Modeling of Novel Thermodynamic Cycles to Produce Power and Cooling Simultaneously
Abstract
:1. Introduction
2. System Description
2.1. Goswami Cycle (Model I)
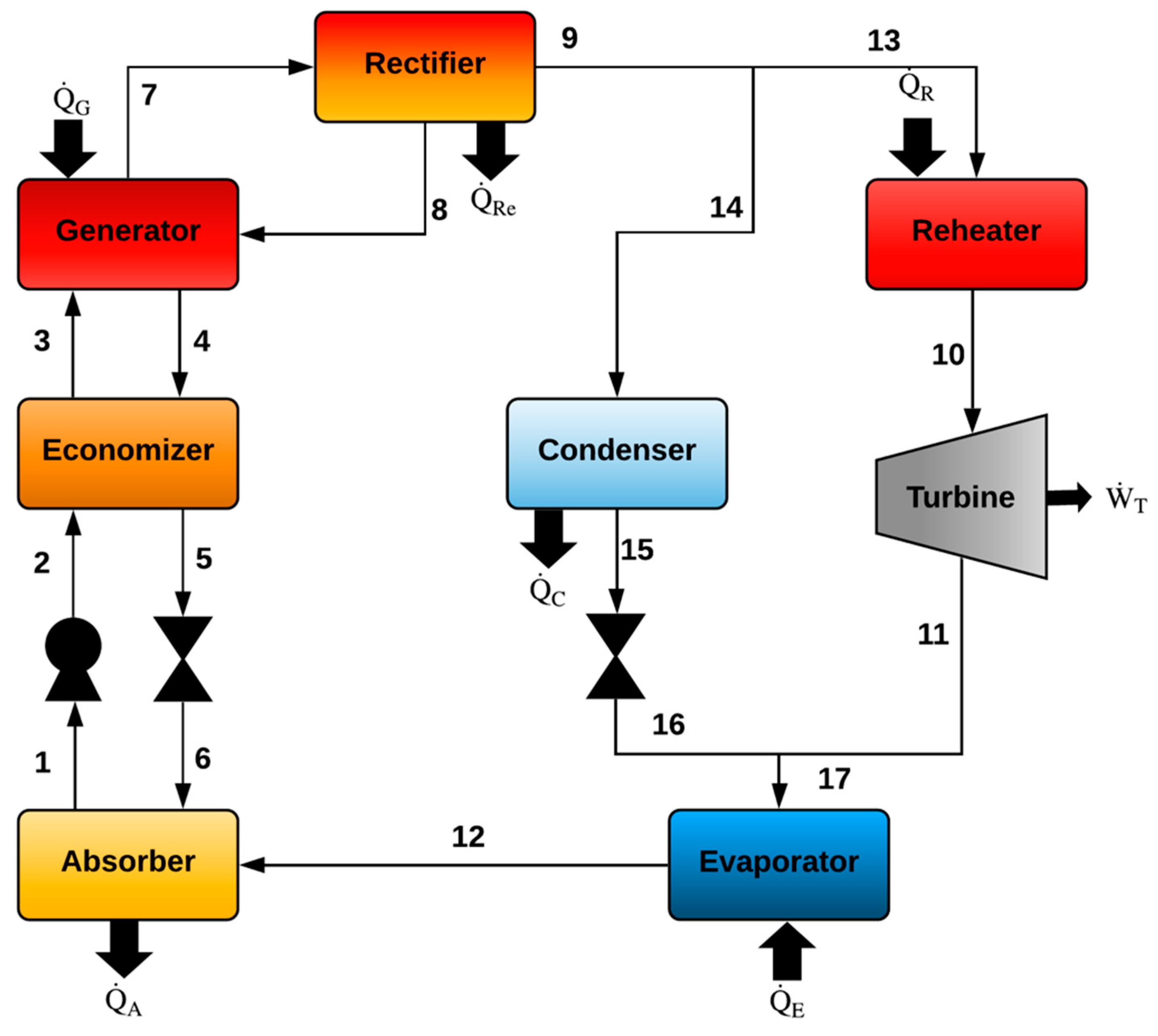
2.2. Goswami Cycle with a Condenser and a Flow Division after the Rectifier (Model II)
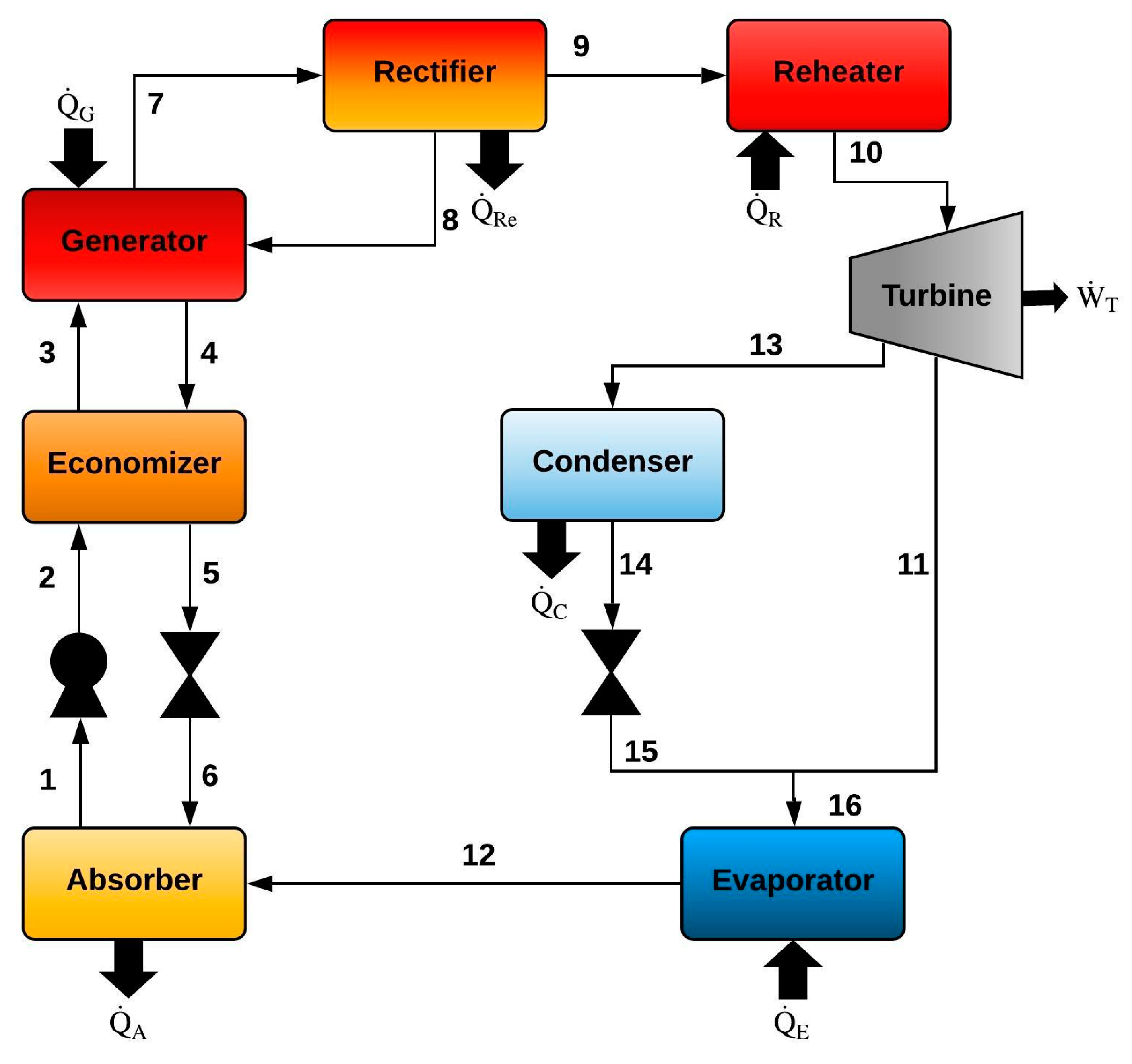
2.3. Goswami Cycle with a Condenser and a Flow Division into the Turbine (Model III)
3. Mathematical Model
3.1. Assumptions
- i.
- Thermodynamic equilibrium conditions are considered in each cycle.
- ii.
- The cycles operate in steady-state conditions.
- iii.
- Heat losses from the components are considered negligible.
- iv.
- The refrigerant is considered in saturation conditions at the exit of the condenser.
- v.
- The solution is considered in saturation conditions at the exit of the absorber and generator.
- vi.
- Pressure losses due to friction are neglected in each component, with the exception of the expansion valves and the turbine.
- vii.
- The throttling process in the valves is isenthalpic.
