Mathematical Model for the Removal of Essential Oil Constituents during Steam Distillation Extraction
Abstract
1. Introduction
1.1. The Essential Oils Industry
1.2. Essential Oil Extraction Technologies
1.3. Published SDE Theories
1.4. Objectives of Research
- Treat the packed bed as a fully mixed entity, whereas in operations involving passing gas through a fixed packed bed of particles, there will be vertical variations in both the gas and the particles in the bed.
- Do not incorporate all of the resistances to transfer in their models.
- Do not recognize that the essential oil separated from the particles is a mixture of components of differing volatilities.
2. Mathematical Model
2.1. Mechanisms of Component Transfer
- Transfer of the oil to the surface of the material by diffusion
- Vaporization of the oil at the surface
- Transfer of the oil from the surface of the material to the steam by convection
2.2. Diffusion
2.3. Equilibrium Relationship
2.4. Resistance to Convective Transfer
2.5. Heating-Up Period
2.6. Application of the Model
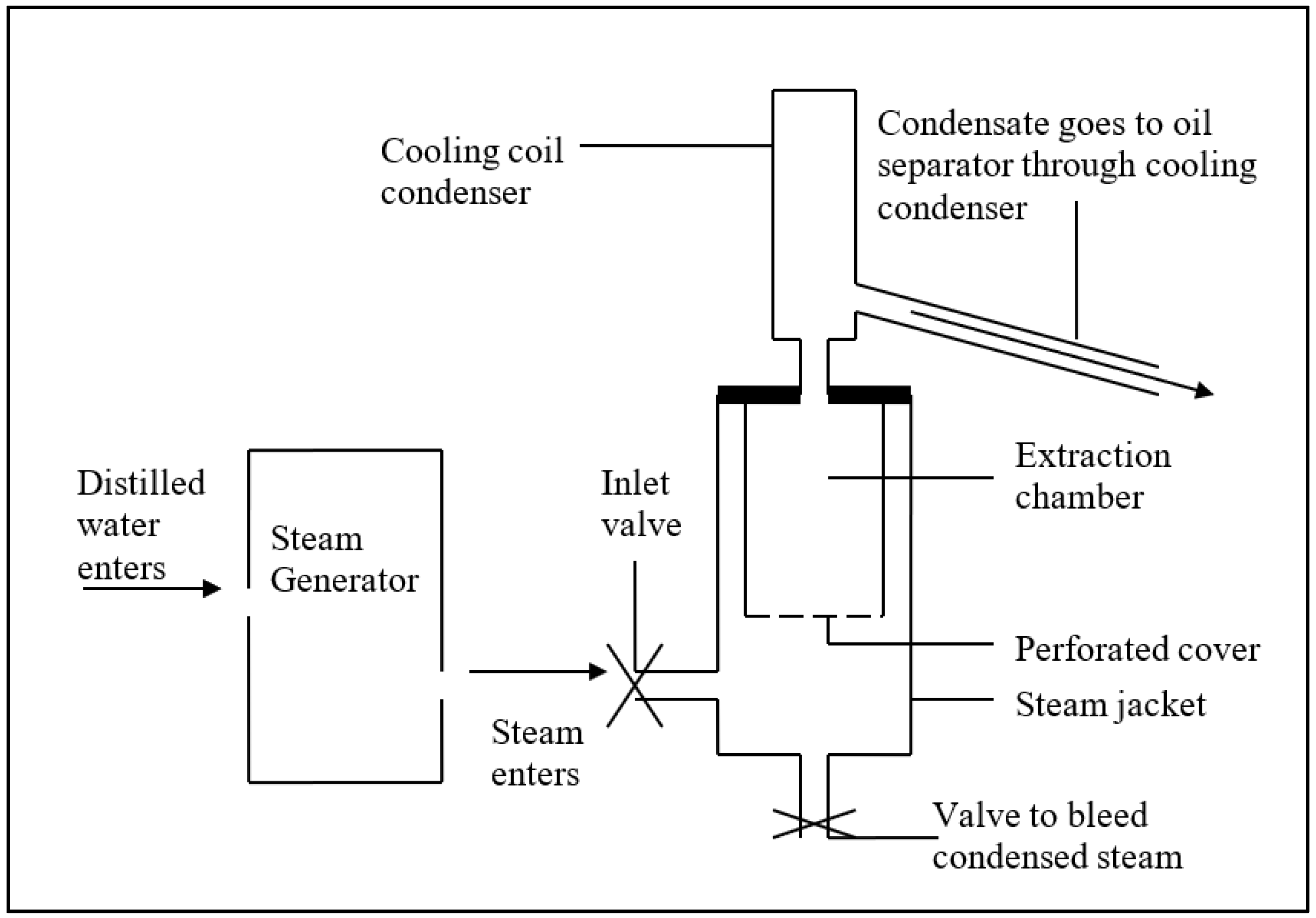
3. Materials and Methods
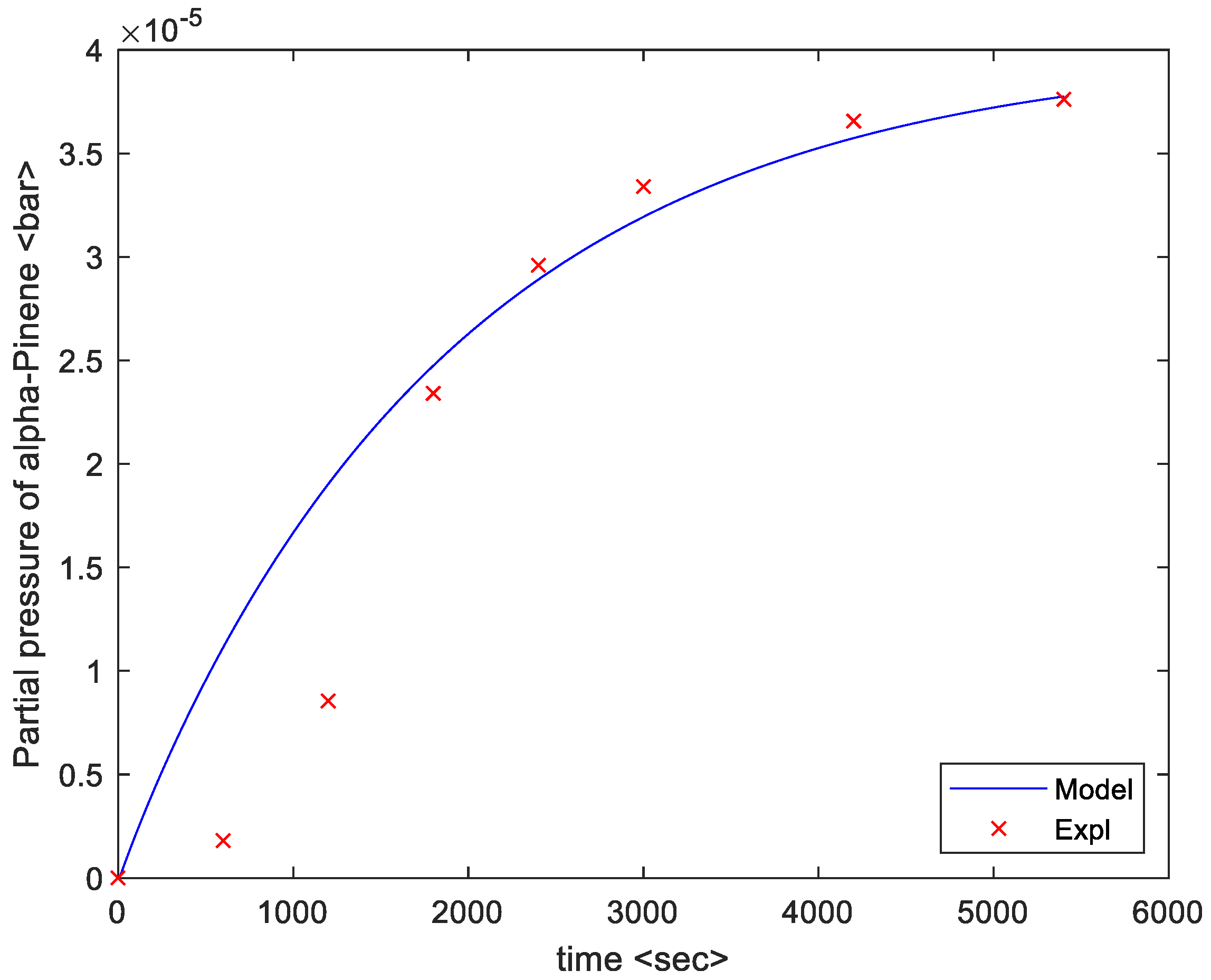
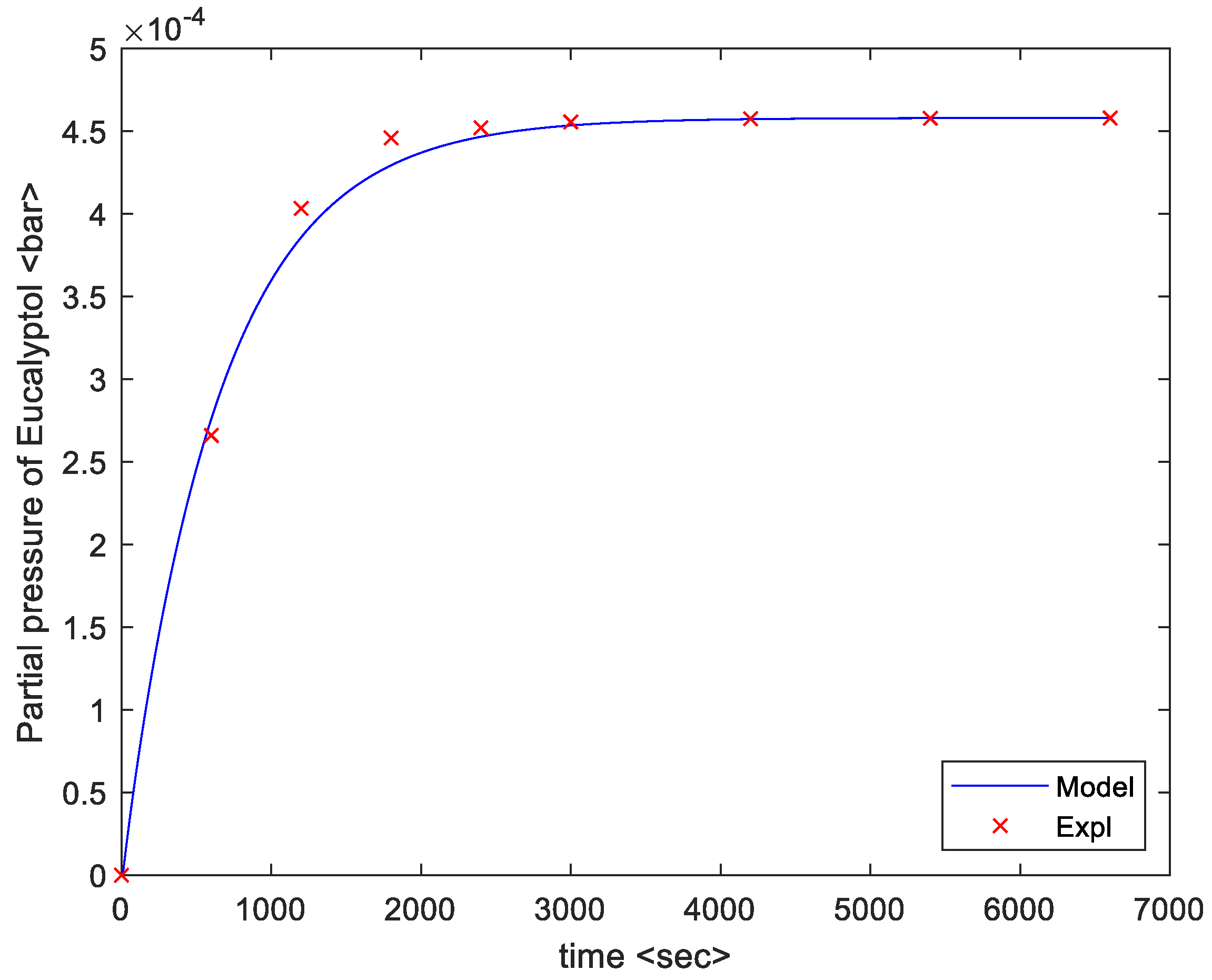
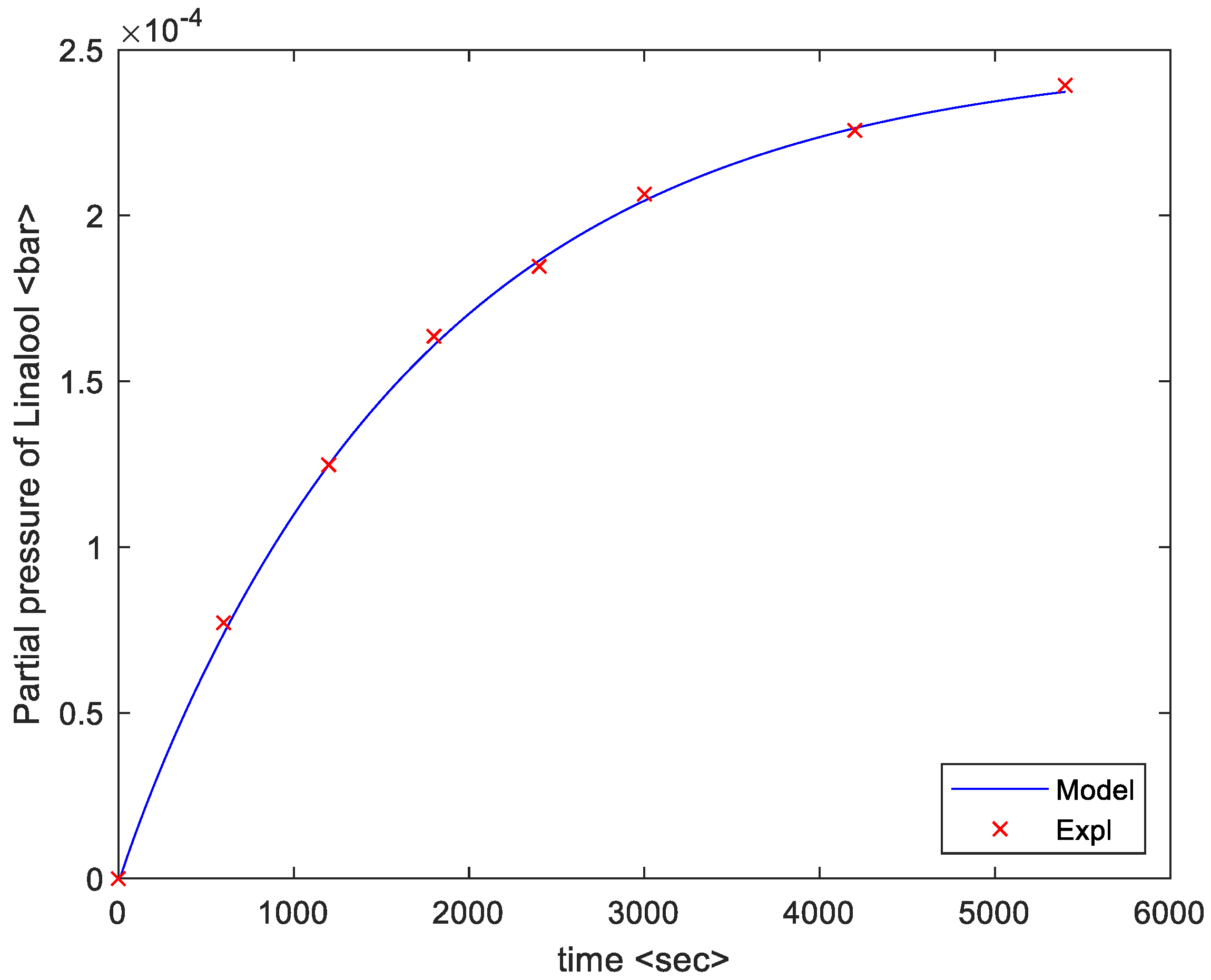
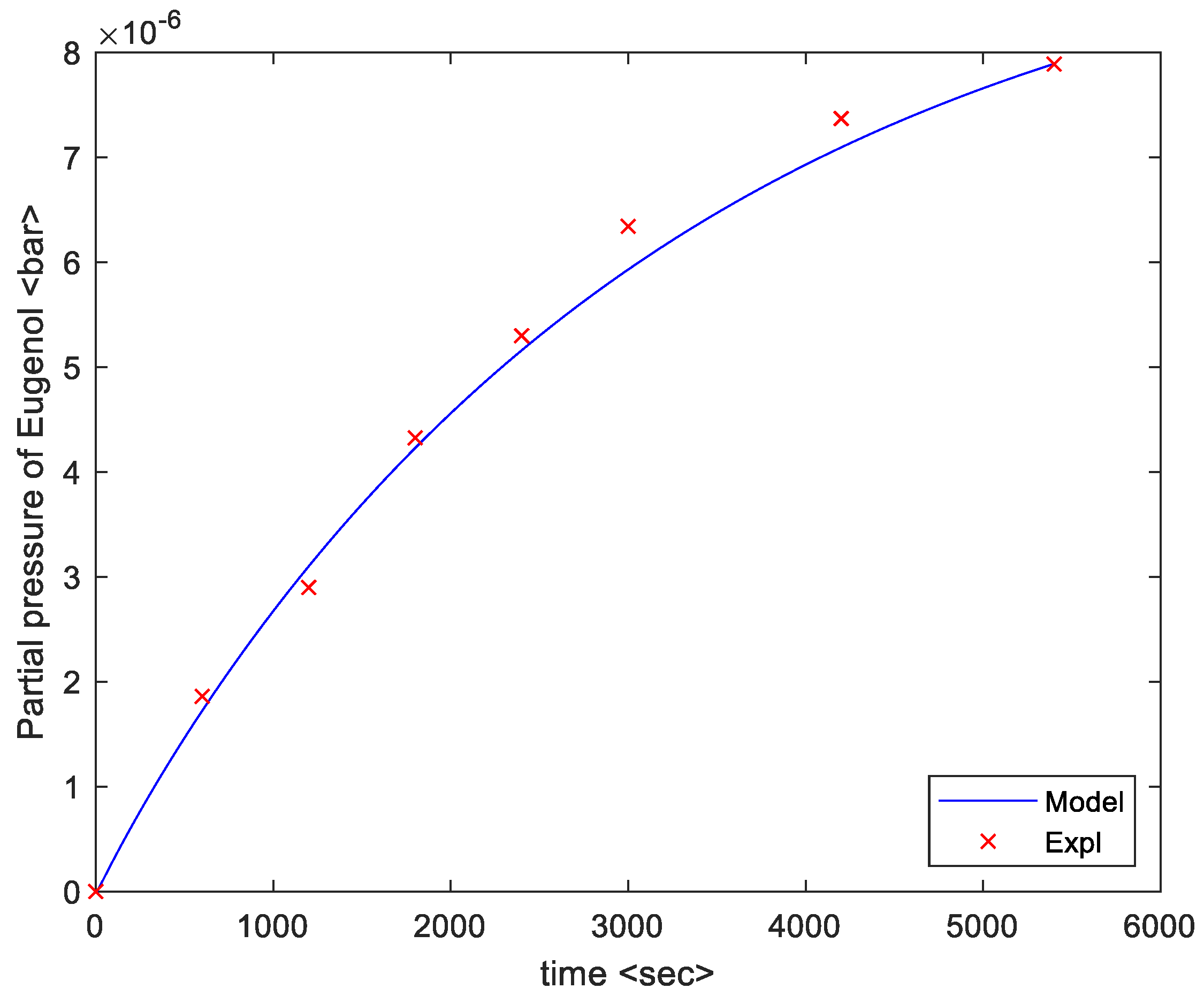
4. Results
5. Discussion
5.1. Comparison of the Proposed Model with Existing Models
5.2. Recommendations
- The effect of the steam pressure, temperature, and flow rate could be investigated by varying the process conditions to observe the nature of extraction of the components and verify our analysis further.
