more abrupt, and the liquid recovery began in all cases at around 380 °C (during the temperature increase ramp). On the other hand, for the dormant polymer samples, especially those of N/I = 1.3, the pressure increase was smoother and more gradual, and the liquid recovery began before, at temperatures between 370–380 °C. These and other observations, as well as some of the quantitative results, reinforce the conclusion that the pyrolysis of PS samples with and without nitroxide ends behave differently.
3.5. Mathematical Modeling Results
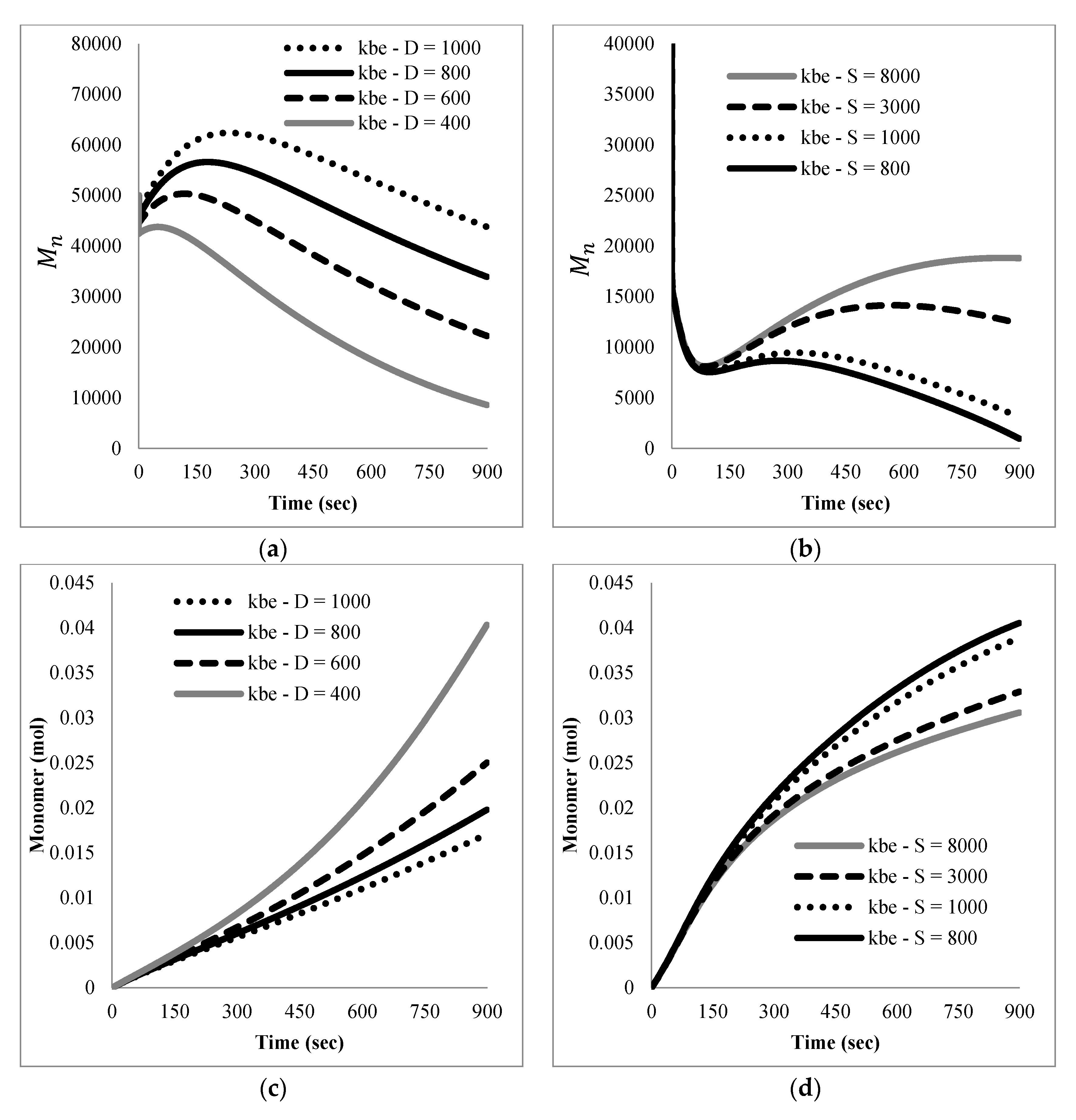
