Abstract
The success of mucoadhesive drug delivery systems relies on the type of polymer used, which becomes adhesive naturally upon hydration. Intended polymers should be able to maintain prolonged contact with biological membranes, and to protect or cater the drug to a prolonged period. Most of the hydro polymers form weak non-covalent bonds, that hinder localization of dosage forms at specific sites resulting in therapeutic inefficiency. This can be overcome by the thiol functionalization of natural polymers. In the present study, natural okra gum (OG) was extracted, followed by thiolation (TOG) and evaluated for mucoadhesion property and its role in enhancing the efficacy of repaglinide as a model drug (short-acting Type II antidiabetic drug). The thiol functionalization of OG (TOG) was confirmed by a Fourier-transform infrared spectroscopy (FTIR) study that showed a polyhedral to a spherical shape that had a rougher surface. Differential scanning calorimetry (DSC) and X-Ray Diffraction (XRD) studies of TOG indicated a decline in endothermic transition temperature and high crystallinity, respectively, in comparison to OG. CSFR (Crushing Strength: Friability Ratio), weight and thickness variations of repaglinidetablets formulated using TOG were >80% and <2.5% respectively. The highest swelling index (107.89 ± 1.99%) and strong mucoadhesion due to high disulfide bonds were observed for repaglinide TOG tablets in comparison to RG OG tablets. In-vitro release studies indicated a controlled drug release from thiolated formulations proportional to the concentration of thiomers that have a good correlation with in-vivo studies. Pharmacokinetic studies indicated higher AUC (area under the curve), longer t1/2 with thiomers. and Level A IVIV (in vitro in vivo) correlation was established from the bioavailability and dissolution data. Consequently, all the obtained results suggest that thiomers based formulations can be promising drug delivery systems, even in targeting onerous mucosal surfaces like nasal, ocular or vaginal.
1. Introduction
The utilization of plant-based gums and mucilage turns out to be imperative as a pharmaceutical excipient, especially in designing a control drug delivery system [1]. The physicochemical properties of these materials can be easily altered to meet the requests of ideal drug delivery systems [2]. Abelmoschus esculentus (L.) Moench, known as “Okra”, belonging to the mallow family is cultivated extensively throughout the tropical and subtropical regions of the world [3]. The okra gum (OG) is an acidic polysaccharide, comprising of glucuronic acid, rhamnose and galactose [4,5]. OG has been used in formulating mucoadhesive beads [6], buccal patches [7] and other controlled drug delivery matrices [8]. OG bears good mucoadhesion property, but there is a need to enhance mucoadhesion potential by suitable approaches such as thiolation, to employ in mucoadhesion drug delivery systems [9]. Nevertheless, to date, there has been no research reported in demonstrating the synthesis of thiolated okra gum (TOG) and evaluating its use in designing mucoadhesive drug delivery systems.
The term bio adhesion depicts the adhesion of the polymer to the biological membrane. Specifically, when the adhesion is limited to the mucosal surface it is named mucoadhesion [10]. The conception of mucoadhesion, as well as mucoadhesion polymers, was developed in the mid 1980s as an intriguing methodology especially for targeting the delivery of drugs at a site or at the absorption window. Mucoadhesive polymers become adhesive on hydration and characterized to have prolonged contact and residence time with the mucous membrane. [11]. Despite a few notable exceptions, gastric mucoadhesive systems do not reach their full potential. The success of the majority of the first generation mucoadhesion polymers was limited owing to their insufficient adhesion (such as hydrogen bonds, ionic interactions, and Van der Waals forces etc.) to gastrointestinal tract (GIT), accordingly, cannot assure localization of dosage forms [12,13].
A feasible new era of mucoadhesive polymers are thiolated polymers [14], (bearing thiol side chain) alleged thiomers have proven to be a promising new class of polymeric excipients. Instead of entrenched polymers, thiomers can form strong covalent bonds via thiol/disulfide exchange reactions with cysteine-rich subdomains of mucus [15,16,17]. As per the need, thiomers ensure the localization of the dosage form for an extended time with good biodegradation. Crosslinking property of thiomers is corresponding to the extent of inter and intra disulfide bond formation [18,19]. Furthermore, thiomers enhance permeation, represses the efflux pump and acts as defensive shield particularly for peptides and proteins from enzymatic attack [20]. In this manner, all the specified focal points render thiomers as a remarkably appropriate excipient for mucoadhesive drug delivery [3]. Considering the applicability of thiomers, the present research envisages enhancing the mucoadhesion strength of extracted okra gum by an appropriate thiolation method.
In the present study Repaglinide is selected as a model drug to evaluate the potential of TOG in formulating a mucoadhesive system. Repaglinidepossesses a unique non-specific anti-diabetic action. Repaglinideacts by stimulating the insulin release by binding to the specific sites on β islet cells of the pancreas and blocks ATP (Adenosine triphosphate) dependent K+ channel, however, its use is limited due to short t 1/2 (1 h) and a bioavailability of 50% [21]. In addition to this, Repaglinide produces hypoglycemic conditions on oral administration and imposes side effects such as skeletal muscle pain, headache and some GIT effects [22,23]. Due to short-lasting action, fast clearance, enzymatic stability and the absorption window in the upper GIT (stomach), makes repaglinide a suitable target for developing gastro retentive dosage form.
The present work is sought to (I) synthesize of thiolated okra gum (TOG), (II) compare mucoadhesive strength of TOG with OG, sodium alginate (SA), (III) compare dissolution profiles by modified basket method in addition to paddle methodology, to mimic the in-vivo mucoadhesion of the dosage form, (IV) perform In vivo buoyancy studies, (V) study the pharmacokinetic parameters and (VI) assess the in vitro and in vivo correlation. The objectives explore the novel application of a multifunctional natural polymer (Okra gum and Thiolated okra gum) in designing the gastro retentive mucoadhesive drug delivery system using repaglinide as a model drug.
2. Material and Methods
2.1. Materials
Repaglinide was kindly gifted by Aurobindo Pharma, Hyderabad, India. Sodium alginate (SA) was procured from Loba Chemie Pvt Ltd.; Mumbai (Maharashtra, India), India. Thioglycolic acid (TGA) and hydrochloric acid (HCl) were acquired from SD Fine-Chem Ltd. (Mumbai, India). Fresh unripe fruits of Abelmoschus esculentus were purchased from the local market of Rajampet (Andhra Pradesh, India) and gum (OG) was extracted. All other solvents and chemicals are of analytical grade.