3.2. Main Equations
3.3. Input Data
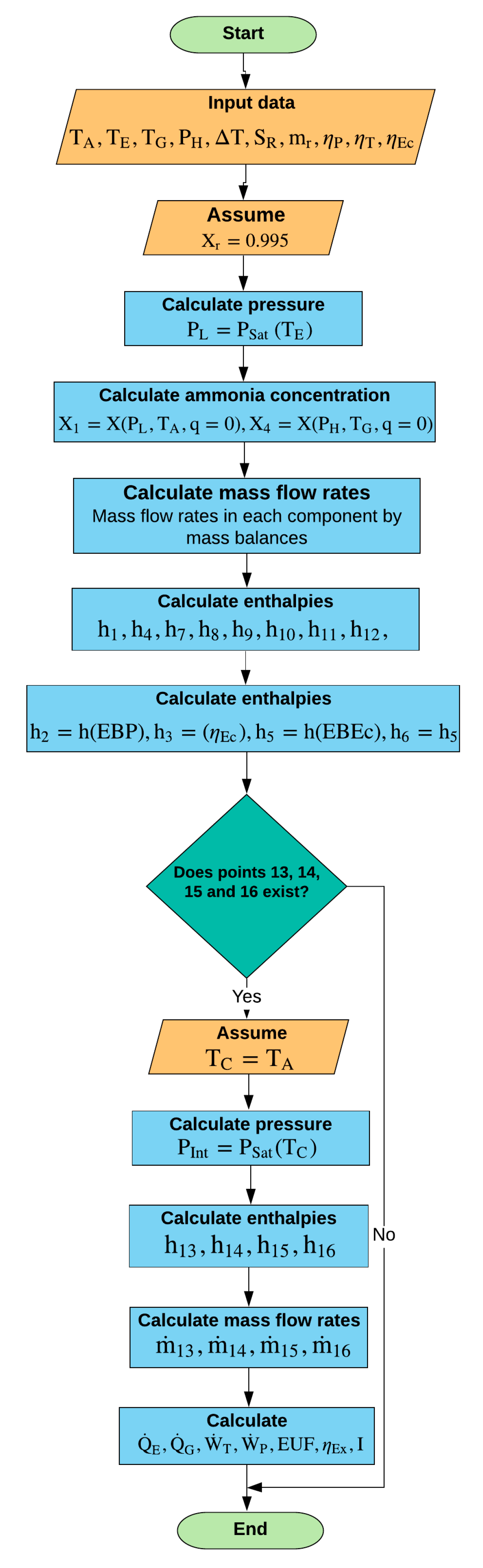
3.4. Algorithm
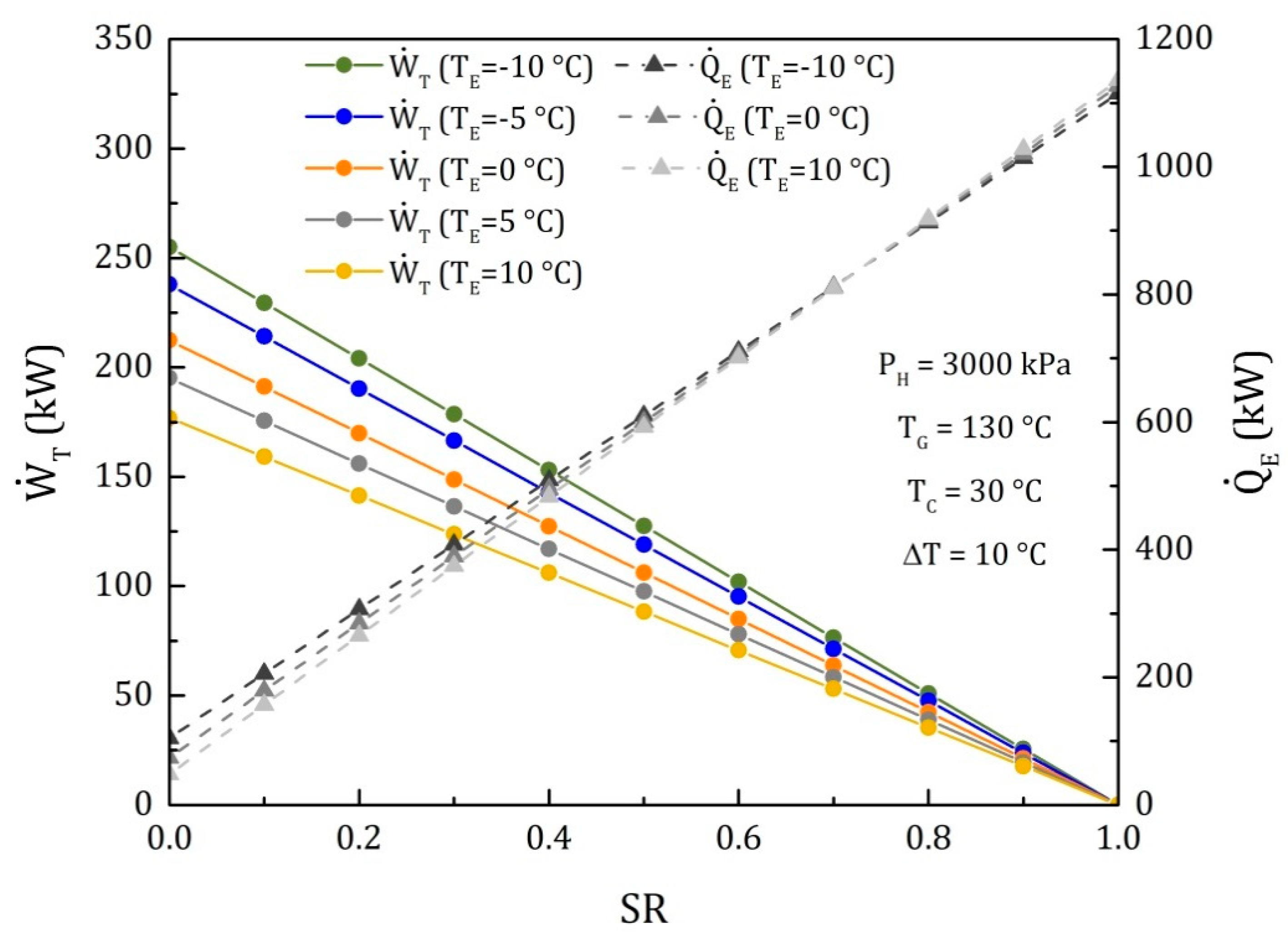
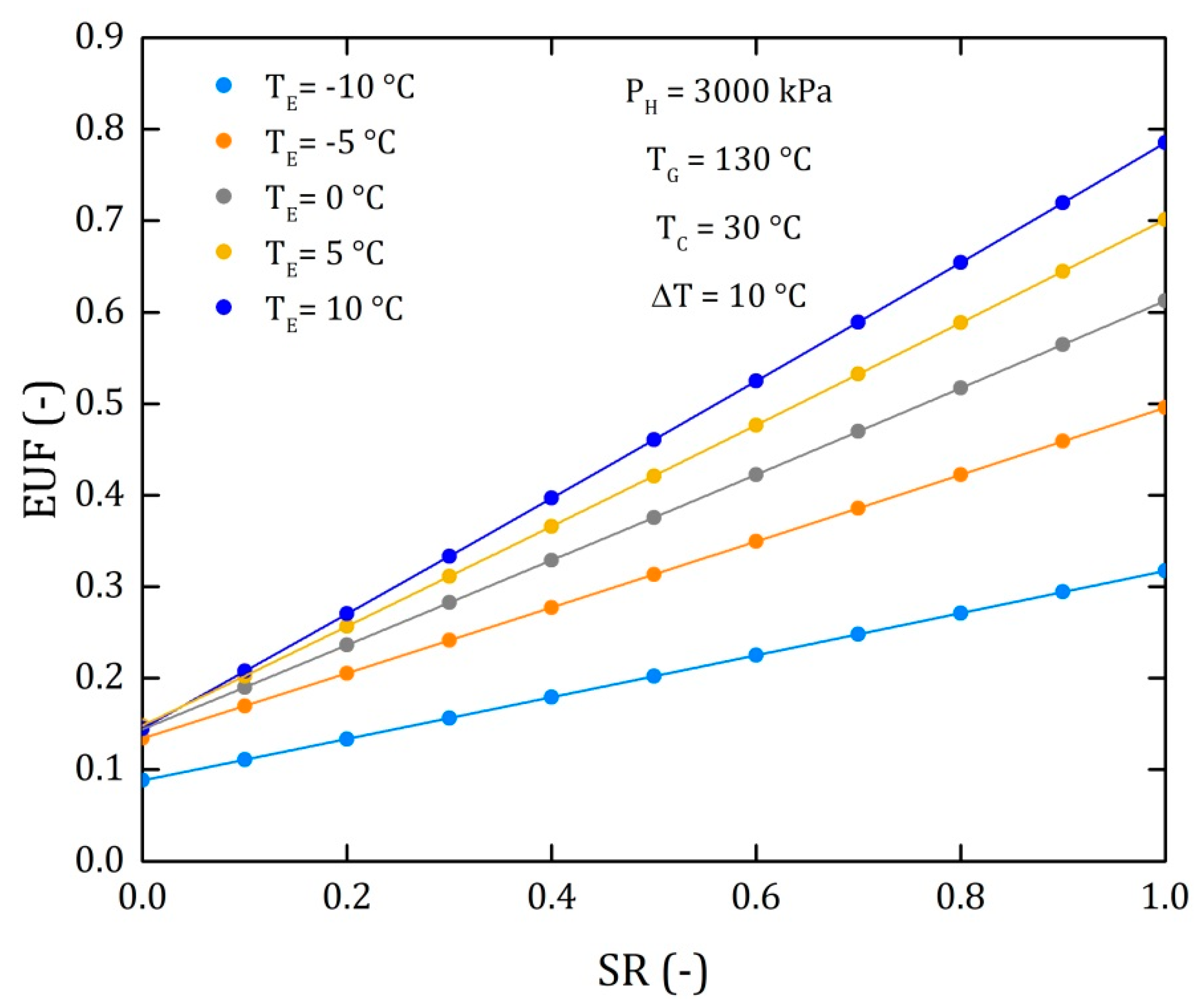
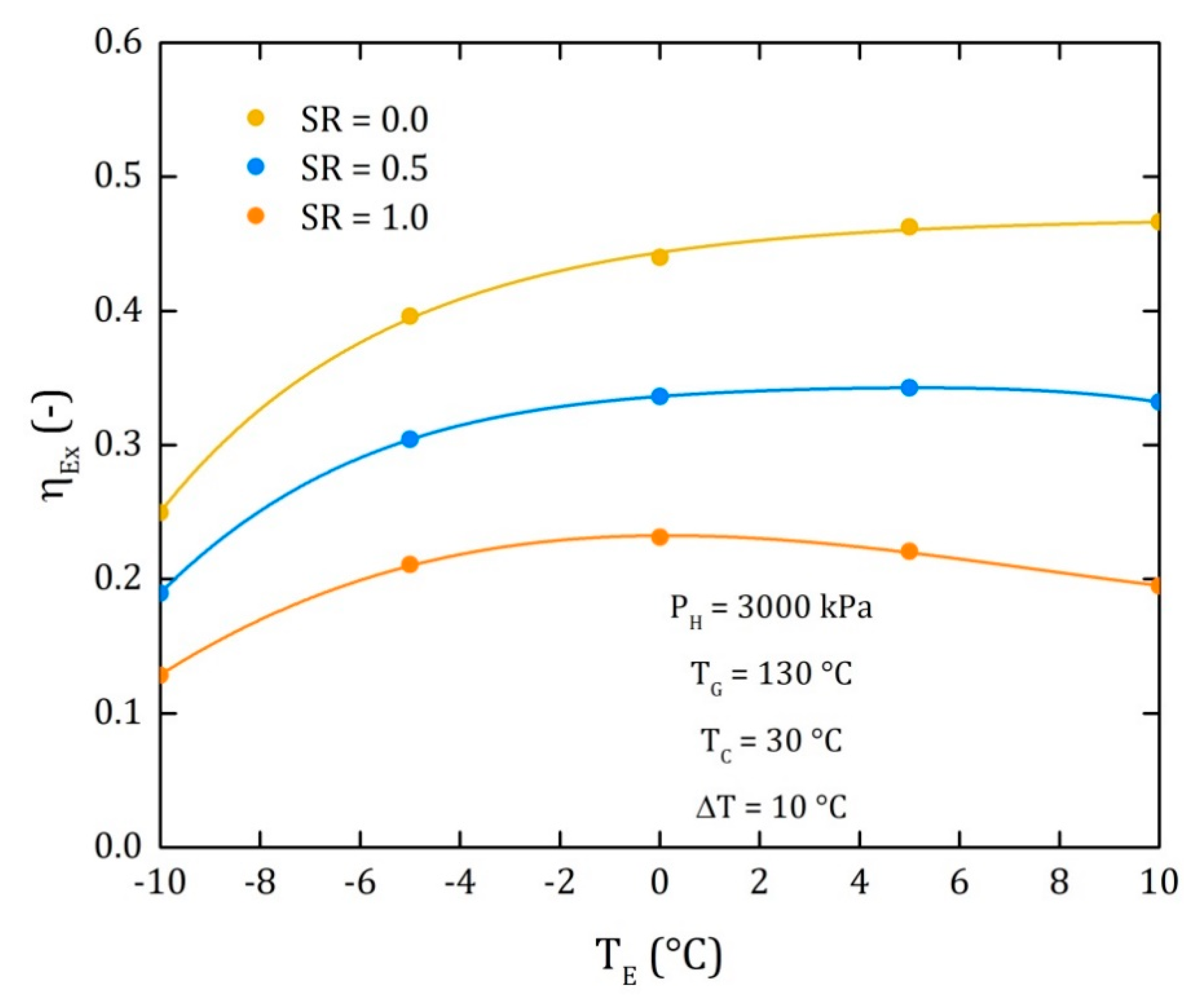
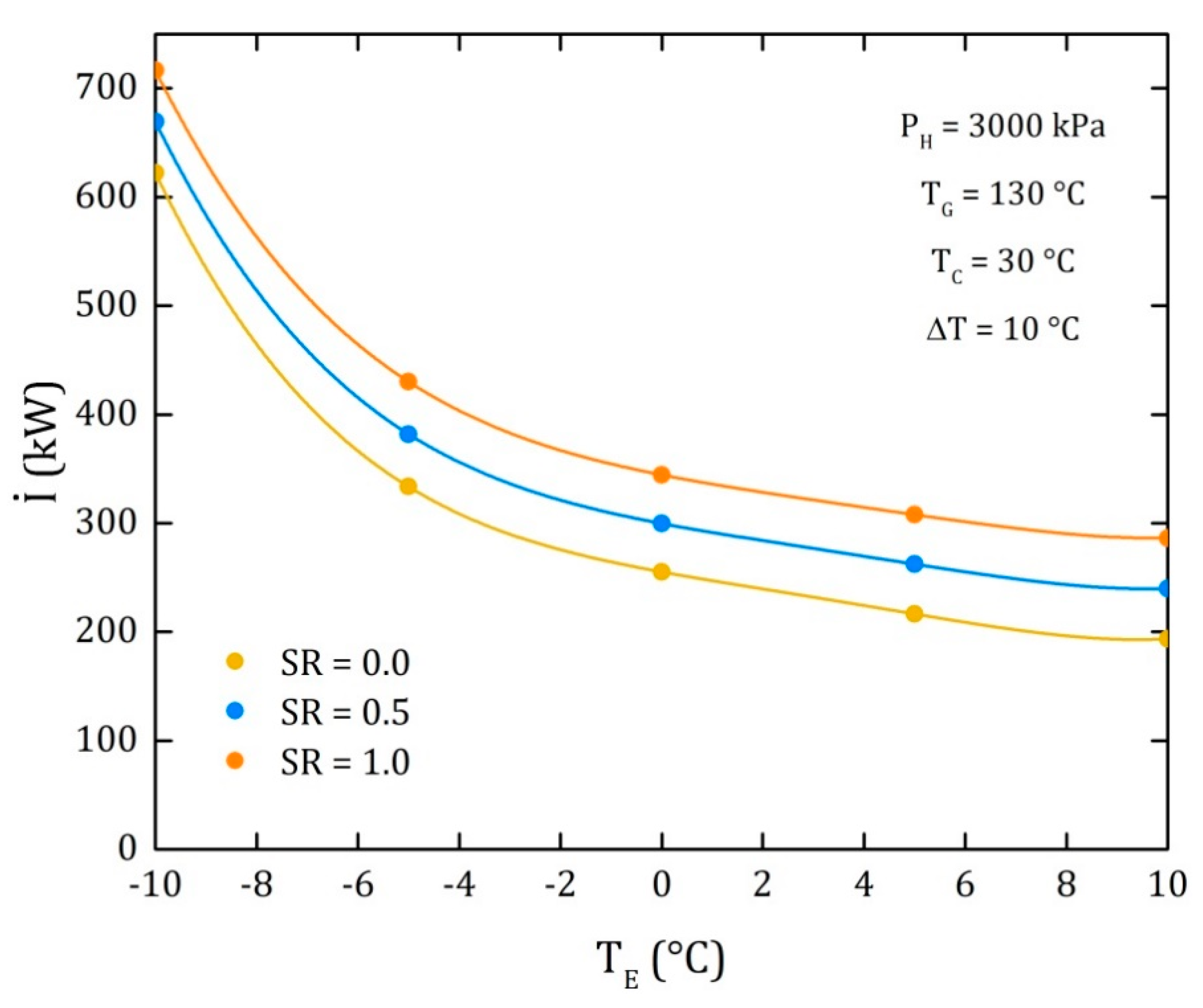
4. Results
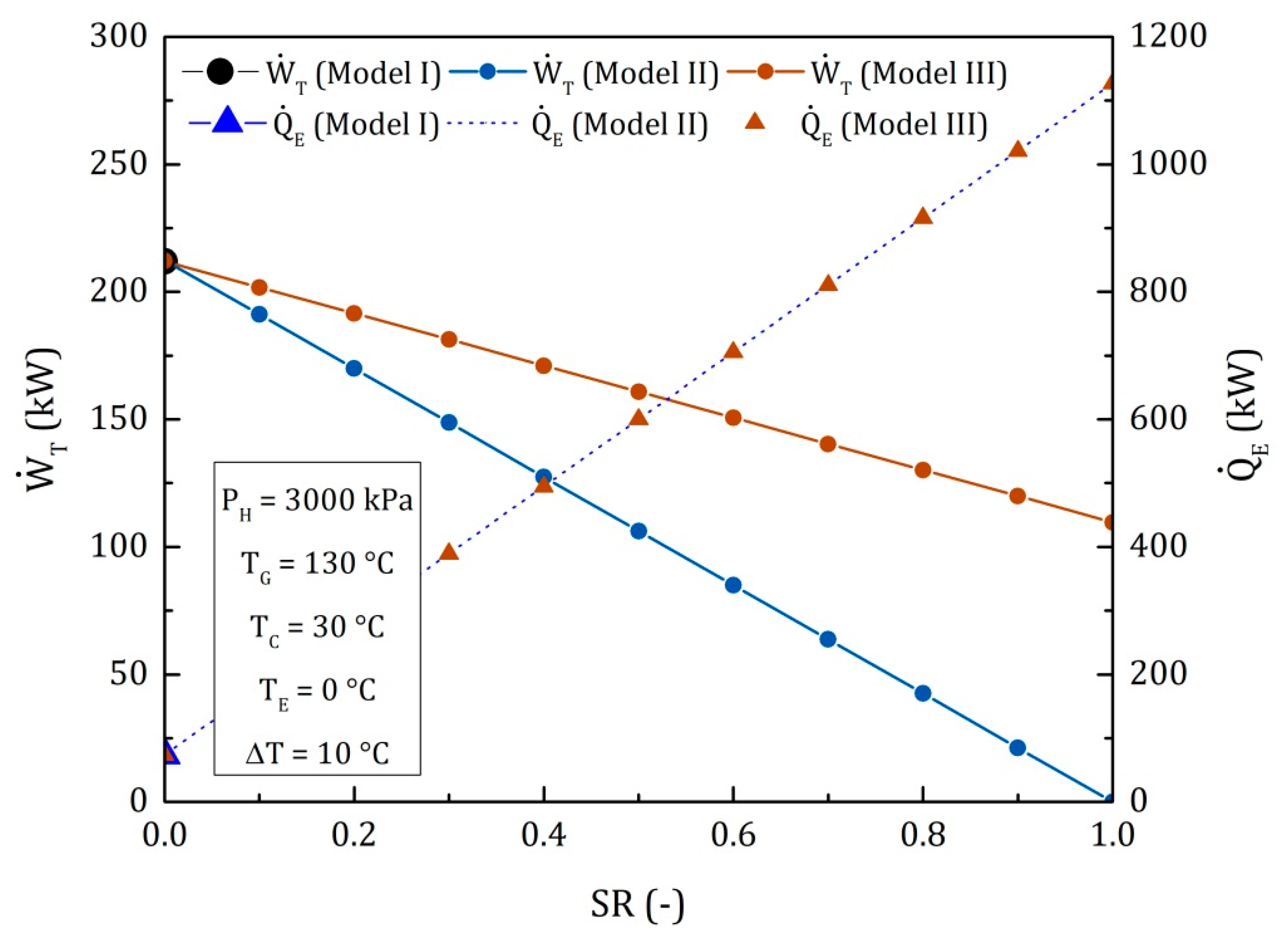
4.1. Goswami Cycle with a Condenser and a Flow Division after the Rectifier (Model II)
4.2. Goswami Cycle with a Condenser and a Flow Division into the Turbine (Model III)
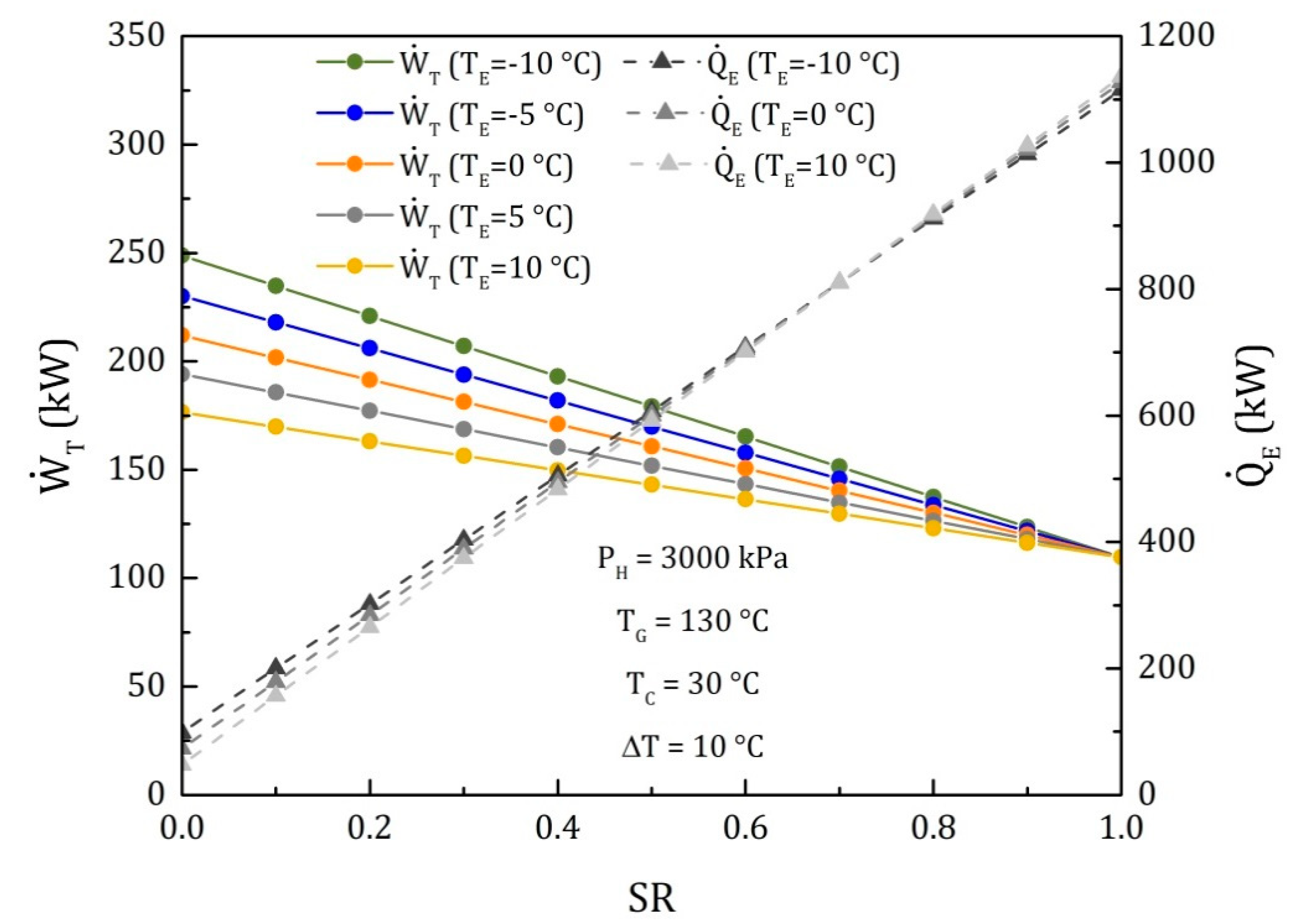
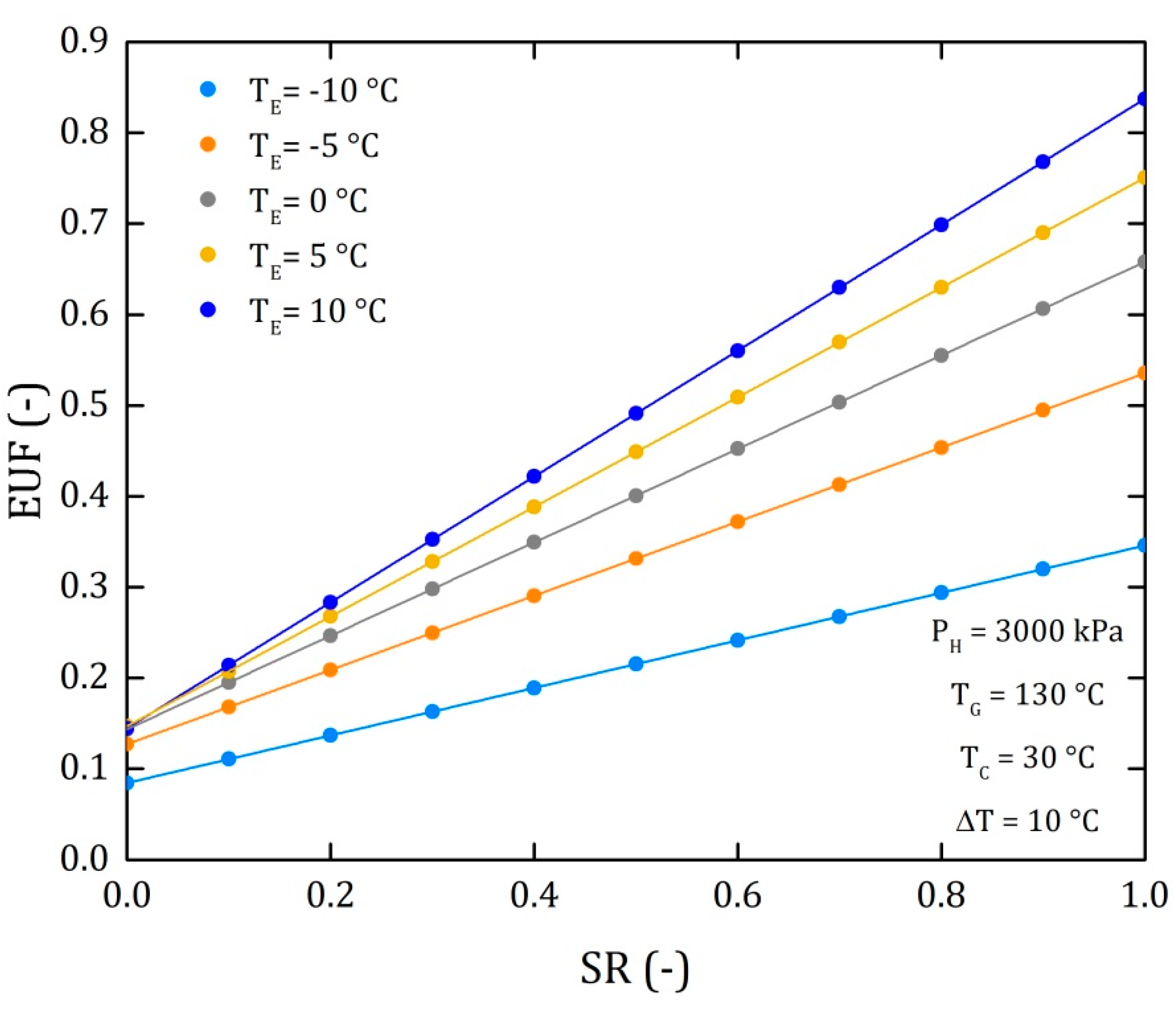
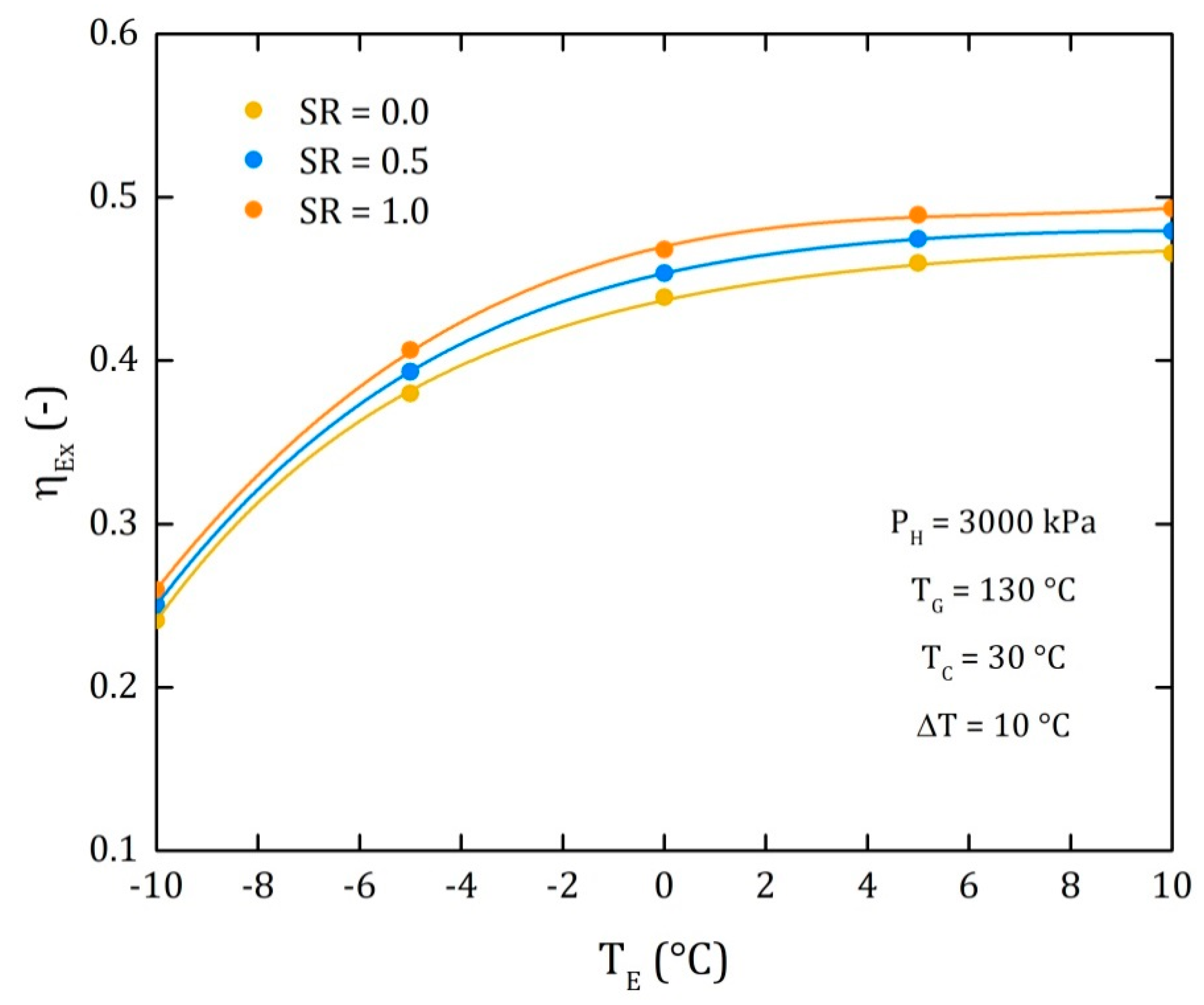
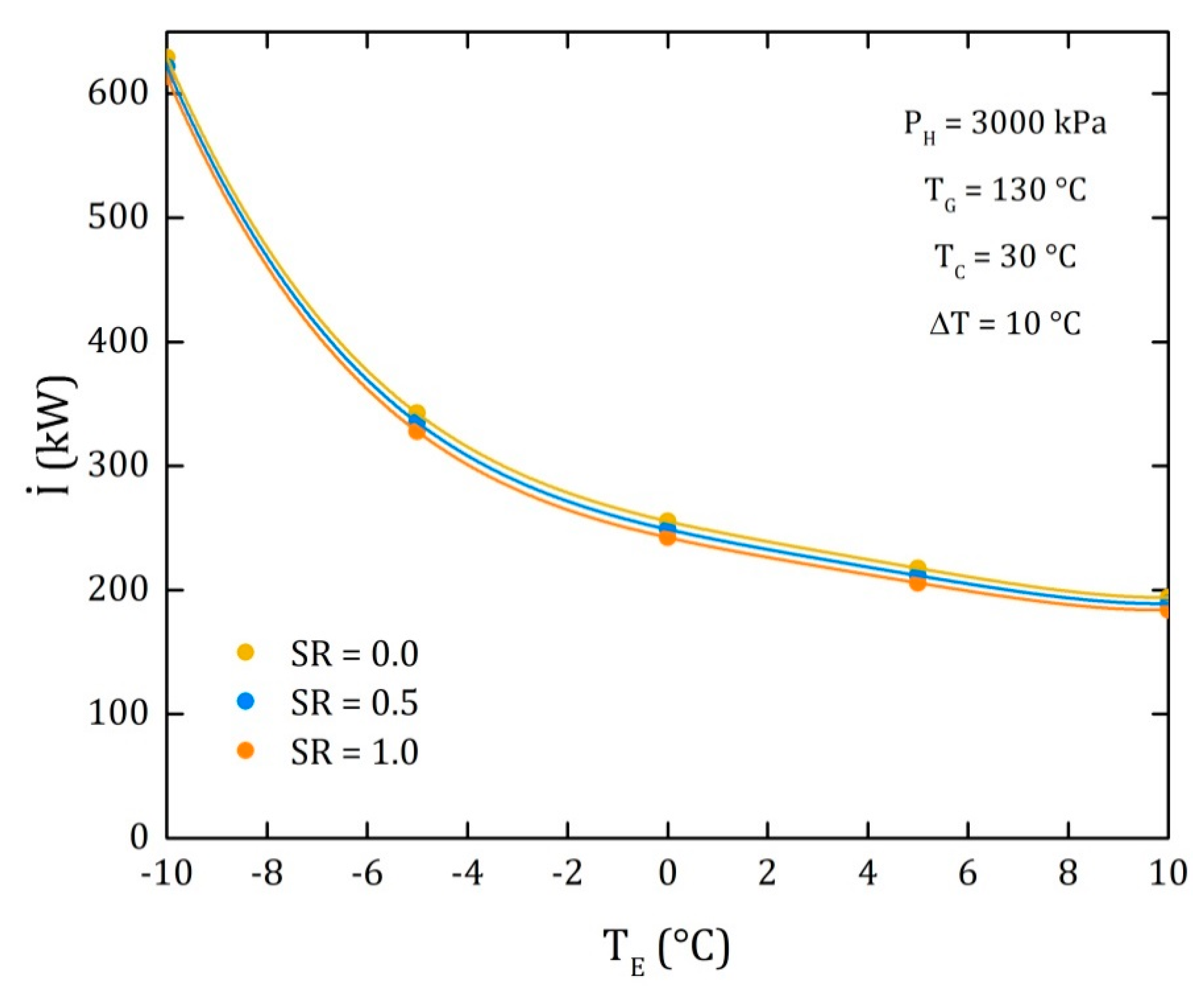
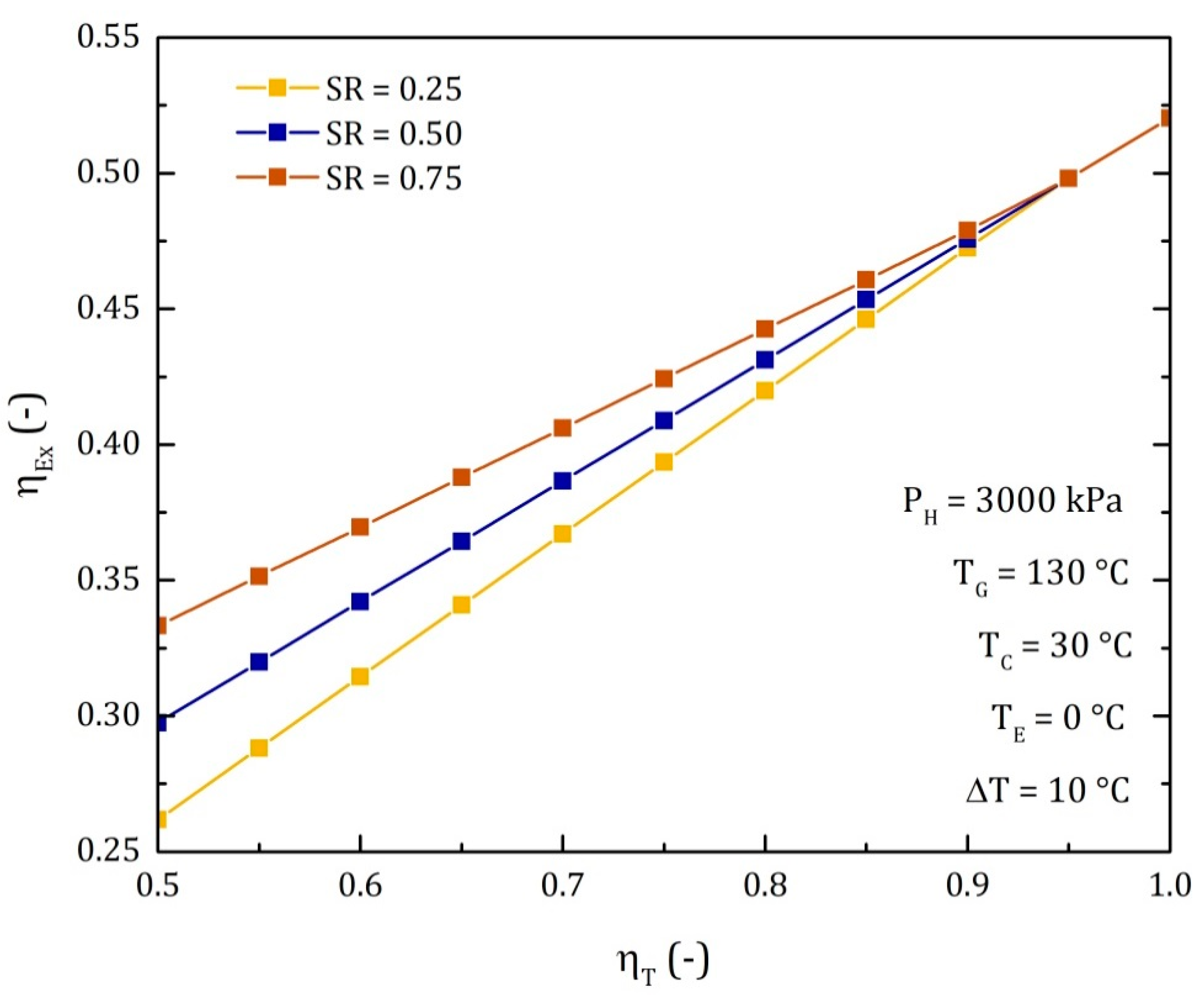
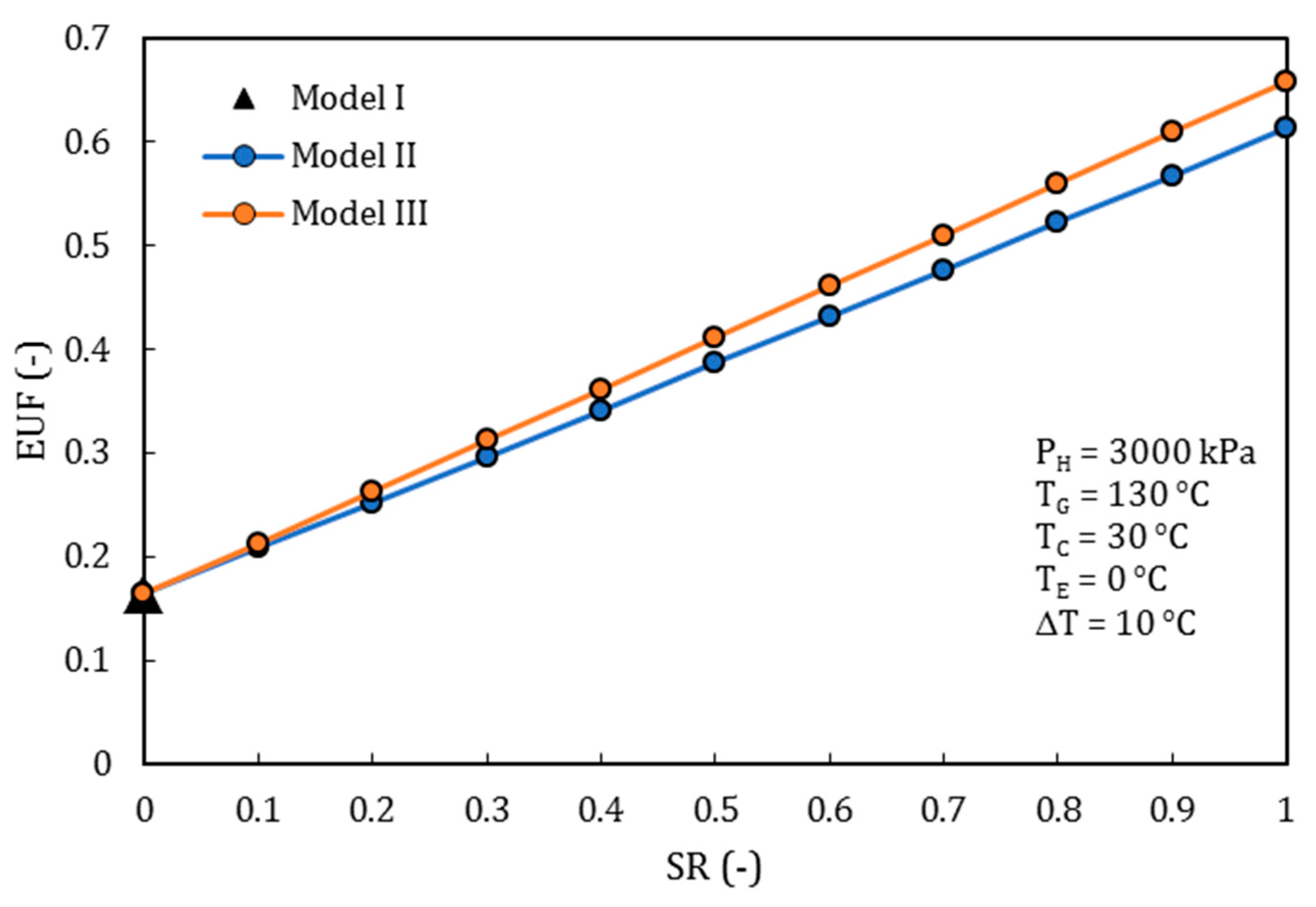
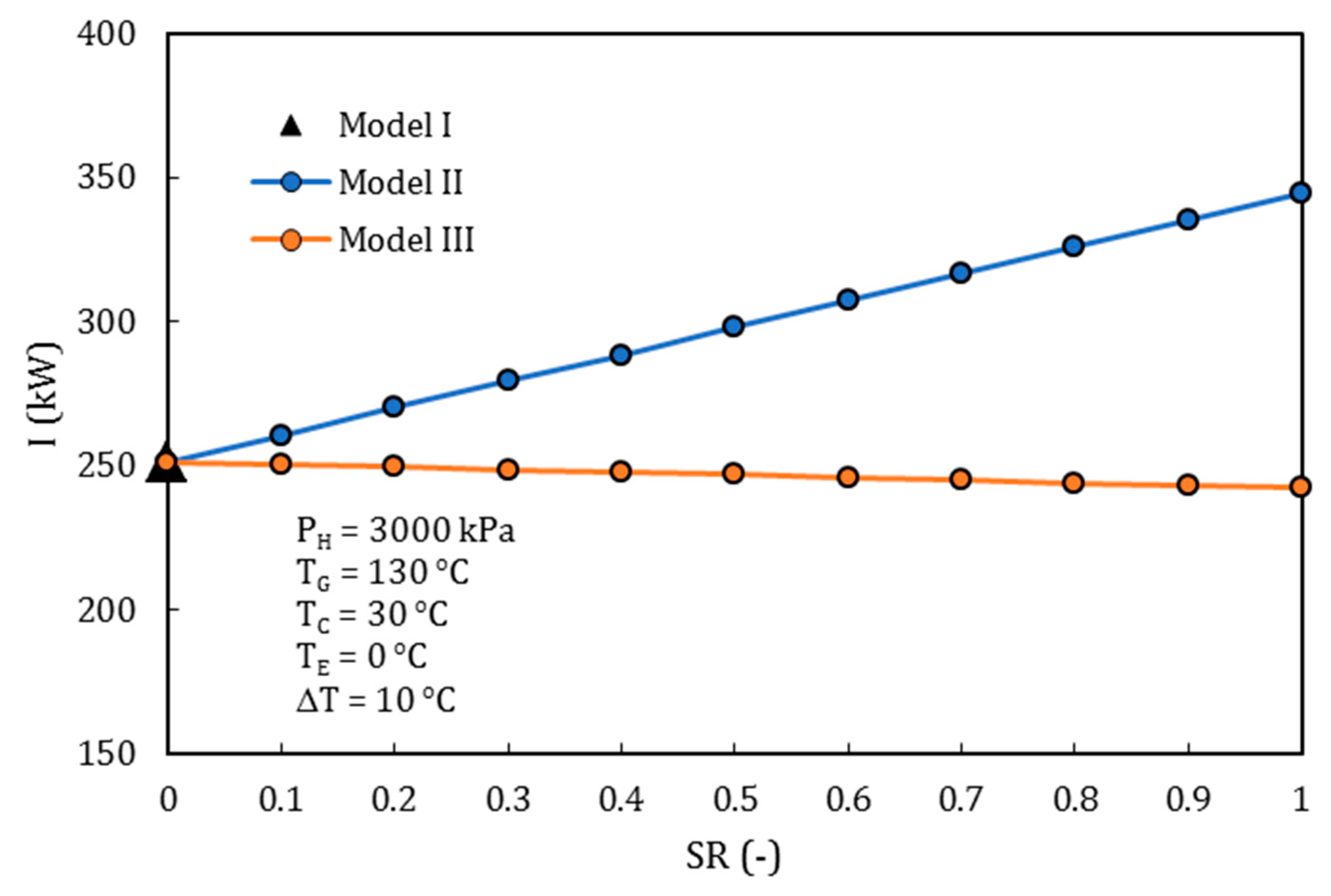
4.3. Comparison of the Performance for the Three Different Thermodynamic Cycles
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| EBP | Energy balance pump |
| EBEc | Energy balance economizer |
| EUF | Energy Utilization Factor (dimensionless) |
| h | Specific enthalpy (kJ/kg) |
| I | Irreversibility (kW) |
| m | Mass flow rate (kg/s) |
| P | Pressure (kPa) |
| Q | Heat power (kW) |
| q | Vapor fraction (dimensionless) |
| RP | Pressure ratio |
| SR | Split ratio (dimensionless) |
| T | Temperature (°C) |
| W | Mechanical power (kW) |
| X | Ammonia concentration (% in weight) |
| Greek letters | |
| Efficiency (dimensionless) | |
| ΔT | Temperature increment (°C) |
| Subscripts | |
| A | Absorber |
| C | Condenser |
| Cool | Cooler |
| E | Evaporator |
| Ec | Economizer |
| EX | Exergy |
| G | Generator |
| H | High |
| Int | Intermediate |
| L | Low |
| Net | Net |
| P | Pump |
| R | Reheater |
| r | Refrigerant |
| Re | Rectifier |
| T | Turbine |
| 0 | Environment state |
Appendix A
| P2 = P3 = P4 = P5 = P7 = P8 = P9 = P10 = 30 bar, P1 = P6 = P11 = P12 = P14 = 4.29 bar, P13 = P15 = 11.67 bar | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Property | 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1 |
| T1 (°C) | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| X1 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 |
| h1 (kJ/kg) | −103.7 | −103.7 | −103.7 | −103.7 | −103.7 | −103.7 | −103.7 | −103.7 | −103.7 | −103.7 | −103.7 |
| s1 (kJ/kg °C) | 0.2908 | 0.2908 | 0.2908 | 0.2908 | 0.2908 | 0.2908 | 0.2908 | 0.2908 | 0.2908 | 0.2908 | 0.2908 |
| m1 (kg/s) | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 |
| T2 (°C) | 30.39 | 30.39 | 30.39 | 30.39 | 30.39 | 30.39 | 30.39 | 30.39 | 30.39 | 30.39 | 30.39 |
| X2 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 |
