Ultrasound-Assisted Extraction of GAC Peel: An Optimization of Extraction Conditions for Recovering Carotenoids and Antioxidant Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials
2.3. Ultrasound-Assisted Extraction of GAC Peel
2.4. Experimental Design Using Response Surface Methodology (RSM)
2.5. Measurement of Total Carotenoid Content
2.6. HPLC Analysis of Carotenoid Composition
2.7. Determination of Antioxidant Activity
2.8. Statistical Analysis
3. Results and Discussion
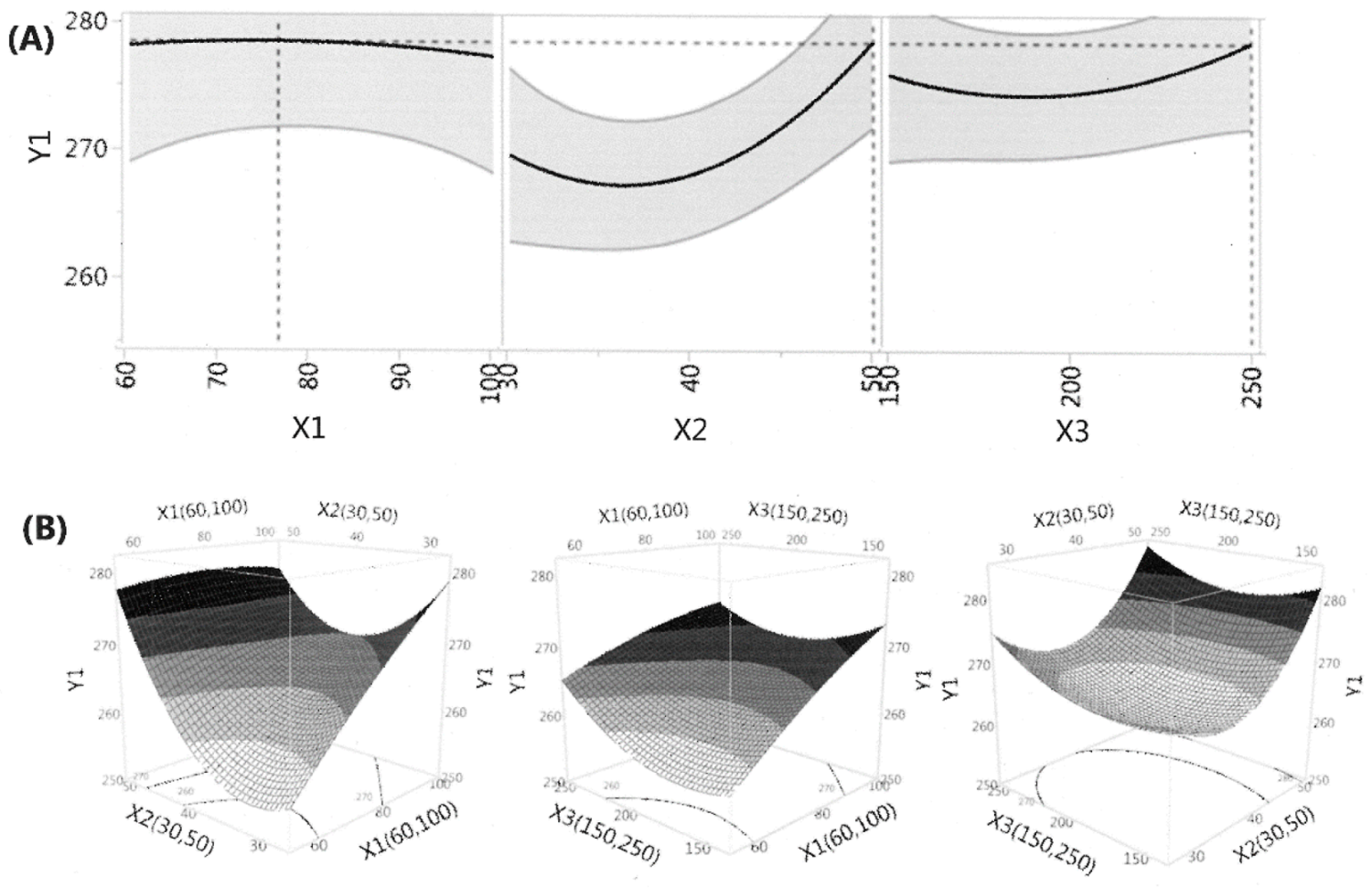
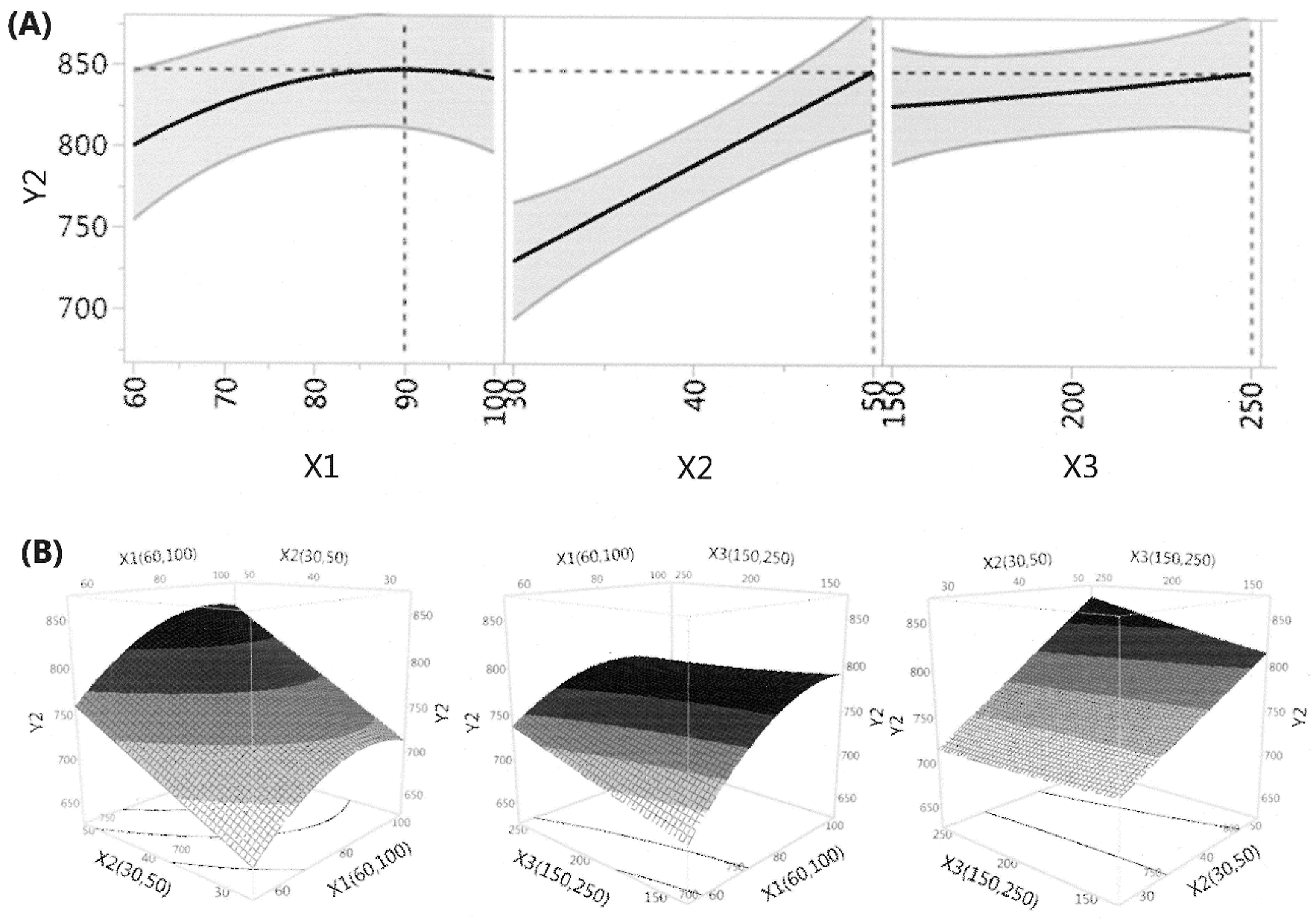
3.1. Fitting the Model for the Prediction of Total Carotenoid Content and Antioxidant Capacity
3.2. Effects of Extraction Conditions on the Total Carotenoid Extraction Yield
3.3. Effects of Extraction Conditions on Antioxidant Capacity of the Extracts
3.4. Optimal Extraction Conditions and Validation of the Model
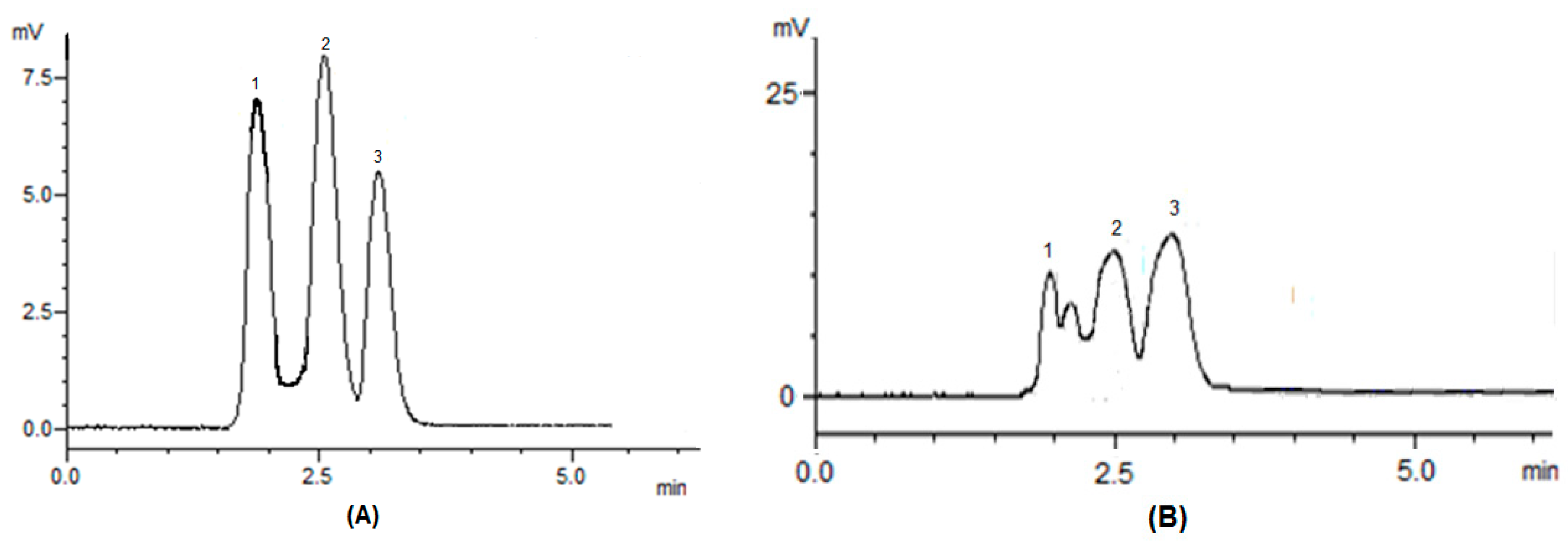
3.5. Carotenoid Composition of the Extract Obtained under Optimal Conditions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vuong, L.T.; Franke, A.A.; Custer, L.J.; Murphy, S.P. Momordica Cochinchinensis Spreng. (Gac) Fruit Carotenoids Reevaluated. J. Food Composit. Anal. 2006, 19, 664–668. [Google Scholar] [CrossRef]
- Kha, T.C.; Nguyen, M.H.; Roach, P.D.; Parks, S.E.; Stathopoulos, C. Gac Fruit: Nutrient and Phytochemical Composition, and Options for Processing. Food Rev. Int. 2013, 29, 92–106. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Gac Fruit (Momordica Cochinchinensis Spreng.): A Rich Source of Bioactive Compounds and Its Potential Health Benefits. Int. J. Food Sci. Technol. 2015, 50, 567–577. [Google Scholar] [CrossRef]