- The proposed model was developed using Raoult’s law which considers an ideal liquid solution that also follows the ideal-gas laws. Even though Treybal (1981) stated that for engineering purposes, this law could be applied, further model development should take this factor into account [22].
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Research, G.V. US Essential Oil Market Size, Share & Trends Analysis Report by Product (Eucalyptus, Lemon), by Application (Flavors, Fragrances), and Segment Forecasts, 2016–2024. Available online: https://www.grandviewresearch.com/industry-analysis/us-essential-oil-market (accessed on 10 January 2016).
- Guenther, E. The Essential Oils. In The Individual Essential Oils of the Plant Families Rutaceae and Labiatae; D. Van Nostrand Company, Inc.: New York, NY, USA, 1949; Volume 3. [Google Scholar]
- Romdhane, M.; Tizaoui, C. The kinetic modelling of a steam distillation unit for the extraction of aniseed (Pimpinella anisum) essential oil. J. Chem. Technol. Biotechnol. 2005, 80, 759–766. [Google Scholar] [CrossRef]
- Huang, Z. Mass Transfer Models for Supercritical Fluid Extraction; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Cassel, E.; Vargas, R. Experiments and Modeling of the Cymbopogon winterianus Essential Oil Extraction by Steam Distillation. J. Mex. Chem. Soc. 2006, 50, 126–129. [Google Scholar]
- Cassel, E.; Vargas, R.M.F.; Martinez, N.; Lorenzo, D.; Dellacassa, E. Steam distillation modeling for essential oil extraction process. Ind. Crop. Prod. 2009, 29, 171–176. [Google Scholar] [CrossRef]
- Sovová, H.; Aleksovski, S.A. Mathematical model for hydrodistillation of essential oils. Flavour Fragr. J. 2006, 21, 881–889. [Google Scholar] [CrossRef]
- Xavier, V.B.; Vargas, R.M.F.; Cassel, E.; Lucas, A.M.; Santos, M.A.; Mondin, C.A.; Santarem, E.R.; Astarita, L.V.; Sartor, T. Mathematical modeling for extraction of essential oil from Baccharis spp. by steam distillation. Ind. Crop. Prod. 2011, 33, 599–604. [Google Scholar] [CrossRef]
- Meziane, I.A.A.; Bali, N.; Belblidia, N.-B.; Abatzoglou, N.; Benyoussef, E.-H. The first-order model in the simulation of essential oil extraction kinetics. J. Appl. Res. Med. Aromat. Plants 2019, 15, 100226. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: London, UK, 1975. [Google Scholar]
- Reid, R.; Prausnitz, J.; Poling, B. The Properties of Gases and Liquids; McGraw-Hill: New York, NY, USA, 1987. [Google Scholar]
- McGaw, D.R. Gas—Particle heat transfer in a crossflow moving packed bed heat exchanger. Powder Technol. 1976, 13, 231–239. [Google Scholar] [CrossRef]
- Maharaj, S.; McGaw, D.R.; Chang Yen, I. Analysis and Potential End-user Applicationsof Steam Distilled Essential Oils of Ocimumbasilicum L. grown in Trinidad, West Indies. Int. J. Sci. Eng. Res. 2017, 8, 367–371. [Google Scholar]