2.2. Synthesis of Thiolated Okra Gum [TOG]
OG was extracted as reported formerly by our research group [21]. TOG was synthesized by the esterification of OG with Thioglycolic acid (TGA) in presence of HCl. Six g of OG were added to 50 mL of hot distilled water. To this 3.6 mL of TGA, 2 mL HCl (7N) were added and permitted to react for about 2 h 30 min at 80 °C temperature [24]. The reaction mixture was poured into 0.5 L methanol to obtain final precipitate and this was washed for 2–3 times with methanol, then dried and stored in a desiccator. Synthesis of TOG was done in an oxygen-free environment (carried out under argon).
2.3. Characterization of TOG
2.3.1. Fourier Transform Infrared Spectroscopy (FT-IR)
The Fourier Transform Infrared Radiation (FTIR) spectral measurements were taken at ambient temperature using an IR spectrophotometer (Perkin Elmer Instruments, North Billerica, MA, USA). These were done qualitatively in order to assess the pattern of peaks and for comparison purposes [25]. The FTIR spectra of OG and TOG were taken by making a KBr disc and analyzed in the range of 400–4000 cm−1.
2.3.2. Differential Scanning Calorimetry (DSC Studies)
DSC studies were carried out for pure OG and TOG. DSC scans were performed by a programmed thermal analyzer system (DSC60 Shimadzu Corporation, Tokyo, Japan). Sealed and perforated aluminum pans were used in the experiments for all the samples. Indium was utilized as a standard to perform temperature calibrations [26]. The entire samples were run at a scanning rate of 10 °C/min from 50–350 °C.
2.3.3. Surface Morphology
Samples were gold-coated to render them electrically conductive and examined under the scanning electron microscopy (SEM) (JSM 6100 JEOL (Tokyo, Japan) at 20 kV to characterize the surface morphology of the extracted OG and prepared TOG [27].
2.3.4. X-Ray Diffraction (XRD) Analysis
XRD analysis was carried out using Inxitu Benchtop XRD/XRF (X-ray fluorescence) to examine the crystallinity of OG and TOG. Polymers were scanned in an ambient state from 5° to 80° diffraction angle with the following conditions: Scan rate of 5° per minute, Voltage: 35 kV, Current: 30 mA [28].
2.3.5. Thiol Content Determination
Ellman’s reagent was used to determine the immobilized thiol groups on the polymer backbone as given by Bernkop-Schnürch et al. [29]. Disulfide content was also measured after reduction with NaBH4 [30].
2.4. Formulation of Gastro Retentive Mucoadhesion Tablets
Repaglinide gastro retentive mucoadhesion tablets were prepared by using 8% Polyvinlypyrroliodne (PVP) K30 in 80% ethanol solution as a granulating medium. Subsequent to being grounded and sifted, required amounts of repaglinide, OG/TOG/Sodium alginate (SA), and all other excipients (Table 1) were blended thoroughly, and consequently passed through sieve #80. The required proportions of the granulating medium were added to the powder blend and screened under #10–12 mesh to obtain wet granules. These granules were dried at 55–60 °C for about 60 min and dampness was maintained between 3% to 5%. The dried granules which were retained on sieve #14–20, were lubricated by adding the prescribed amount of magnesium stearate and talc. Finally, the tablets were compressed by using flat-faced 8-mm punches in the Rotary tablet punching machine (Chamunda pilot press, Ahmedabad, Gujarat, India) and the compression force was adjusted to control the hardness within 8–9 kg/cm2.

Table 1.
Formulation of Repaglinide mucoadhesive tablets *.
2.5. Evaluation Tests
2.5.1. In-Process Quality Control Tests
All the formulated tablets were evaluated for thickness, hardness, friability, and uniformity of weight [31]. The thickness and hardness of tablets were assessed by using digital screw gauge and Monsanto hardness tester. Friability and weight variation were done in Roche friabilator (Electrolab-EF-2, Navi Mumbai, India) and digital electronic balance (Citizen scales Pvt. Ltd., Kolkata, West Bengal, India), respectively. Additionally, diametric fracture (DF) was examined visually and CSFR (Crushing Strength: Friability ratio) was also reported based on the obtained hardness and friability values.
2.5.2. Swelling Study
The gravimetric method was used to determine the water-absorbing capacity. Initially weighed (W0) tablets of all the prepared batches were fixed to a needle and immersed in a beaker containing 100 mL of simulated gastric fluid (SGF) at 37 °C [32]. At the end of 8 h, tablets were removed out of the medium and blotted to take out of excess water. The amount of water uptake was determined gravimetrically, and the degree of swelling was calculated in terms of percentage weight gain according to the following equation,
2.5.3. Ex Vivo Mucoadhesion Time
Formulations were wetted with 0.1N HCl then adhered to the freshly excised goat gastric mucosa (which was glued to a glass slide by means of cyanoacrylate) by applying little pressure with a fingertip for about 25–30 sec analyzed for mucoadhesion time by placing the glass in a beaker, containing 200 mL 0.1 N HCl at 37 ± 0.5 °C [33,34]. The gastric environment was stimulated by applying a moderate stirring rate of 50 rpm and retention of tablets was monitored for about 12 h. Ex vivo residence time of prepared tablets was also analyzed by modifying the dissolution apparatus (TDT-08L USP type I, Electro lab, Navi Mumbai, Maharashtra, India) [35]. Freshly excised gastric mucosa was glued to the external surface of the basket and the hydrated tablets were attached with a little force. Subsequently, the basket was immersed in the relevant media (0.5 L 0.1 N HCl at 37 ± 0.5 °C). The time requisite for detachment was noted after exposing the basket to 100 rpm.
2.5.4. Measurement of Bioadhesion
The ex-vivo adhesion studies were conducted using a modified balance method [35]. Freshly excised goat gastric mucosa was stretched and attached to the lower glass slide. This glass slide was placed beneath the left arm of the balance. To the right arm, a conical flask was attached and connected with an upper glass slide on the left arm. The balance was calibrated to counter the equal weights of assembly prior to the placement of the tablet. Both the arms can be adjusted in such a way that the upper glass slide should superimpose with the lower glass slide [Figure 1]. Tablet was glued to the upper glass slide and allowed to come in contact with the wetted mucus layer on the lower glass slide for about 1–2 min. The weight of the right arm was increased successively by adding water into the conical flask with the help of the syringe and weight required for the detachment was noted. The weight required (W) to detach the tablet was calculated by subtracting the weight of the tablet from the weight of water added to the conical flask. The force in terms of Newton’s was calculated by the using following formula,
where W is the amount of water.