- Zhao, L.-M.; Han, L.-N.; Ren, F.-Z.; Chen, S.-H.; Liu, L.-H.; Wang, M.-X.; Sang, M.-X.; Shan, B.-E. An Ester Extract of Cochinchina Momordica Seeds Induces Differentiation of Melanoma B16 F1 Cells Via Mapks Signaling. Asian Pac. J. Cancer Prev. 2012, 13, 3795–3802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubola, J.; Siriamornpun, S. Phytochemicals and Antioxidant Activity of Different Fruit Fractions (Peel, Pulp, Aril and Seed) of Thai Gac (Momordica Cochinchinensis Spreng). Food Chem. 2011, 127, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Chuyen, H.V.; Roach, P.D.; Golding, J.B.; Parks, S.E.; Nguyen, M.H. Effects of Four Different Drying Methods on the Carotenoid Composition and Antioxidant Capacity of Dried Gac Peel. J. Sci. Food Agric. 2017, 97, 1656–1662. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Roach, P.D.; Golding, J.B.; Parks, S.E.; Nguyen, M.H. Effects of Pretreatments and Air Drying Temperatures on the Carotenoid Composition and Antioxidant Capacity of Dried Gac peel. J. Food Proc. Preserv. 2017, 41, e13226. [Google Scholar] [CrossRef]
- Nowacka, M.; Wedzik, M. Effect of Ultrasound Treatment on Microstructure, Colour and Carotenoid Content in Fresh and Dried Carrot Tissue. Appl. Acoust. 2016, 103, 163–171. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, D.S.; Kozukue, N.; Kim, H.J.; Nishitani, Y.; Mizuno, M.; Levin, C.E.; Friedman, M. Protein, Free Amino Acid, Phenolic, B-Carotene, and Lycopene Content, and Antioxidative and Cancer Cell Inhibitory Effects of 12 Greenhouse-Grown Commercial Cherry Tomato Varieties. J. Food Composit. Anal. 2014, 34, 115–127. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Roach, P.D.; Golding, J.B.; Parks, S.E.; Nguyen, M.H. Optimisation of Extraction Conditions for Recovering Carotenoids and Antioxidant Capacity from Gac Peel Using Response Surface Methodology. Int. J. Food Sci. Technol. 2017, 52, 972–980. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to Extract Bioactive Compounds from Food by-Products of Plant Origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Wani, S.A.; Bishnoi, S.; Kumar, P. Ultrasound and Microwave Assisted Extraction of Diosgenin from Fenugreek Seed and Fenugreek-Supplemented Cookies. J. Food Meas. Charact. 