| h2 (kJ/kg) | −99.69 | −99.69 | −99.69 | −99.69 | −99.69 | −99.69 | −99.69 | −99.69 | −99.69 | −99.69 | −99.69 |
| s2 (kJ/kg °C) | 0.2934 | 0.2934 | 0.2934 | 0.2934 | 0.2934 | 0.2934 | 0.2934 | 0.2934 | 0.2934 | 0.2934 | 0.2934 |
| m2 (kg/s) | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 |
| T3 (°C) | 100.2 | 100.2 | 100.2 | 100.2 | 100.2 | 100.2 | 100.2 | 100.2 | 100.2 | 100.2 | 100.2 |
| X3 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 | 0.534 |
| h3 (kJ/kg) | 225.1 | 225.1 | 225.1 | 225.1 | 225.1 | 225.1 | 225.1 | 225.1 | 225.1 | 225.1 | 225.1 |
| s3 (kJ/kg °C) | 1.255 | 1.255 | 1.255 | 1.255 | 1.255 | 1.255 | 1.255 | 1.255 | 1.255 | 1.255 | 1.255 |
| m3 (kg/s) | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 | 5.096 |
| T4 (°C) | 130 | 130 | 130 | 130 | 130 | 130 | 130 | 130 | 130 | 130 | 130 |
| X4 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 |
| h4 (kJ/kg) | 364.3 | 364.3 | 364.3 | 364.3 | 364.3 | 364.3 | 364.3 | 364.3 | 364.3 | 364.3 | 364.3 |
| s4 (kJ/kg °C) | 1.617 | 1.617 | 1.617 | 1.617 | 1.617 | 1.617 | 1.617 | 1.617 | 1.617 | 1.617 | 1.617 |
| m4 (kg/s) | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 |
| T5 (°C) | 42.9 | 42.9 | 42.9 | 42.9 | 42.9 | 42.9 | 42.9 | 42.9 | 42.9 | 42.9 | 42.9 |
| X5 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 |
| h5 (kJ/kg) | −39.79 | −39.79 | −39.79 | −39.79 | −39.79 | −39.79 | −39.79 | −39.79 | −39.79 | −39.79 | −39.79 |
| s5 (kJ/kg °C) | 0.4908 | 0.4908 | 0.4908 | 0.4908 | 0.4908 | 0.4908 | 0.4908 | 0.4908 | 0.4908 | 0.4908 | 0.4908 |
| m5 (kg/s) | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 |
| T6 (°C) | 43.38 | 43.38 | 43.38 | 43.38 | 43.38 | 43.38 | 43.38 | 43.38 | 43.38 | 43.38 | 43.38 |
| X6 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 | 0.4214 |
| h6 (kJ/kg) | −39.79 | −39.79 | −39.79 | −39.79 | −39.79 | −39.79 | −39.79 | −39.79 | −39.79 | −39.79 | −39.79 |
| s6 (kJ/kg °C) | 0.5004 | 0.5004 | 0.5004 | 0.5004 | 0.5004 | 0.5004 | 0.5004 | 0.5004 | 0.5004 | 0.5004 | 0.5004 |
| m6 (kg/s) | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 | 4.096 |
| T7 (°C) | 100.2 | 100.2 | 100.2 | 100.2 | 100.2 | 100.2 | 100.2 | 100.2 | 100.2 | 100.2 | 100.2 |
| X7 | 0.9863 | 0.9863 | 0.9863 | 0.9863 | 0.9863 | 0.9863 | 0.9863 | 0.9863 | 0.9863 | 0.9863 | 0.9863 |
| h7 (kJ/kg) | 1425 | 1425 | 1425 | 1425 | 1425 | 1425 | 1425 | 1425 | 1425 | 1425 | 1425 |
| s7 (kJ/kg °C) | 4.292 | 4.292 | 4.292 | 4.292 | 4.292 | 4.292 | 4.292 | 4.292 | 4.292 | 4.292 | 4.292 |
| m7 (kg/s) | 1.029 | 1.029 | 1.029 | 1.029 | 1.029 | 1.029 | 1.029 | 1.029 | 1.029 | 1.029 | 1.029 |