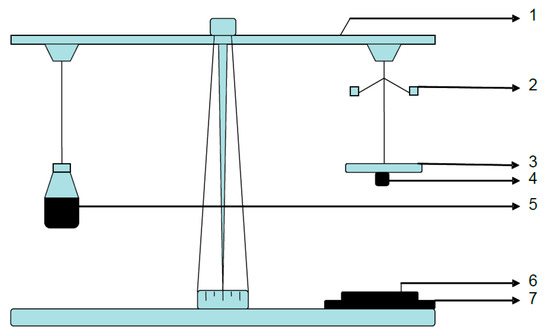
Figure 1.
Experimental setup of Detachment Force Measurement. [1. Modified physical balance, 2. Counter weights, 3. Upper Glass Slide, 4. Tablet, 5. Conical flask to hold water, 6. Goat intestine, 7. Lower glass slide].
2.5.5. Cytotoxicity Study
Cell viability studies for OG and TOG were performed by colorimetric assay, which measures the reduction of yellow MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) to purple-colored formazan crystals by mitochondrial succinate dehydrogenase. MTT solution was prepared by dissolving MTT in serum-free DMEM (Dulbecco’s Modified Eagle Medium) at a concentration of 5 mg/mL. HepG2 cells were plated at a density of 1.0 × 104 cells/mL into all 96 well plates and incubated at 95% RH, 37 °C, 5% CO2. Different concentrations of OG and TOG were incubated in 0.1% w/v DMEM solution after washing the cell layers with warm DMEM solution. Consequently, to each well 10 µL of MTT solution (5 mg/mL) was added and allowed to incubate [36]. Plates incubated only with the warm medium were identified as control. The cell viability percentage was calculated using the following formula. Absorbance at 570 nm was measured with a Synergy LX Multi-Mode Reader (BioTek Instruments, Winooski, VT, USA) using the dedicated UV-Vis monochromator.
2.5.6. In Vitro drug release studies
Drug release from all the prepared batches was performed by paddle strategy using United States Pharmacopeia (USP) dissolution apparatus II (TDT-08L, Electro lab, India) at 50 rpm with 900 mL of 0.1N HCl as dissolution medium. Constant temperature (37 ± 0.5 °C) was maintained throughout the study period. Aliquots (10 mL) were withdrawn at various time intervals and the samples were filtered through a 0.45 µm millipore filter, followed by appropriate dilution and analyzed for drug content by UV spectrophotometer (UV-1800, Shimadzu, Japan) at 223 nm. In vitro dissolution studies were further carried out by the modified basket method, (TDT-08L, USP type I, Electro lab, India) to mimic the in-vivo adhesion of dosage form. A piece of freshly excised gastric mucosa was glued internally at the bottom and sides of the basket. The study was further conducted by adhering the wetted tablet to the mucosa membrane and samples were analyzed using the same paddle method. Both the dissolution methods were compared for resemblance in drug release by calculating the difference factor (f1) and similarity factor (f2), considering the paddle method as a reference and basket method as a test. Rate constants, correlation coefficients (r2) for all the kinetics models were calculated and the release mechanism can be concluded from the Peppas model.
2.6. In-Vivo Studies
All the in vivo studies were approved by the Institutional Ethical Committee of Annamacharya College of Pharmacy, Rajmapet, Andhra Pradesh, India (IAEC/ANCP/2015-16/1).
2.6.1. In Vivo Mucoadhesion Study
This was done on healthy albino rabbits weighing between 2.5–3 kg of either sex, and the animal was fasted for about 12 h, but enabled free access to water all across the study. Initially X-ray of the abdomen was taken to confirm the nonexistence of any radio-opaque materials. Barium sulfate was incorporated into the formulation (without repaglinide) to make the tablet visible under X-ray and administered by using an endotracheal tube along with 15 mL of water. The animal was anesthetized and X-ray photographs of the abdomen at different time intervals were taken by placing the animal in an upright posture and this was done under the guidance of radiologists.
2.6.2. Bioavailability Studies
Optimized formulation was evaluated for different pharmacokinetic parameters like Cmax, tmax, AUCt, AUMCt, MRT and t1/2, and so forth, by conducting bioavailability studies in healthy albino rabbits (weighing 2.2–2.5 kg) [37]. A total of eighteen animals were used and housed separately under the environment conditions (at 25 °C, 12 h light and dark cycle) fed with test formulation. About 2 mL of the blood sample was collected through the peripheral ear vein prior to administration (0 h) and at regular intervals up to 22 h, after oral administration of RGM-6, Repide and intravascular administration of equivalent dose of repaglinide in solution form. Samples were transferred immediately into heparin containing test tubes. After centrifugation at 5000 rpm for 30 min, plasma samples were harvested, stored at −20 °C and analyzed.
Chromatographic system: Chromatographic separations were conducted by employing the Agilent 1100 HPLC (High-performance liquid chromatography). The Agilent 1100 HPLC system with UV detector, installed with Ez Chrome software for data acquisition and quantification of peaks. Hypersil® BDS C18 (150 mm × 4.6 mm, 5 μm, Thermo scientific, Navi Mumbai, India) used for chromatographic separations and method validation. Solvent and sample filtrations were done by using Ultipor® N66® 0.2 μm and 0.45 μm membranes, respectively. Chromatographic analysis was performed by using C18 Column (150 mm× 4.6 mm, 5µm) protected with a guard column with 30:70 v/v [Phosphate buffer (pH 2.5): Methanol] mobile phase at a 1mL/min flow rate [37]. To each sample tube, about 5 mL of ethyl acetate was added and contents were vortex mixed for 3 min and then centrifuged at 3000 rpm for 3 min. The formed organic layer was collected, evaporated on a water bath under nitrogen stream and then reconstituted with 50 µL of methanol. About 10 µL volume was injected, analyzed at 245 nm and total run time was 8 min. The data were calculated by PK Solver, a freely available menu-driven add-in program for Microsoft Excel written in Visual Basic for Applications (VBA) [38].
2.6.3. In Vitro In Vivo Correlation (IVIVC)
Level A correlation represents a point to point relationship and it is the most informative and highest level of correlation as per Food and Drug Administration (FDA) guidelines. Level A correlation was developed by deconvolution of the in vivo plasma profile using a model-independent method such as the Wagner Nelson method and compared with the in vitro dissolution rate [39].