2016, 10, 527–532. [Google Scholar] [CrossRef]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T.J. Investigation of the Effects of Ultrasound on Vegetal Tissues During Solvent Extraction. Ultrason. Sonochem. 2001, 8, 137–142. [Google Scholar] [CrossRef]
- Kumcuoglu, S.; Yilmaz, T.; Tavman, S. Ultrasound Assisted Extraction of Lycopene from Tomato Processing Wastes. J. Food Sci. Technol. 2014, 51, 4102–4107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordóñez-Santos, L.E.; Pinzón-Zarate, L.X.; González-Salcedo, L.O. Optimization of Ultrasonic-Assisted Extraction of Total Carotenoids from Peach Palm Fruit (Bactris Gasipaes) by-Products with Sunflower Oil Using Response Surface Methodology. Ultrason. Sonochem. 2015, 27, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-Products of Plant Food Processing as a Source of Functional Compounds—Recent Developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Microwave-Assisted Extraction and Ultrasound-Assisted Extraction for Recovering Carotenoids from Gac Peel and Their Effects on Antioxidant Capacity of the Extracts. Food Sci. Nutr. 2018, 6, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Khuri, A.I.; Mukhopadhyay, S. Response Surface Methodology. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Wang, T.; Liang, H.; Yuan, Q. Optimization of Ultrasonic-Stimulated Solvent Extraction of Sinigrin from Indian Mustard Seed (Brassica Juncea, L.) Using Response Surface Methodology. Phytochem. Anal. 2011, 22, 205–213. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Thanh, D.T.; Bhuyan, D.J.; Goldsmith, C.D.; Sadeqzadeh, E.; Scarlett, C.J.; Bowyer, M.C. Optimization of Ultrasound-Assisted Extraction Conditions for Euphol from the Medicinal Plant, Euphorbia Tirucalli, Using Response Surface Methodology. Ind. Crops Prod. 2015, 63, 197–202. [Google Scholar] [CrossRef]