| T8 (°C) | 85.36 | 85.36 | 85.36 | 85.36 | 85.36 | 85.36 | 85.36 | 85.36 | 85.36 | 85.36 | 85.36 |
| X8 | 0.6903 | 0.6903 | 0.6903 | 0.6903 | 0.6903 | 0.6903 | 0.6903 | 0.6903 | 0.6903 | 0.6903 | 0.6903 |
| h8 (kJ/kg) | 206.7 | 206.7 | 206.7 | 206.7 | 206.7 | 206.7 | 206.7 | 206.7 | 206.7 | 206.7 | 206.7 |
| s8 (kJ/kg °C) | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 |
| m8 (kg/s) | 0.02945 | 0.02945 | 0.02945 | 0.02945 | 0.02945 | 0.02945 | 0.02945 | 0.02945 | 0.02945 | 0.02945 | 0.02945 |
| T9 (°C) | 85.36 | 85.36 | 85.36 | 85.36 | 85.36 | 85.36 | 85.36 | 85.36 | 85.36 | 85.36 | 85.36 |
| X9 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 |
| h9 (kJ/kg) | 1365 | 1365 | 1365 | 1365 | 1365 | 1365 | 1365 | 1365 | 1365 | 1365 | 1365 |
| s9 (kJ/kg °C) | 4.129 | 4.129 | 4.129 | 4.129 | 4.129 | 4.129 | 4.129 | 4.129 | 4.129 | 4.129 | 4.129 |
| m9 (kg/s) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| T10 (°C) | 95.36 | 95.36 | 95.36 | 95.36 | 95.36 | 95.36 | 95.36 | 95.36 | 95.36 | 95.36 | 95.36 |
| X10 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 |
| h10 (kJ/kg) | 1401 | 1401 | 1401 | 1401 | 1401 | 1401 | 1401 | 1401 | 1401 | 1401 | 1401 |
| s10 (kJ/kg °C) | 4.223 | 4.223 | 4.223 | 4.223 | 4.223 | 4.223 | 4.223 | 4.223 | 4.223 | 4.223 | 4.223 |
| m10 (kg/s) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| T11 (°C) | 1.617 | 1.617 | 1.617 | 1.617 | 1.617 | 1.617 | 1.617 | 1.617 | 1.617 | 1.617 | 1.617 |
| X11 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 |
| h11 (kJ/kg) | 1173 | 1173 | 1173 | 1173 | 1173 | 1173 | 1173 | 1173 | 1173 | 1173 | 1173 |
| s11 (kJ/kg °C) | 4.298 | 4.298 | 4.298 | 4.298 | 4.298 | 4.298 | 4.298 | 4.298 | 4.298 | 4.298 | 4.298 |
| m11 (kg/s) | 1 | 0.9 | 0.8 | 0.7 | 0.6 | 0.5 | 0.4 | 0.3 | 0.2 | 0.1 | 0 |
| T12 (°C) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| X12 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 |
| h12 (kJ/kg) | 1268 | 1268 | 1268 | 1268 | 1268 | 1268 | 1268 | 1268 | 1268 | 1268 | 1268 |
| s12 (kJ/kg °C) | 4.633 | 4.633 | 4.633 | 4.633 | 4.633 | 4.633 | 4.633 | 4.633 | 4.633 | 4.633 | 4.633 |
| m12 (kg/s) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| T13 (°C) | 38.59 | 38.59 | 38.59 | 38.59 | 38.59 | 38.59 | 38.59 | 38.59 | 38.59 | 38.59 | 38.59 |
| X13 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 |
| h13 (kJ/kg) | 1291 | 1291 | 1291 | 1291 | 1291 | 1291 | 1291 | 1291 | 1291 | 1291 | 1291 |
| s13 (kJ/kg °C) | 4.285 | 4.285 | 4.285 | 4.285 | 4.285 | 4.285 | 4.285 | 4.285 | 4.285 | 4.285 | 4.285 |