2.7. Reproducibility of Thiolated Batches
RGM-5R, RGM-5S and RGM-6R, RGM-6S were the reproduced batches of RGM-5 and RGM-6, respectively. All the prepared batches were evaluated for ex-vivo residence time, mucoadhesion strength, drug release at the end of 16 h (%) and in vivo mucoadhesion study to compare the results with RGM-5 and RGM-6.
3. Results and Discussion
3.1. Synthesis of TOG
Thiolation of extracted okra gum was accomplished by ester bond formation between -OH groups of OG and -COO groups of TGA with an average yield of 75%. The final product was found to be a cream to brown color, with improved flow properties (Angle of repose-32° for OG and 27° for TOG, Hausner′s ratio-1.16 for OG and 1.06 for TOG) in contrast to unmodified okra gum [20].
3.2. Characterization of TOG
FTIR spectrum of OG (Figure 2a) shows a characteristic band around 3414 cm−1 due to OH stretching vibrations, band at 2945 cm−1 due to -CH stretching, C=O stretching at 1742 cm−1, C-H bend at 1456 cm−1. Figure 2b [FTIR spectrum of TOG] contains all peaks the same as that of OG but additionally shows a sharp peak at 2600 cm−1 indicating the presence of -SH group. DSC thermogram for OG and TOG were shown in Figure 3a,b, respectively. OG and TOG demonstrated an exotherm at 63.3 °C and 80.7 °C; endotherm at 273.1 °C and 267.3 °C, respectively. Water loss from the sample can be observed as an endothermic peak. TOG thermogram showed a decline in endothermic transition temperature. No sharp peaks were observed in the X-ray diffractogram of OG, indicating its amorphous nature, while in the XRD spectrum of TOG, sharp characteristic peaks with slightly elevated intensity were observed at 22° and 27° demonstrating the increase in crystallinity of thiolated compound. Figure 4a–c compares the surface morphology and shape of OG and TOG. From the micrographs, the shape of OG particles was found to be almost spherical but at the same time, some flakes of irregular sizes can also be observed. The surface morphology of TOG reveals its polyhedral to a spherical shape and rougher surface in comparison to OG.
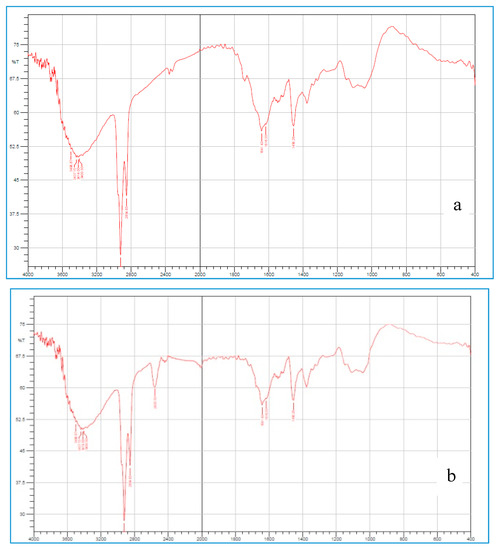
Figure 2.
Fourier transform infrared (FTIR) spectra of okra gum (OG) (a) and thiol functionalization of OG (TOG) (b).
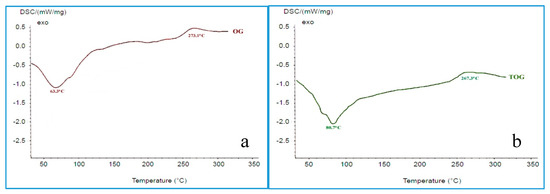
Figure 3.
Differential scanning calorimetry (DSC) thermograph of OG (a) and TOG (b).
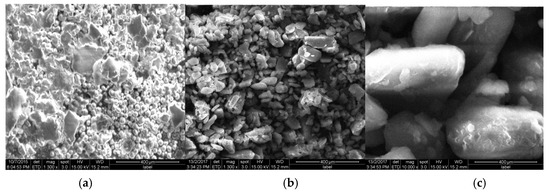
Figure 4.
Scanning electron microscopy (SEM) of OG (a) and TOG at low magnification (b) and TOG at high magnification (c).
3.3. Thiol Content Determination
Immobilized thiol group content on 1 g of TOG was quantified and found to be 128.31 ± 10.85 µmol, and the number of disulfide bonds was not significant. In general, thiomers are subjected to oxidation of thiol groups into disulfide bonds, but maintaining an oxygen-free environment during the synthesis of TOG might explain negligible disulfide bonds. The thiolation reaction strictly depends on the extent of hydroxyl groups attached.
3.4. Formulation and In-Vitro Evaluations
Six formulations (RGM-1 to RGM-6) were prepared by wet granulation technique with two different concentrations (25% and 40%) of extracted OG, TOG and SA. Prepared granules for all the formulations showed good flow property as evident from angle of repose (ranged from 26° to 29°), Hausner’s ratio (1.11 to 1.18) and compressibility index values (9%–12%). The compression force was adjusted to manage crushing strength in the range of 8–9 kg/cm2 and passed the DF test. Maximum and minimum percentage friability for all the formulations were reported between 0.084% and 0.1%, with no quantifiable variation among them. CSFR (ratio of crushing strength to friability) was found to be >80, which demotes for the substantial mechanical strength of the tablets (Figure 5). Weight and thickness variations were maintained at less than 2.5%.
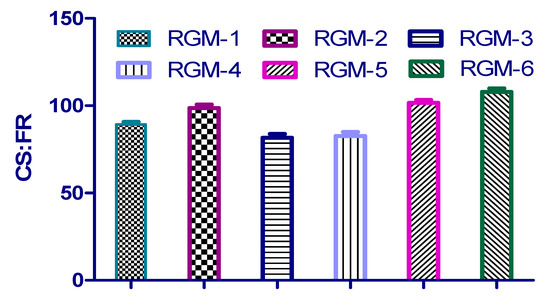
Figure 5.
Assessment of the crushing strength friability ratio (CSFR) for prepared batches.