- Hasegawa, Y.; Tajima, K.; Toi, N.; Sugimura, Y. Characteristic Components Found in the Essential Oil of Ocimum basilicum L. Flavour Fragr. J. 1997, 12, 195–200. [Google Scholar] [CrossRef]
- ÖZcan, M.; Chalchat, J.-C. Essential Oil Composition of Ocimum basilicum L. and Ocimum minimum L. in Turkey. Czech J. Food Sci. 2002, 20. [Google Scholar] [CrossRef]
- Ozcan, M.; Chalchat, J.C. Essential oil composition of a new chemotype of Basil (Ocimum basilicum L.) cultivating in Turkey. J. Essent. Oil Bear. Plants 2004, 7, 155–159. [Google Scholar] [CrossRef]
- Sanda, K.; Koba, K.; Nambo, P.; Gaset, A. Chemical investigation of Ocimum species growing in Togo. Flavour Fragr. J. 1998, 13, 226–232. [Google Scholar] [CrossRef]
- Silva, M.G.D.V.; Matos, F.J.D.A.; Lacerda Machado, M.I.; Craveiro, A.A. Essential oils of Ocimum basilicum L., O. basilicum. var. minimum L. and O. basilicum. var. purpurascens Benth. grown in north-eastern Brazil. Flavour Fragr. J. 2003, 18, 13–14. [Google Scholar] [CrossRef]
- Kéita, S.M.; Vincent, C.; Schmit, J.-P.; Bélanger, A. Essential oil composition of Ocimum basilicum L., O. gratissimum L. and O. suave L. in the Republic of Guinea. Flavour Fragr. J. 2000, 15, 339–341. [Google Scholar] [CrossRef]
- Schulz, H.; Schrader, B.; Quilitzsch, R.; Pfeffer, S.; Krüger, H. Rapid Classification of Basil Chemotypes by Various Vibrational Spectroscopy Methods. J. Agric. Food Chem. 2003, 51, 2475–2481. [Google Scholar] [CrossRef] [PubMed]
- Priyanka; Khanam, S. Selection of suitable model for different matrices of raw materials used in supercritical fluid extraction process. Sep. Sci. Technol. 2018, 53, 71–96. [Google Scholar] [CrossRef]
- Treybal, R.E. Mass Transfer Operations; International Edition; McGraw-Hill: New York, NY, USA, 1981. [Google Scholar]



| Components | k Values*10−5/ms−1 | D Values*10−12/m2s−1 |
|---|---|---|
| α-pinene | 3.81 | 5.00 |
| α-terpinene | 2.59 | 2.98 |
| Eucalyptol | 5.14 | 6.99 |
| Linalool | 4.03 | 5.42 |
| Geraniol | 1.60 | 1.00 |
| Eugenol | 2.59 | 2.98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maharaj, S.; McGaw, D. Mathematical Model for the Removal of Essential Oil Constituents during Steam Distillation Extraction. Processes 2020, 8, 400. https://doi.org/10.3390/pr8040400
Maharaj S, McGaw D. Mathematical Model for the Removal of Essential Oil Constituents during Steam Distillation Extraction. Processes. 2020; 8(4):400. https://doi.org/10.3390/pr8040400
Chicago/Turabian StyleMaharaj, Sharad, and David McGaw. 2020. "Mathematical Model for the Removal of Essential Oil Constituents during Steam Distillation Extraction" Processes 8, no. 4: 400. https://doi.org/10.3390/pr8040400
APA StyleMaharaj, S., & McGaw, D. (2020). Mathematical Model for the Removal of Essential Oil Constituents during Steam Distillation Extraction. Processes, 8(4), 400. https://doi.org/10.3390/pr8040400