| m13 (kg/s) | 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1 |
| T14 (°C) | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| X14 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 |
| h14 (kJ/kg) | 141.8 | 141.8 | 141.8 | 141.8 | 141.8 | 141.8 | 141.8 | 141.8 | 141.8 | 141.8 | 141.8 |
| s14 (kJ/kg °C) | 0.4995 | 0.4995 | 0.4995 | 0.4995 | 0.4995 | 0.4995 | 0.4995 | 0.4995 | 0.4995 | 0.4995 | 0.4995 |
| m14 (kg/s) | 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1 |
| T15 (°C) | 0.1604 | 0.1604 | 0.1604 | 0.1604 | 0.1604 | 0.1604 | 0.1604 | 0.1604 | 0.1604 | 0.1604 | 0.1604 |
| X15 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 | 0.995 |
| h15 (kJ/kg) | 141.8 | 141.8 | 141.8 | 141.8 | 141.8 | 141.8 | 141.8 | 141.8 | 141.8 | 141.8 | 141.8 |
| s15 (kJ/kg °C) | 0.5463 | 0.5463 | 0.5463 | 0.5463 | 0.5463 | 0.5463 | 0.5463 | 0.5463 | 0.5463 | 0.5463 | 0.5463 |
| m15 (kg/s) | 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1 |
| (kW) | 1806 | 1806 | 1806 | 1806 | 1806 | 1806 | 1806 | 1806 | 1806 | 1806 | 1806 |
| (kW) | 35.85 | 35.85 | 35.85 | 35.85 | 35.85 | 35.85 | 35.85 | 35.85 | 35.85 | 35.85 | 35.85 |
| (kW) | 1633 | 1633 | 1633 | 1633 | 1633 | 1633 | 1633 | 1633 | 1633 | 1633 | 1633 |
| (kW) | 0 | 114.9 | 229.9 | 344.8 | 459.7 | 574.7 | 689.6 | 804.6 | 919.5 | 1034 | 1149 |
| (kW) | 96.12 | 96.12 | 96.12 | 96.12 | 96.12 | 96.12 | 96.12 | 96.12 | 96.12 | 96.12 | 96.12 |
| (kW) | 1655 | 1655 | 1655 | 1655 | 1655 | 1655 | 1655 | 1655 | 1655 | 1655 | 1655 |
| (kW) | 20.21 | 20.21 | 20.21 | 20.21 | 20.21 | 20.21 | 20.21 | 20.21 | 20.21 | 20.21 | 20.21 |
| (kW) | 228 | 216.2 | 204.4 | 192.5 | 180.7 | 168.9 | 157 | 145.2 | 133.4 | 121.5 | 109.7 |
| FUE | 0.1646 | 0.2142 | 0.2638 | 0.3133 | 0.3629 | 0.4124 | 0.462 | 0.5115 | 0.5611 | 0.6106 | 0.6602 |
| EfEX | 0.4805 | 0.4793 | 0.4782 | 0.4771 | 0.476 | 0.4749 | 0.4738 | 0.4726 | 0.4715 | 0.4704 | 0.4693 |
| I (kW) | 236.1 | 236.6 | 237.1 | 237.6 | 238.1 | 238.6 | 239.1 | 239.6 | 240.1 | 240.6 | 241.1 |
References
- Kalina, A.I. Combined cycle and waste heat recovery power systems based on a novel thermodynamic energy cycle utilizing low-temperature heat for power generation. In 1983 Joint Power Generation Conference; Society of Mechanical Engineers: New York, NY, USA, 1983. [Google Scholar]
- Kalina, A.I. Combined-Cycle system with novel bottoming cycle. J. Eng. Gas Turbines Power 1984, 106, 737–742. [Google Scholar] [CrossRef]
- El-Sayed, Y.M.; Tribus, M. Thermodynamic properties of water-ammonia mixtures: Theoretical implementation for use in power cycles analysis. Presented at the Winter Annual Meeting of the American Society of Mechanical Engineers, Miami Beach, FL, USA, 9 December 1985; p. 89. [Google Scholar]