The swelling behavior of mucoadhesion polymers has an enormous influence on the adhesive and cohesiveness properties, hence water uptake of prepared formulations was evaluated. Figure 6 demonstrates the percentage of swelling of prepared batches, at different time intervals. Formulations (RGM-1 and RGM-2) with unmodified OG, swollen quickly, yet not able to retain the integrity because of rapid hydration followed by disintegration/erosion of the tablet. At the end of 6 h swelling behavior was found to be 89.27% ± 1.54% and 98.67% ± 1.99%. The swelling index for RGM-3 and RGM-4 was found to be 81.72% ± 2.07% and 82.63% ± 2.26% at the end of 8 h. At the same time, the highest swelling percentage of 112.50% ± 3.00% (RGM-5) and 131.8% ± 2.06% (RGM-6) was observed in the tablets which were formulated with thiolated polymer (TOG), which concludes the enhancement in hydration ability. The presence of thiol groups can accountable for the formation of a polymeric matrix which high water retention and strong intrachain disulfide bond formation. Furthermore, thiolated formulations remained stable for a longer period of time without erosion in comparison to remaining formulations.
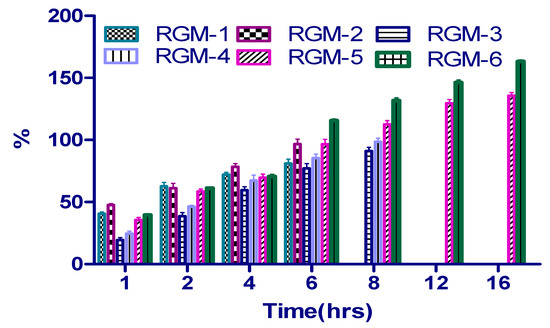
Figure 6.
Swelling index profile for RGM-1 to RGM-6.
3.5. Evaluation of Ex-Vivo Mucoadhesion Time and Mucoadhesion Strength
Ex vivo mucoadhesion time was determined by glass slide and modified dissolution apparatus and results were comparable. Mucoadhesion property of OG containing formulations may due to the formation of weak non-covalent bonds with cysteine-rich subdomains of mucus, accordingly showing less mucoadhesion time in comparison to other formulations. RGM-5 and RGM-6 showed the highest residence time as they were capable of forming strong covalent bonds by thiol exchange reactions with mucus. As displayed in Table 2 residence time and force of adhesion were increased nearly by 2–3 folds as a result of thiolation [40]. Disintegration and erosion were not observed throughout the study. As thiolated formulations will form strong disulfide bonds with mucus, accordingly maximum force is required for the detachment from the mucus. Both residence time and mucoadhesion strength were increased accordingly with the concentrations of TOG in RGM-5 and RGM-6 due to high -SH content which favors the number of disulfide bonds with the mucus gel layer [41]. Formulations with SA showed moderate mucoadhesion time and strength.

Table 2.
Comparison of ex-vivo mucoadhesion time and mucoadhesion strength.
3.6. Cytotoxicity Studies
Since TOG is a new polymer its possible toxic effect was investigated on HepG2 cells. Cell viability after 2 h of exposure to OG and TOG was assessed with MTT assay, which quantifies the metabolic activity of mitochondrial cells. Percentage cell viability for OG at 20 µg/mL and 120 µg/mL was found to be 90.50% and 72.42%, respectively [Figure 7]. There is a remarkable increase in cell viability with TOG. About 81.75% of cells were viable with a high concentration of TOG (120 µg/mL), this can be due to functional modification and confirms the safety of TOG polymer.
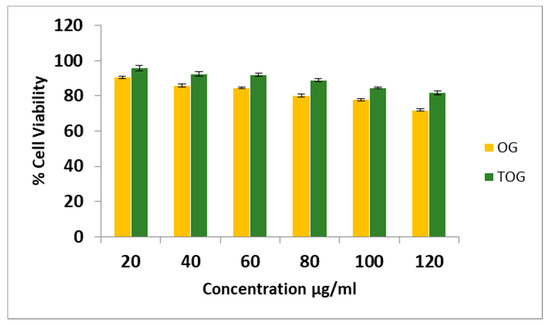
Figure 7.
Cytotoxic study of OG and TOG at different concentrations on the HepG2 cell line. Each point represents the mean ± SD.
3.7. In Vitro Drug Release Studies
In vitro dissolution studies were carried out in a modified basket method in addition to paddle methodology, to mimic the in vivo mucoadhesion of the dosage form. Figure 8a,b compares the dissolution profile of all prepared batches by both paddle and modified methods. Drug release from unmodified OG tables was found to be 98.97% and 99.68% at the end of 7 and 9 h respectively. RGM-1 and RGM-2 have initially controlled the drug release (up to 4 h) due to rapid hydration and swelling. But they failed to maintain the same integrity of the tablet matrix and followed by burst drug release. These outcomes were found to be in accordance with earlier ex-vivo mucoadhesion time. Formulations containing 25% and 40% of SA showed controlled drug release up to the end of 10 and 13 h respectively. The augmented drug release retardation (RGM-5, 16 h; RGM-6, 19 h) was observed with TOG formulated tablets. Thiolation results information of three-dimensional gel structure and inter or interchain disulfide bonds (this may probably improve the crosslinking and cohesiveness of the matrix). Consequently, this in turn increases the pathway for the diffusion of media. The drug release pattern in modified basket correlates with the paddle method but in a more controlled manner. Total tablet surface will be exposed to the media in the paddle method is contrary to in vivo conditions, where one surface of the tablet will be always in contact with the mucosa and eventually less surface will be available for dissolution. Hence, the paddle method is probably not an appropriate method for correlating in vitro drug release within the in vivo drug absorbed (IVIVC). In view of this, a modified dissolution method was employed by sticking a piece of freshly excised mucosa to the inner surface of the basket. The difference factor (f1) and the similarity factor (f2) were also calculated (MS office excel work sheet-2010) to compare the release profiles of both the methods (Table 3). From the tabulated values of f2 and f1, one can conclude that the modified basket method was signified with the paddle method, but with low f2 (64.05–70.37) and high f1 values (7.08–10.63). In general f2 and f1 values in the range of 50–100 and 0–15 respectively, but with these low signified values, one can’t expect a good correlation with in vivo release studies. This will make us think about the modified dissolution method for preciseness in IVIVC.
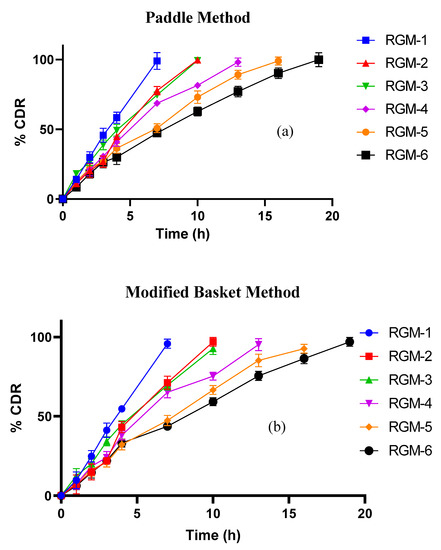
Figure 8.