- Tuyen, C.K.; Nguyen, M.H.; Roach, P.D.; Stathopoulos, C.E. Effects of Gac Aril Microwave Processing Conditions on Oil Extraction Efficiency, and B-Carotene and Lycopene Contents. J. Food Eng. 2013, 117, 486–491. [Google Scholar]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of Abts, Dpph, Frap, and Orac Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Composit. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, G.; Ye, X.; Kakuda, Y.; Meng, R. Stability of All-Trans-B-Carotene under Ultrasound Treatment in a Model System: Effects of Different Factors, Kinetics and Newly Formed Compounds. Ultrason. Sonochem. 2010, 17, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhao, G.; Chen, F.; Wang, Z.; Wu, J.; Hu, X. Different Effects of Microwave and Ultrasound on the Stability of (All-E)-Astaxanthin. J. Agric. Food Chem. 2006, 54, 8346–8351. [Google Scholar] [CrossRef] [PubMed]
- Chuyen, H.V.; Tran, X.T.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Yield of Carotenoids, Phenolic Compounds and Antioxidant Capacity of Extracts from Gac Peel as Affected by Different Solvents and Extraction Conditions. J. Adv. Agric. Technol. 2017, 4, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Vuong, L.T.; Dueker, S.R.; Murphy, S.P. Plasma Beta-Carotene and Retinol Concentrations of Children Increase after a 30-D Supplementation with the Fruit Momordica Cochinchinensis (Gac). Am. J. Clin. Nutr. 2002, 75, 872–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and Its Antioxidant Role in the Prevention of Cardiovascular Diseases—A Critical Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1868–1879. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, Zeaxanthin, and Meso-Zeaxanthin: The Basic and Clinical Science Underlying Carotenoid-Based Nutritional Interventions against Ocular Disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef] [Green Version]