- El-Sayed, Y.M.; Tribus, M. A theoretical comparison of the Rankine and Kalina cycles. In Proceedings of the Analysis of Energy Systems, Design and Operation, Presented at the Winter Annual Meeting of the American Society of Mechanical Engineers, Miami Beach, FL, USA, 9 December 1985; p. 97. [Google Scholar]
- Marston, C.H. Parametric analysis of the Kalina cycle. J. Eng. Gas Turbines Power 1990, 112, 107–116. [Google Scholar] [CrossRef]
- Park, Y.M.; Sonntag, R.E. A preliminary study of the Kalina power cycle in connection with a combined cycle system. Int. J. Energy Res. 1990, 14, 153–162. [Google Scholar] [CrossRef]
- Rogdakis, E.D.; Antonopoulos, K.A. A high efficiency NH3/H2O absorption power cycle. Heat Recovery Syst. CHP 1991, 11, 263–275. [Google Scholar] [CrossRef]
- Nag, P.K.; Gupta, A.V. Exergy analysis of the Kalina cycle. Appl. Therm. Eng. 1998, 18, 427–439. [Google Scholar] [CrossRef]
- Dejfors, C.; Thorin, E.; Svedberg, G. Ammonia-Water power cycles for direct-fired cogeneration applications. Energy Convers. Manag. 1998, 39, 1675–1681. [Google Scholar] [CrossRef]
- Goswami, D. Solar thermal power technology: Present status and ideas for the future. Energy Sources 1998, 20, 137–145. [Google Scholar] [CrossRef]
- Xu, F.; Goswami, D.Y.; Bhagwat, S.S. A combined power/cooling cycle. Energy 2000, 25, 233–246. [Google Scholar] [CrossRef]
- Lu, S.; Goswami, D.Y. Theoretical analysis of ammonia based combined power/refrigeration cycle at low refrigeration temperatures. Sol. Eng. 2002, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Hasan, A.A.; Goswami, D.Y.; Vijayaraghavan, S. First and second law analysis of a new power and refrigeration thermodynamic cycle using a solar heat source. Sol. Energy 2002, 73, 385–393. [Google Scholar] [CrossRef]
- Hasan, A.A.; Goswami, D.Y. Exergy analysis of a combined power and refrigeration thermodynamic cycle driven by a solar heat source. J. Sol. Energy Eng. 2003, 125, 55–60. [Google Scholar] [CrossRef]
- Tamm, G.; Goswami, D.Y.; Lu, S.; Hasan, A.A. Theoretical and experimental investigation of an ammonia-water power and refrigeration thermodynamic cycle. Sol. Energy 2004, 76, 217–228. [Google Scholar] [CrossRef]
- Vijayaraghavan, S.; Goswami, D.Y. Organic working fluids for a combined power and cooling cycle. J. Energy Resour. Technol. 2005, 127, 125–130. [Google Scholar] [CrossRef]