In vitro drug release profile for prepared bathes by (a) paddle method and (b) modified basket method.

Table 3.
Difference factor (f1) and the similarity factor (f2) values between the paddle method and the modified basket method.
The in vitro drug release data were evaluated kinetically using various mathematical models such as zero order, first order, Higuchi and Korsmeyer-Peppas models [21]. The ’n’ value (release exponent from Peppas model) and r2 (regression values) for all the formulations (both paddle and modified basket method) were shown in Table 4. RGM-1 and RGM-2 formulations are shown ’n’ value in the range of 0.9916 to 1.1775, which confirms non-fickian super case II transport and drug release depend on swelling and erosion of the polymer. Thiolated formulations showed diffusion and swelling release mechanism (n value was found to be in between 0.7548 to 0.8844) except RGM-6B (n value-0.9118). Release exponent for all the formulations was seen in the vicinity of 0.9759 and 0.9987, affirming the closeness to the fulfillment of perfect zero-order kinetics. RGM-6 was identified as an optimal formulation for further in vivo studies on account of its promising mucoadhesion time and drug release retardation.

Table 4.
Kinetic data for prepared batches.
3.8. In Vivo Mucoadhesion Studies
The mucoadhesion behavior of RGM-6 was observed in the rabbit stomach using the X-ray technique. The X-ray photograph was taken prior to the administration of formulation (Figure 9a). The next X-ray photograph was taken after 1hr of administration (Figure 9b). Obtained radiographic images at the end of 4 h revealed that the tablet was slightly altered in its position but remain adhesive to the mucosa. Swelling of the tablet visualized with a translucent swelling layer around it at the end of 8 h. Tablet was able to withstand the peristaltic movements of GIT, as it forms a strong disulfide bond with the mucus.
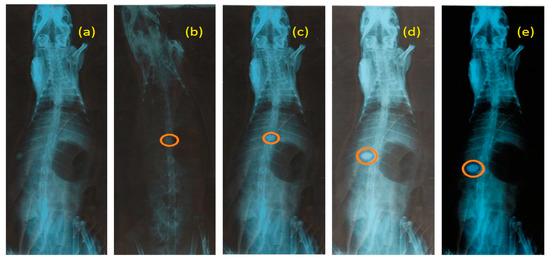
Figure 9.
X-ray radiograms (a) Before administering RGM-6, (b), (c), (d) and (e) showing mucoadhesion of RGM-6 at 1 h, 2 h, 4 h, and 8 h respectively.
3.9. Bioavailability Studies
Repaglinide was estimated by using the RP-HPLC (Reversed-phase high-performance liquid chromatography) method. The precision of the method can be confirmed as there was no interference of the blank solution at the retention time. The drug retention time of RG was found to be 5.809 min [42]. Representative chromatogram of repaglinide monitored at 245 nm was shown in Figure 10.
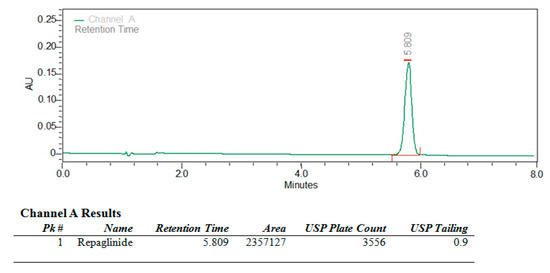
Figure 10.
A typical chromatogram of repaglinide by RP-HPLC.
Calculated pharmacokinetic parameters for the marketed product (RepideTM) and RGM-6 were shown in Table 5. The comparison of pharmacokinetic data of RGM-6 with marketed products clearly indicates that peak plasma concentration of repaglinide from the marketed product was reached very quickly and more rapidly eliminated in contrast to RGM-6. Area under the curve (AUC) for RGM-6 was increased more than 6.5 times, which indicates better absorption in improving the relative bioavailability [Figure 11]. This enhanced bioavailability is due to prolonged contact time of thiomer with mucus which in turn favors for the longer residence of dosage form, extended-release and absorption. Ke for RGM-6 was reduced to 0.888 (1/h) but elimination half-life (t1/2) was increased to 2.65 h, which is desirable for controlled drug delivery systems. Vd is almost similar for both RGM-6 and marketed products. Rate of absorption (Ka) for RGM-6 was significantly decreased to 0.88 in contrast with Repide (1.94). In view of the higher membrane permeability of repaglinide, the dissolution rate or solubility may be the rate-limiting step for its absorption. The enhanced absorption of repaglinide in thiolated formulations may be attributed to the improvement of its residence time. Enhanced gastric retention of thiolated formulations, enhances the time for repaglinide to get dissolve before entering into the intestine, which favors enhanced bioavailability.

Table 5.
Pharmacokinetic parameters for marketed (Repide) and test (RGM-6) formulations.
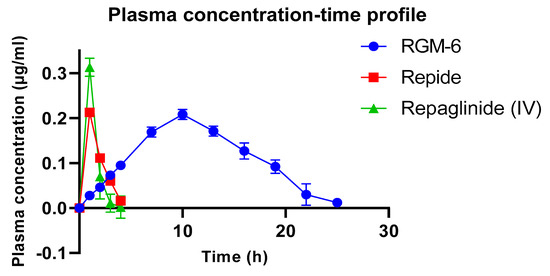
Figure 11.
Plasma concentration-time profile for RGM-6 and REPIDE following oral administration (n = 6).
3.10. IVIVC
Level A in vitro–in vivo correlation was plotted on a graph by considering the fraction of drug released (in vitro) (for both RGM-6 P and RGM-6 B) on the x-axis and fraction of drug absorbed (in vivo) on the y-axis for the same period of time. The graph was represented in Figure 12b. (RGM-6 B) showed a strong linear relationship between the fraction of drug released and a fraction of drug absorbed with a regression value of 0.9961, while IVIVC for RGM-6 P shown a lesser regression value of 0.9914. Hence, the modified dissolution method is probably an appropriate method for correlating in-vitro drug release with in-vivo drug absorbed than the conventional paddle method.