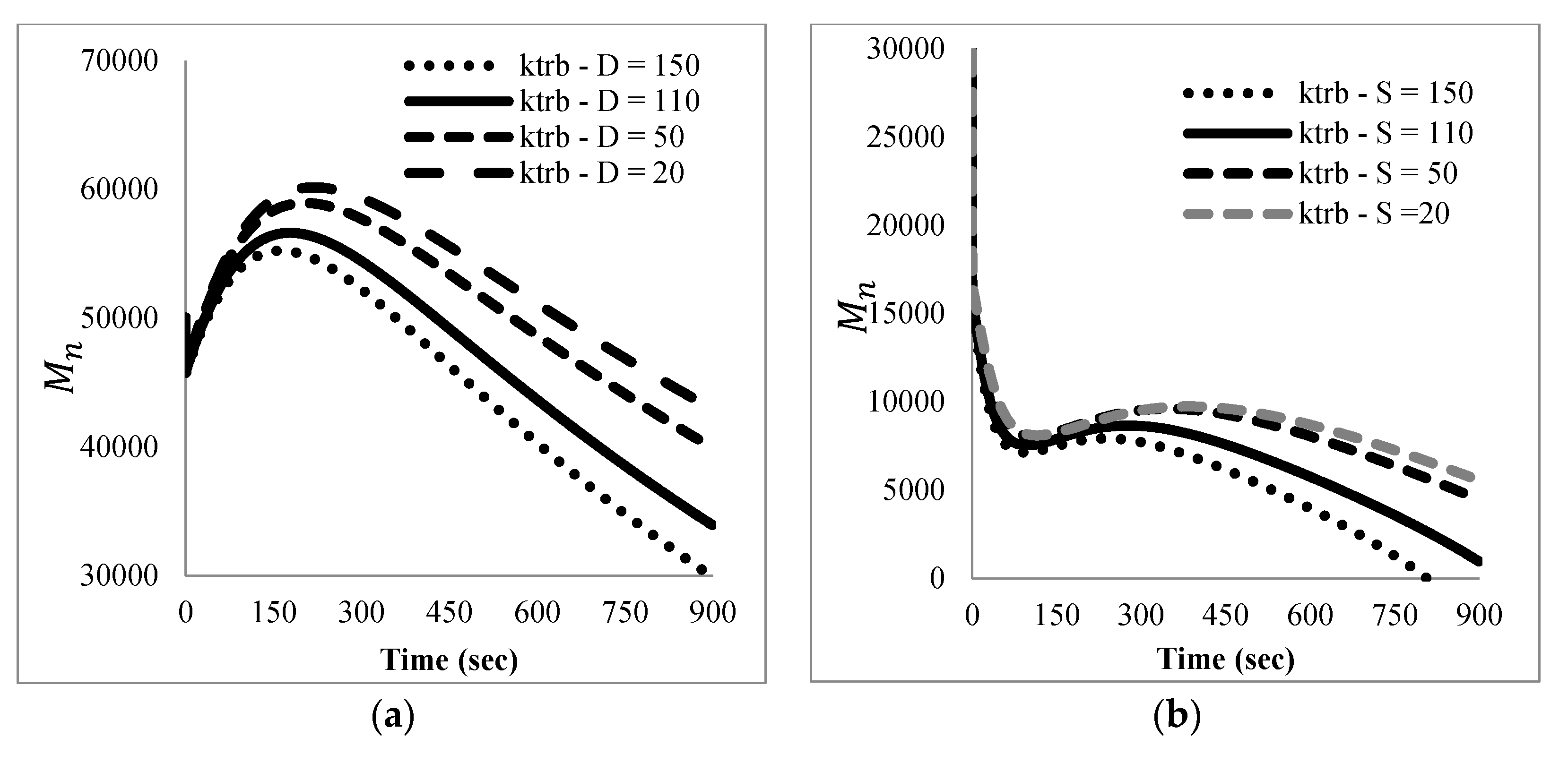
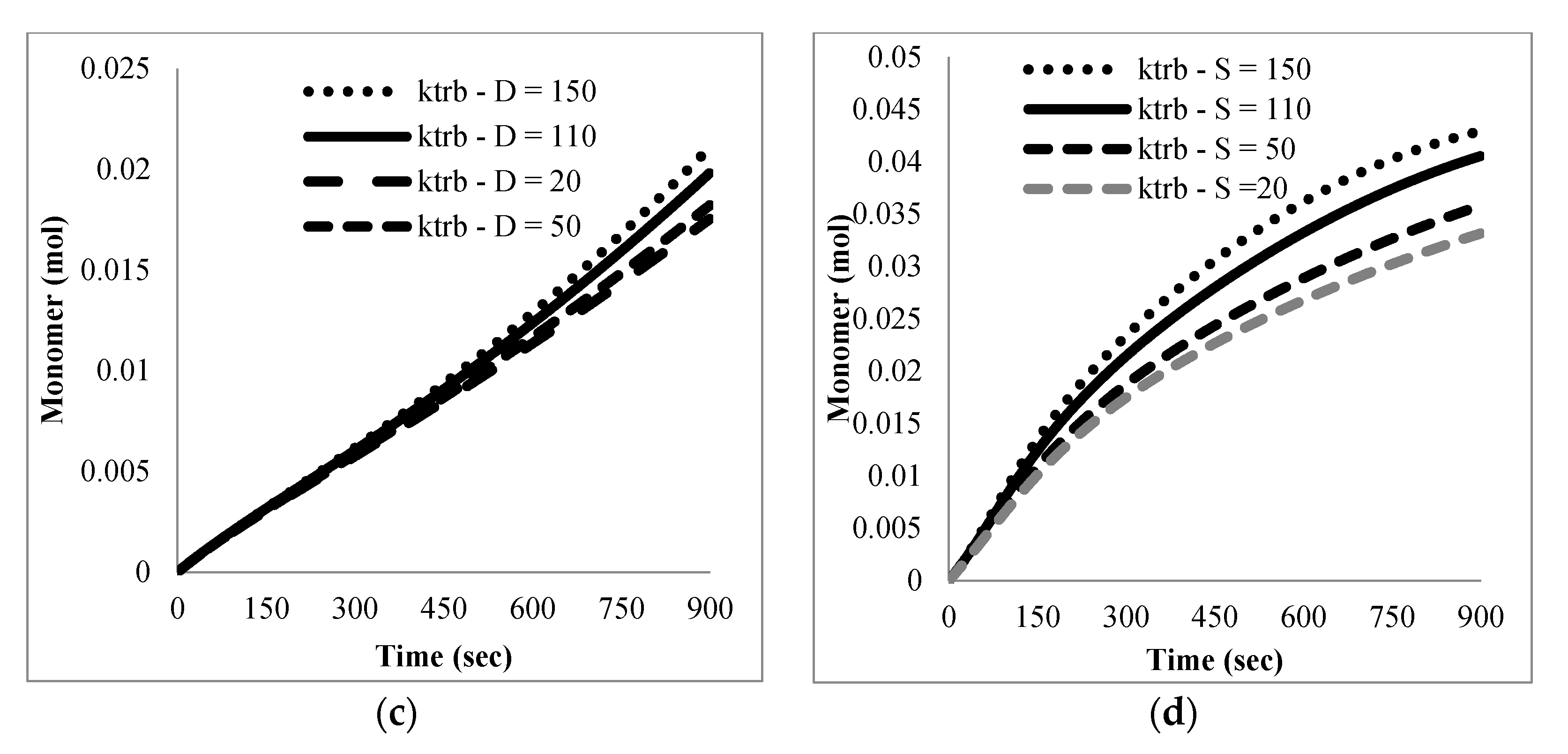
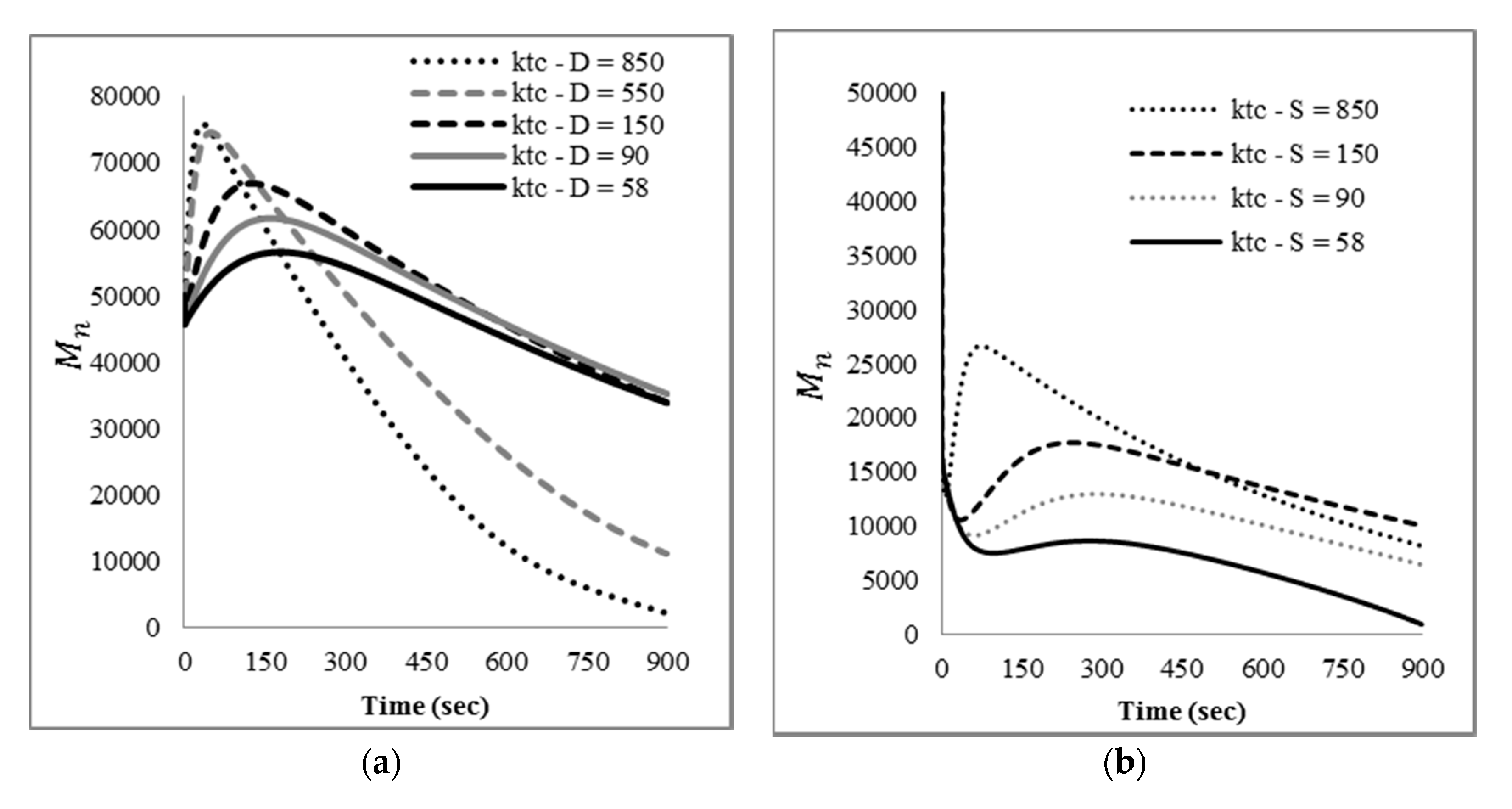
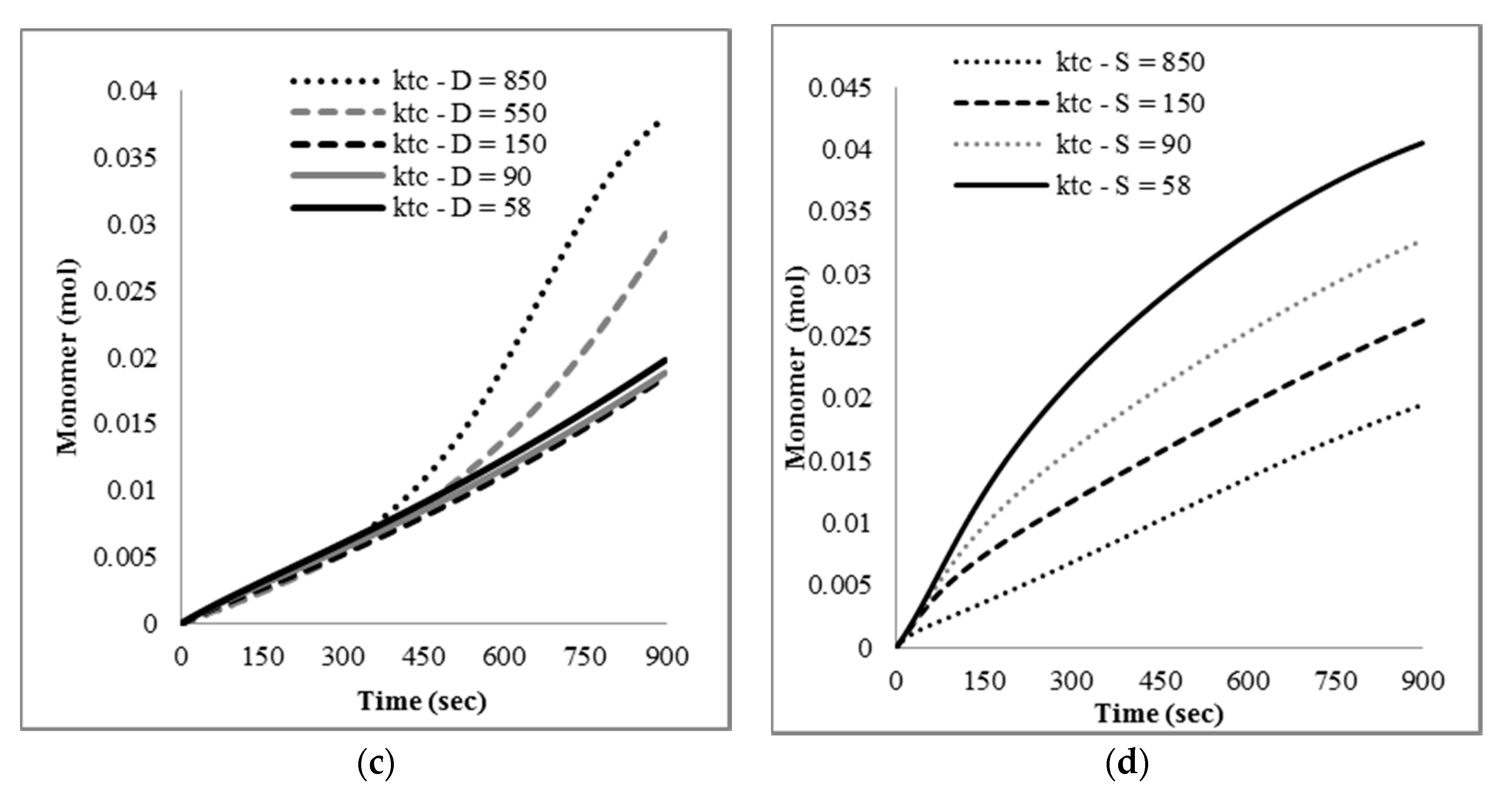
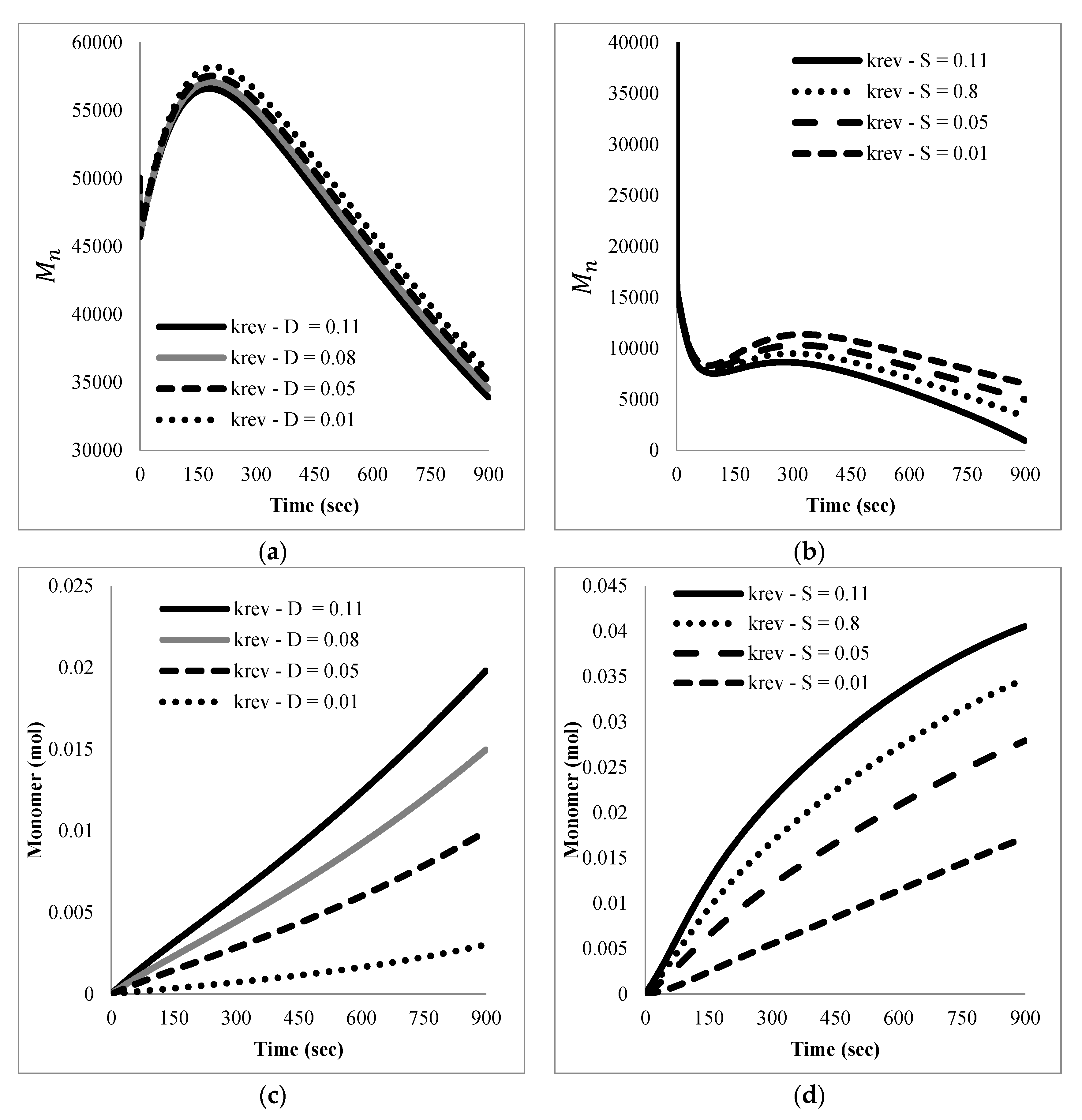
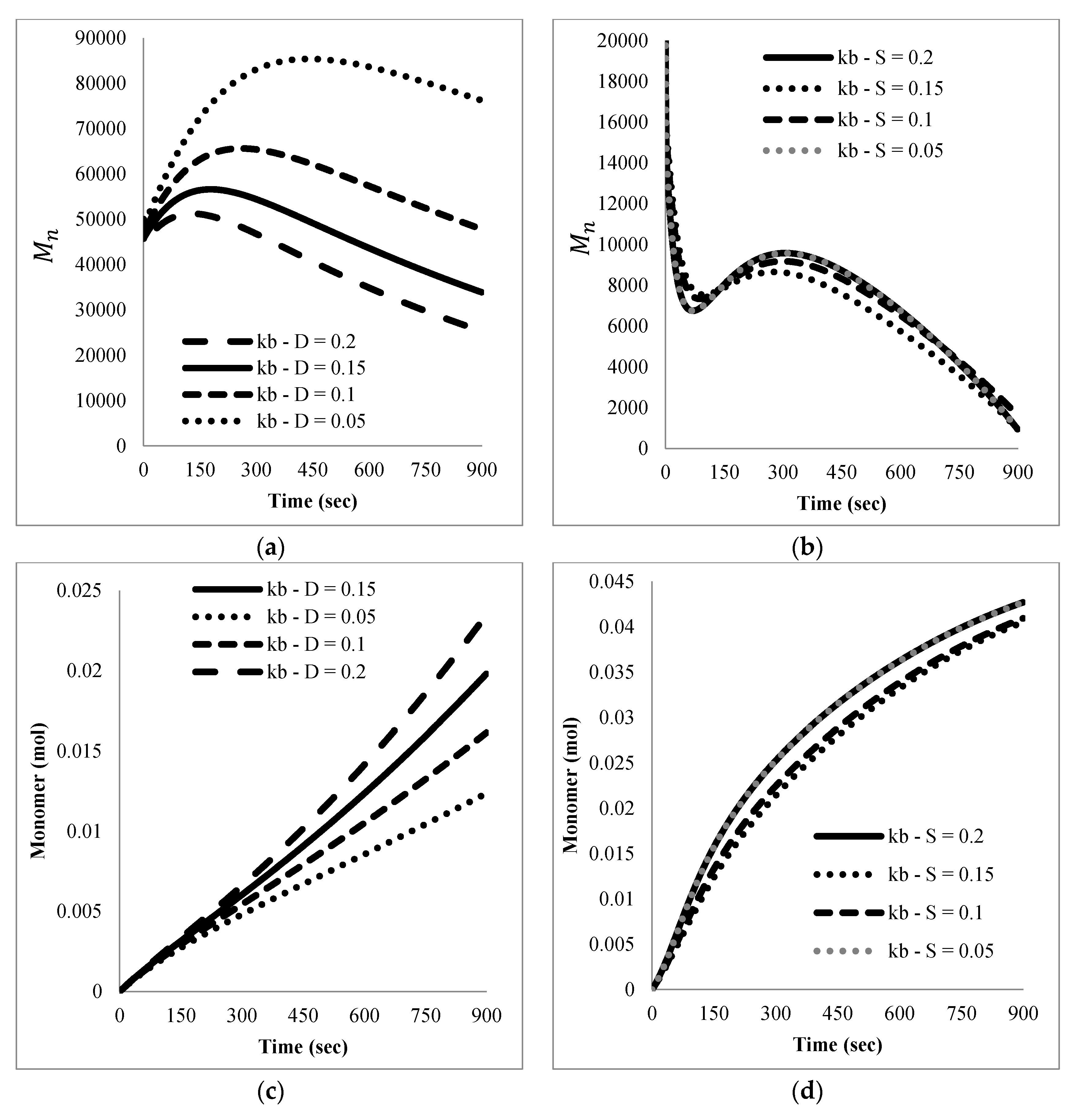
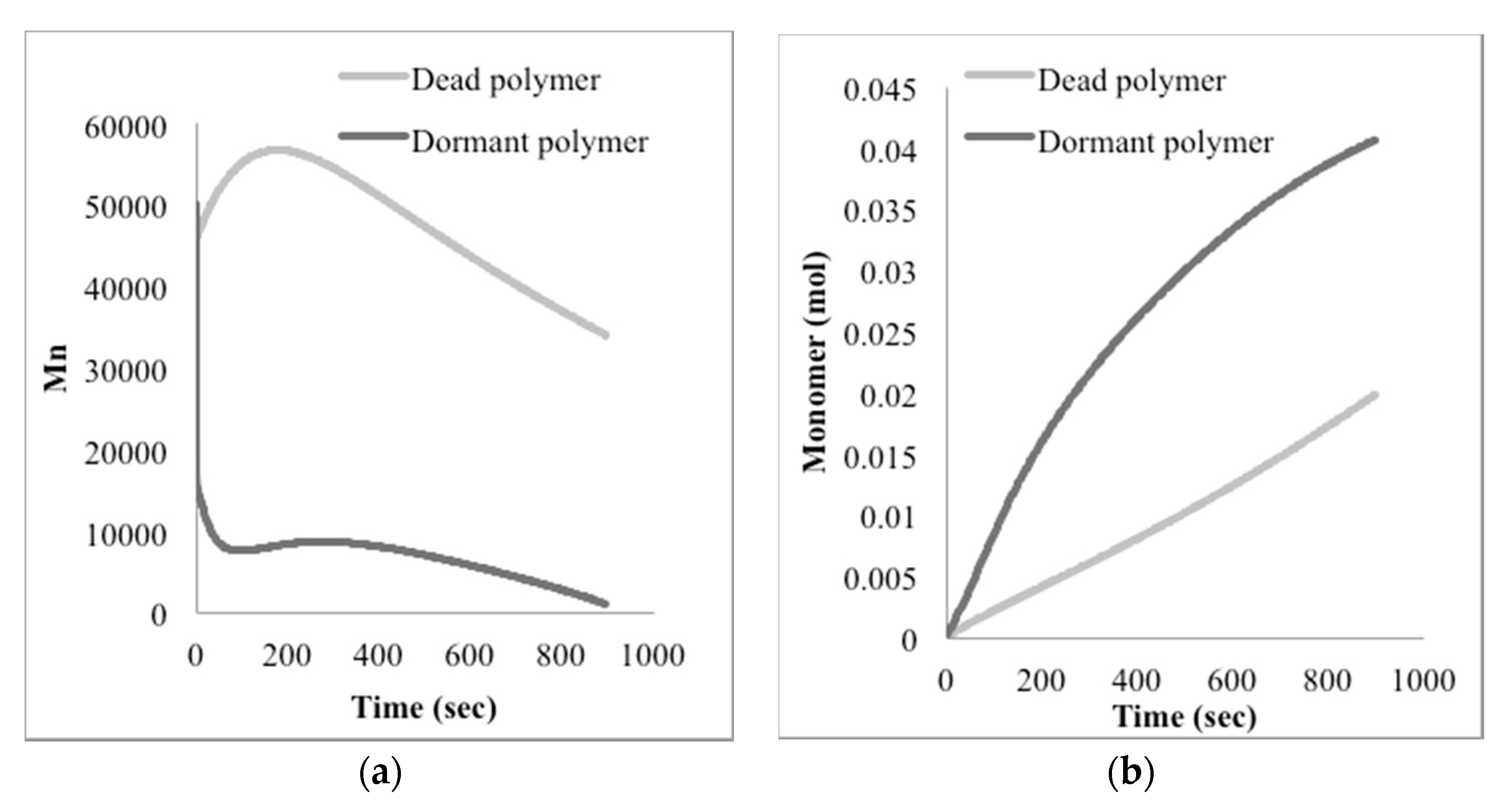
The mathematical model developed here was intended as a first approach to qualitatively and quantitatively understand the experimental results observed. At this stage, only main experimental trends, particularly reaction time and the effect of nitroxide chain-ends on reaction time, were taken into account to tune the model parameters, since not much detailed information, such as time evolution of the molecular weight distribution or of the product distribution (in terms of individual chain-lengths), was available. Ongoing work in our group is directed towards getting more detailed experimental information as well as more detailed mathematical models.
With respect to the kinetic parameters, values are reported in the literature for some of them.
Table 4 summarizes some rate constants for PS depolymerization available from the literature that were estimated in different reaction systems.
Literature values for the kinetic constants were tested using the mathematical model in terms of moments described by the Equations (5)–(8) and (13)–(24); however, the sets of parameter values available in the literature were either incomplete or corresponded to kinetic models with differences with respect to the one proposed here. When they were tried in the present kinetic model, they yielded inconsistent results in the sense of high stiffness and/or non-convergence of the numerical integration of the differential equations, as well as some results without proper physical meaning.