- Martin, C.; Goswami, D.Y. Effectiveness of cooling production with a combined power and cooling thermodynamic cycle. Appl. Therm. Eng. 2006, 26, 576–582. [Google Scholar] [CrossRef]
- Vidal, A.; Best, R.; Rivero, R.; Cervantes, J. Analysis of a combined power and refrigeration cycle by the exergy method. Energy 2006, 31, 3401–3414. [Google Scholar] [CrossRef]
- Zhang, N.; Lior, N. Development of a novel combined absorption cycle for power generation and refrigeration. J. Energy Resour. Technol. 2007, 129, 254–265. [Google Scholar] [CrossRef] [Green Version]
- Boza, J.J.; Lear, W.E.; Sherif, S.A. Performance of a novel semiclosed gas-turbine refrigeration combined cycle. J. Energy Resour. Technol. 2008, 130. [Google Scholar] [CrossRef]
- Demirkaya, G.; Vasquez Padilla, R.; Goswami, D.Y.; Stefanakos, E.; Rahman, M.M. Analysis of a combined power and cooling cycle for low-grade heat sources. Int. J. Energy Res. 2011, 35, 1145–1157. [Google Scholar] [CrossRef]
- Ryu, C.; Tiffany, D.R.; Crittenden, J.F.; Lear, W.E.; Sherif, S.A. Dynamic modeling of a novel cooling, heat, power, and water microturbine combined cycle. J. Energy Resour. Technol. 2010, 132. [Google Scholar] [CrossRef]
- Padilla, R.V.; Archibold, A.R.; Demirkaya, G.; Besarati, S.; Goswami, D.Y.; Rahman, M.M.; Stefanakos, E.L. Performance analysis of a Rankine cycle integrated with the Goswami combined power and cooling cycle. J. Energy Resour. Technol. 2012, 134. [Google Scholar] [CrossRef]
- Mendoza, L.C.; Ayou, D.S.; Navarro-Esbrí, J.; Bruno, J.C.; Coronas, A. Small capacity absorption systems for cooling and power with a scroll expander and ammonia based working fluids. Appl. Therm. Eng. 2014, 72, 258–265. [Google Scholar] [CrossRef]
- Ayou, D.S.; Bruno, J.C.; Coronas, A. Combined absorption power and refrigeration cycles using low-and mid-grade heat sources. Sci. Technol. Built Environ. 2015, 21, 934–943. [Google Scholar] [CrossRef]
- Muye, J.; Ayou, D.S.; Saravanan, R.; Coronas, A. Performance study of a solar absorption power-cooling system. Appl. Therm. Eng. 2016, 97, 59–67. [Google Scholar] [CrossRef]
- Barkhordarian, O.; Behbahaninia, A.; Bahrampoury, R. A novel ammonia-water combined power and refrigeration cycle with two different cooling temperature levels. Energy 2017, 120, 816–826. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, H.; Wang, R.; Wang, L.; Gong, L.; Lu, Y.; Roskilly, A.P. Investigation on an innovative cascading cycle for power and refrigeration cogeneration. Energy Convers. Manag. 2017, 145, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Shankar, R.; Srinivas, T. Performance investigation of Kalina cooling cogeneration cycles. Int. J. Refrig. 2018, 86, 163–185. [Google Scholar] [CrossRef]