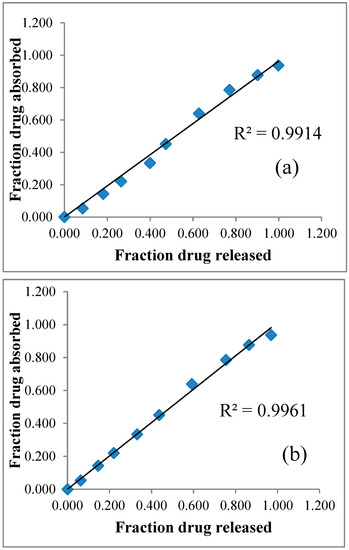
Figure 12.
Level A IVIVC for (a) RGM-6 P (b) RGM-6 B.
3.11. Reproducibility of Thiolated Batches
Reproducibility of thiolated polymer interms of its mucoadhesion property and sustained nature can be confirmed by performing ex vivo residence time, mucoadhesion strength, drug release at 16 h and in vivo mucoadhesion residence foro the reproducible batches. Ex vivo residence time was estimated by glass slide method and modified methods, and the similar result was found to be similar to RGM-5 and RGM-6. Mucoadhesion strength of reproduced batches of RGM-5, RGM-6 was varied only in the range of 2.56%–4.37% and drug release at the 16 h was slightly changed (1.35% & 1.14%) but found be within the acceptable range. There is no change with respect to the in vivo mucoadhesion time and all the results were shown in Table 6.

Table 6.
Reproducibility of Thiolated Batches by ex-vivo residence time, mucoadhesion strength, percentage drug release at end of 16 h and in-vivo mucoadhesion study.
4. Conclusions
Thiolation was achieved by esterification of OG with TGA leading to conjugate with the improved characteristics in comparison to the original material. Thiomes are capable of forming inter and/or intramolecular disulfide bonds that can contribute to enhanced swelling, mucoadhesion strength and sustained release. The mucoadhesion potential of OG was increased by 2–3 folds as a result of thiolation. In vivo studies revealed that thiolated formulations were able to withstand peristaltic movements of GIT up to 8 h, as it forms strong disulfide bonds with the mucous membrane. Cytotoxicity studies confirm the safety of prepared TOG. Pharmacokinetic studies for RGM-6 confirm that there is a decline in the Ka and Ke, which is desirable for control release. IVIVC study confirms that the modified dissolution method is probably a better method for correlating in-vitro drug release with in-vivo drug absorbed than the conventional paddle method. Thus the use of plant-based polymeric gum in thiolated form can be a good, safe replacement for synthetic polymers in the development of mucoadhesive dosage forms for poorly soluble drugs intended for GIT targeted controlled release.
Author Contributions
N.R.N.: Methodology, Validation, Formal analysis, Investigation, Writing- Original draft. C.G.: Formal analysis, Methodology, reviewing. M.K.: Conceptualization, Resources, Writing- review & editing, Supervision, Data curation, Project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schnurch, A.B.; Hornof, M.; Zoidl, T. Thiolated polymers–thiomers: Synthesis and in vitro evaluation of chitosan-2-iminothiolane conjugates. Int. J. Pharm. 2003, 260, 229–237. [Google Scholar] [CrossRef]
- Clausen, A.E.; Kast, C.E.; Schnurch, A.B. The role of glutathione in the permeation enhancing effect of thiolated polymers. Pharm. Res. 2002, 19, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Ghori, M.U.; Alba, K.; Smith, A.M.; Conway, B.R.; Kontogiorgos, V. Okra extracts in pharmaceutical and food applications. Food Hydrocoll. 2014, 42, 342–347. [Google Scholar] [CrossRef]
- Kontogiorgos, V.; Margelou, I.; Georgiadis, N.; Ritzoulis, C. Rheological characterization of okra pectins. Food Hydrocoll. 2012, 29, 356–362. [Google Scholar] [CrossRef]
- Lorenzo, C.A.; Fernandez, B.B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef]
- Ogaji, I.; Nnoli, O. Film coating potential of okra gum using paracetamol tablets as a model drug. Asian J. Pharm. 2010, 4, 130–134. [Google Scholar] [CrossRef]
- Sinha, P.; Ubaidulla, U.; Nayak, A.K. Okra (Hibiscus esculentus) gum-alginate blend mucoadhesive beads for controlled glibenclamide release. Int. J. Biol. Macromol. 2015, 72, 1069–1075. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, D.; Brar, V. Bioadhesive okra polymer based buccal patches as platform for controlled drug delivery. Int. J. Biol. Macromol. 2014, 70, 408–419. [Google Scholar] [CrossRef]
- Raghavendra Naveen, N.; Gopinath, C.; Subba Rao, D. Isolation and assessment of natural mucoadhesive agent isolated from Abelmoschus esculents. J. Pharm. Res. 2017, 11, 438–443. [Google Scholar]
- Sharma, R.; Ahuja, M. Thiolated pectin: Synthesis, characterization and evaluation as a mucoadhesive polymer. Carbohydr. Polym. 2011, 85, 658–663. [Google Scholar] [CrossRef]
- Iqbal, J.; Shahnaz, G.; Dunnhaupt, S.; Muller, C.; Hintzen, F.; Schnurch, A.B. Preactivated thiomers as mucoadhesive polymers for drug delivery. Biomaterials 2012, 33, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Schnurch, A.B. Thiomers: A new generation of mucoadhesive polymers. Adv. Drug Deliv. Rev. 2005, 57, 1569–1582. [Google Scholar]
- Kim, K.; Ryu, J.H.; Lee, H. Chitosan-catechol: A polymer with long-lasting mucoadhesive properties. Biomaterials 2015, 52, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra Naveen, N.; Gopinath, C.; Subba Rao, D. A spotlight on thiolated natural polymers and their relevance in mucoadhesive drug delivery system. Fut. J. Pharm. Sci. 2018, 4, 47–52. [Google Scholar]
- Ijaz, M.; Matuszczak, B.; Rahmat, D.; Mahmood, A.; Bonengel, S.; Hussain, S.; Huck, C.W.; Schnurch, A.B. Synthesis and characterization of thiolated-cyclodextrin as a novel mucoadhesive excipient for intra-oral drug delivery. Carbohydr. Polym. 2015, 132, 187–195. [Google Scholar] [CrossRef]
- Schnurch, A.B.; Krauland, A.H.; Leitner, V.M.; Palmberger, T. Thiomers: Potential excipients for non-invasive peptide delivery systems. Eur. J. Pharm. Biopharm. 2004, 58, 253–263. [Google Scholar] [CrossRef]