- Shankar, R.; Srinivas, T.; Reddy, V. Investigation of solar cooling cogeneration plant. Appl. Sol. Energy 2018, 54, 65–70. [Google Scholar] [CrossRef]
- Cengel, Y.A.; Boles, M.A. Thermodynamics, 7th ed.; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Ibrahim, O.M.; Klein, S.A. Thermodynamic properties of ammonia-water mixtures. ASHRAE Trans. Symp. 1993, 21, 1495. [Google Scholar]
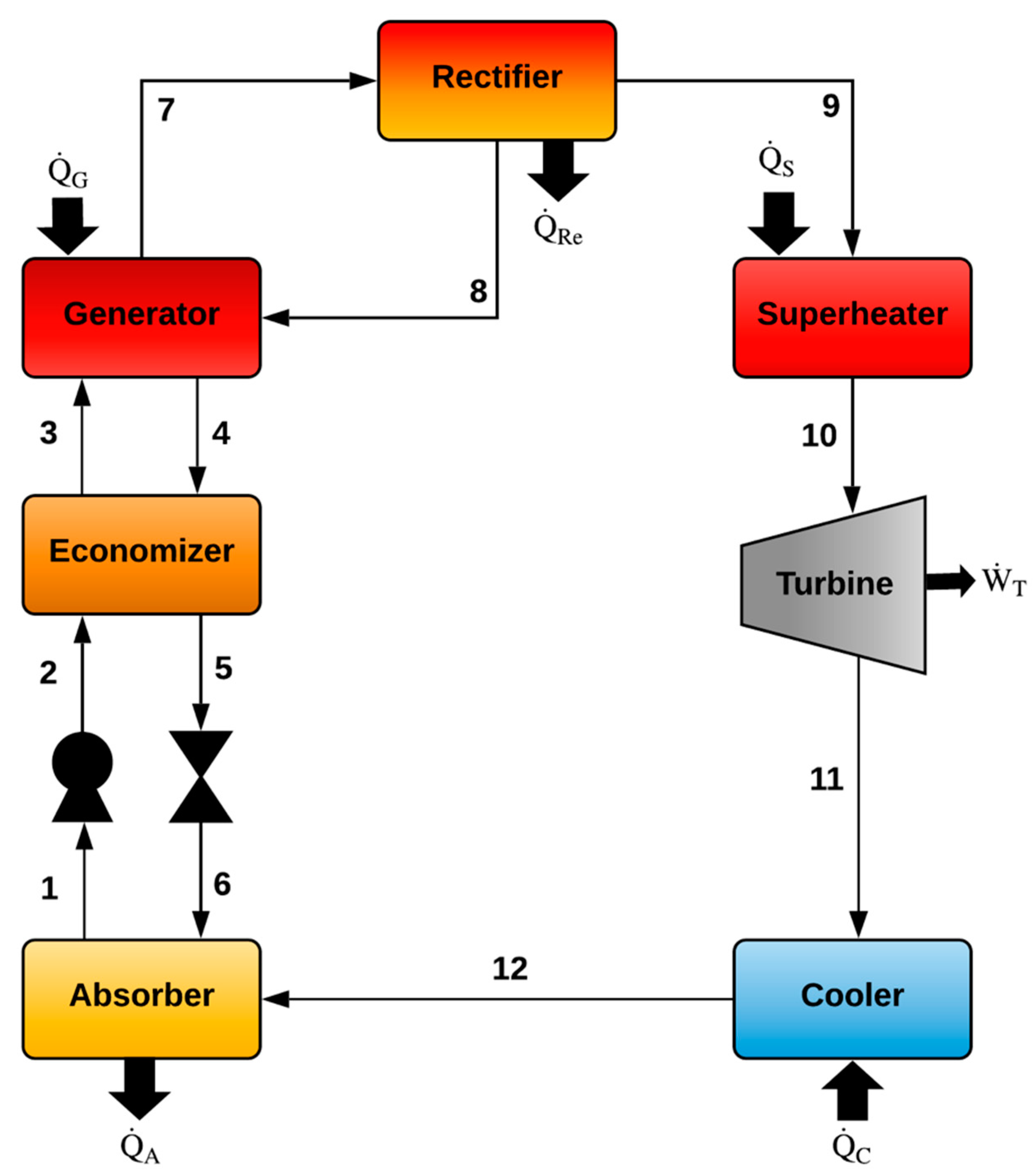


| Goswami Cycle (Model I) | |
|---|---|
| Generator (G) | Absorber (A) |
| Rectifier (Re) | Economizer (EC) |
| Pump (P) | |
| Reheater (R) | |
| Turbine (T) | |
| Cooler or Evaporator (C) | |
| Goswami Cycle with a Condenser and a Flow Division after the Rectifier (Model II) | |
|---|---|
| Generator (G) | Absorber (A) |
| Rectifier (Re) | Economizer (EC) |
| Pump (P) | |
| Reheater (R) | |
| Condenser (C) | |
| Turbine (T) | |
| Evaporator (E) | |
| Goswami Cycle with a Condenser and a Flow Division into the Turbine (Model III) | |
|---|---|
| Generator (G) | Absorber (A) |
| Rectifier (Re) | Economizer (EC) |
| Pump (P) | |
| Reheater (R) | |
| Condenser (C) | |
| Turbine (T) | PR = PH/PInt |
| Evaporator (E) | |
| Variable | Operation Range | Increment |
|---|---|---|
| PH (kPa) | 2000–4000 | 500 |
| TE (°C) | −10–10 | 5 |
| TA = TC (°C) | 20–40 | 5 |
| TG (°C) | 90–150 | 10 |
| ΔT (°C) | 10–50 | 20 |
| SR (-) | 0–1 | 0.1 |
| ηP (-) | 0.80 | |
| ηEC (-) | 0.70 | |
| ηT (-) | 0.85 | |
| xR | 0.995 | |
| mr (kg/s) | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, W.; Sánchez-Sánchez, K.; Hernández-Magallanes, J.A.; Jiménez-García, J.C.; Pacheco, A. Modeling of Novel Thermodynamic Cycles to Produce Power and Cooling Simultaneously. Processes 2020, 8, 320. https://doi.org/10.3390/pr8030320
Rivera W, Sánchez-Sánchez K, Hernández-Magallanes JA, Jiménez-García JC, Pacheco A. Modeling of Novel Thermodynamic Cycles to Produce Power and Cooling Simultaneously. Processes. 2020; 8(3):320. https://doi.org/10.3390/pr8030320
Chicago/Turabian StyleRivera, Wilfrido, Karen Sánchez-Sánchez, J. Alejandro Hernández-Magallanes, J. Camilo Jiménez-García, and Alejandro Pacheco. 2020. "Modeling of Novel Thermodynamic Cycles to Produce Power and Cooling Simultaneously" Processes 8, no. 3: 320. https://doi.org/10.3390/pr8030320
APA StyleRivera, W., Sánchez-Sánchez, K., Hernández-Magallanes, J. A., Jiménez-García, J. C., & Pacheco, A. (2020). Modeling of Novel Thermodynamic Cycles to Produce Power and Cooling Simultaneously. Processes, 8(3), 320. https://doi.org/10.3390/pr8030320