- Borchard, G.; Lueben, H.L.; De Boer, A.G.; Verhoef, J.C.; Lehr, C.M.; Junginger, H.R. The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption. III: Effects of chitosan-glutamate and carbomer on epithelial tight junctions in vitro. J. Control Release 1996, 39, 131–138. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, Y.; Ping, Q.; Xiao, Y.; Huang, X. Mucoadhesive effect of thiolated PEG stearate and its modified NLC for ocular drug delivery. J. Control. Release 2009, 137, 217–223. [Google Scholar] [CrossRef]
- Anitha, A.; Deepa, N.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Development of mucoadhesive thiolated chitosan nanoparticles for biomedical applications. Carbohydr. Polym. 2011, 83, 66–73. [Google Scholar] [CrossRef]
- Kumar, R.; Sinha, V.R. Thiomer: A potential carrier for therapeutic delivery. React. Funct. Polym. 2013, 73, 1156–1166. [Google Scholar] [CrossRef]
- Naveen, N.R.; Gopinath, C.; Rao, D.S. Design expert supported mathematical optimization of repaglinide gastro retentive floating tablets: In vitro and in vivo evaluation. Fut. J. Pharm. Sci. 2017, 2, 140–147. [Google Scholar]
- Wei, H.; Mengmeng, W.; Shiqing, H.; Lifang, Y. Matrix tablets for sustained release of repaglinide: Preparation, pharmacokinetics and hypoglycemic activity in beagle dogs. Int. J. Pharm. 2015, 478, 297–307. [Google Scholar]
- Megha, S.; Seema, K.; Agnimitra, D. In-vitro and in-vivo evaluation of repaglinide loaded floating microspheres prepared from different viscosity grades of HPMC polymer. Saudi Pharm. J. 2015, 23, 675–682. [Google Scholar]
- Dicharry, R.M.; Ye, P.; Saha, G.; Waxman, E.; Asandei, A.D.; Parnas, R.S. Wheat gluten-thiolated poly(vinyl alcohol) blends with improved mechanical properties. Biomacromolecules 2006, 7, 2837–2844. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.K.; Mandapalli, P.K.; Manthri, R.; Reddy, V.P. Development and in vivo evaluation of gastro retentive delivery systems for cefuroxime axetil. Saudi Pharm. J. 2013, 21, 53–59. [Google Scholar] [CrossRef]
- Bahulkara, S.S.; Munota, N.M.; Surwaseba, S.S. Synthesis, characterization of thiolated karaya gum and evaluation of effect of pH on its mucoadhesive and sustained release properties. Carbohydr. Polym. 2015, 130, 183–190. [Google Scholar] [CrossRef]
- Mahajan, H.S.; Tyagi, V.K.; Patil, R.R.; Dusunge, S.B. Thiolated xyloglucan: Synthesis, characterization and evaluation as mucoadhesive in situ gelling agent. Carbohydr. Polym. 2013, 19, 618–625. [Google Scholar] [CrossRef]
- Zaharuddin, N.D.; Noordin, M.I.; Kadivar, A. The Use of Hibiscus esculentus (Okra) Gum in Sustaining the Release of Propranolol Hydrochloride in a Solid Oral Dosage. BioMed Res. Int. 2014, 2014, 735891. [Google Scholar]
- Bernkop-Schnürch, A.; Schwarz, V.; Steininger, S. Polymers with thiol groups: A new generation of mucoadhesive polymers? Pharm. Res. 1999, 16, 876–881. [Google Scholar] [CrossRef]
- Habeeb, A. A sensitive method for localization of disulfide containing peptides in column effluents. Anal. Biochem. 1973, 56, 56,60–65. [Google Scholar]
- Vaithiyalingam, S.R.; Sayeed, V.A. Critical factors in manufacturing multi-layer tablets assessing material attributes in-process controls, manufacturing process and product performance. Int. J. Pharm. 2010, 398, 9–13. [Google Scholar]
- Kaur, H.; Yadav, S.; Ahuja, M.; Dilbaghi, N. Synthesis, characterization and evaluation of thiolated tamarind seed polysaccharide as a mucoadhesive polymer. Carbohydr. Polym. 2012, 90, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Han, R.Y.; Fang, Y.J.; Sung, K.C.; Hu, O.Y.P. Mucoadhesive buccal disks for novel nalbuphine prodrug controlled delivery: Effect of formulation variables on drug release and mucoadhesive performance. Int. J. Pharm. 1999, 171, 201–209. [Google Scholar] [CrossRef]
- Patel, V.M.; Prajapati, B.G.; Patel, M.M. Formulation, evaluation, and comparison of bilayered and multilayered mucoadhesive buccal devices of propranolol hydrochloride. AAPS PharmSciTech. 2017, 8, E1–E8. [Google Scholar] [CrossRef] [PubMed]
- Kafedjiiski, K.; Krauland, A.H.; Hoffer, M.H.; Schnürch, A.B. Synthesis and in vitro evaluation of a novel thiolated chitosan. Biomaterials 2005, 26, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Sarti, F.; Staaf, A.; Sakloetsakun, D.; Bernkop-Schnürch, A. Thiolated hydroxy ethylcellulose: Synthesis and in vitro evaluation. Eur. J. Pharm. Biopharm. 2010, 76, 421–427. [Google Scholar] [CrossRef]
- Abou Auda, H.S.; Najjar, T.A.; Al Khamis, K.I.; Al Hadiya, B.M.; Ghilzai, N.M.; Al Fawzan, N.F. Liquid chromatographic assay of nifedipine in human plasma and its application to pharmacokinetic studies. J. Pharm. Biomed. Anal. 2000, 22, 241–249. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Ghosh, A.; Choudhury, G.K. In vitro-In vivo Correlation (IVIVC): A Review. J. Pharm. Res. 2009, 2, 1255–1260. [Google Scholar]
- Rahmat, D.; Sakloetsakun, D.; Shahnaz, G.; Perera, G.; Kaindl, R.; Schnurch, A.B. Design and synthesis of a novel cationic thiolated polymer. Int. J. Pharm. 2011, 411, 10–17. [Google Scholar] [CrossRef]
- Raval, J.A.; Patel, M.M. Formulation and characterization of gastroretentive discs containing famotidine. Braz. Arch. Biol. Technol. 2011, 54, 293–300. [Google Scholar] [CrossRef]
- Ramanjireddy, T.; Dhachinamoorthi, D.; Chandrasekhar, K.B. Pharmacokinetic study of repaglinide floating drug delivery system in rabbits by RPHPLC method. J. Chin. Pharm. Sci. 2012, 21, 162–168. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).