| Coded Variable Levels | Independent Variables | ||
|---|---|---|---|
| X1 (min) | X2 (°C) | X3 (W) | |
| −1 | 60 | 30 | 150 |
| 0 | 80 | 40 | 200 |
| +1 | 100 | 50 | 250 |
| Run | Pattern * | X1 (min) | X2 (°C) | X3 (W) | Y1 (mg/100 g DW) | Y2 (µM TE/100 g DW) | ||
|---|---|---|---|---|---|---|---|---|
| Experimental | Predicted | Experimental | Predicted | |||||
| 1 | −−0 | 60 | 30 | 200 | 257.4 | 257.0 | 689.2 | 684.0 |
| 2 | −0− | 60 | 40 | 150 | 258.6 | 258.8 | 715.3 | 707.4 |
| 3 | −0+ | 60 | 40 | 250 | 262.8 | 264.7 | 742.3 | 748.4 |
| 4 | −+0 | 60 | 50 | 200 | 274.7 | 272.9 | 762.3 | 769.4 |
| 5 | 0−− | 80 | 30 | 150 | 266.6 | 266.8 | 718.6 | 731.7 |
| 6 | 0−+ | 80 | 30 | 250 | 272.4 | 270.9 | 730.4 | 729.6 |
| 7 | 000 | 80 | 40 | 200 | 262.0 | 264.4 | 760.0 | 777.0 |
| 8 | 000 | 80 | 40 | 200 | 268.7 | 264.4 | 785.6 | 777.0 |
| 9 | 000 | 80 | 40 | 200 | 262.6 | 264.4 | 785.4 | 777.0 |
| 10 | 0+− | 80 | 50 | 150 | 274.9 | 276.4 | 807.8 | 808.6 |
| 11 | 0++ | 80 | 50 | 250 | 278.6 | 278.4 | 855.6 | 842.5 |
| 12 | +−0 | 100 | 30 | 200 | 272.0 | 273.8 | 739.7 | 732.6 |
| 13 | +0+ | 100 | 40 | 250 | 271.5 | 271.3 | 773.3 | 781.3 |
| 14 | +0− | 100 | 40 | 150 | 272.9 | 271.0 | 796.7 | 790.6 |
| 15 | ++0 | 100 | 50 | 200 | 274.5 | 274.9 | 831.7 | 836.9 |
| Statistical Parameters | Total Carotenoid | Antioxidant Capacity |
|---|---|---|
| R2 | 0.92 | 0.96 |
| P of model | 0.024 | 0.005 |
| P of lack of fit | 0.746 | 0.513 |
| Regression Coefficient | Total Carotenoid | Antioxidant Capacity | ||
|---|---|---|---|---|
| Estimated Value | Prob > | Estimated Value | Prob > | |
| Intercept | ||||
| βo | 264.4 * | <0.0001 | 777.0 * | <0.0001 |
| Linear | ||||
| β1 | 4.689 * | 0.0071 | 29.031 * | 0.0028 |
| β2 | 4.277 * | 0.0103 | 47.433 * | 0.0003 |
| β3 | 1.544 | 0.2084 | 7.928 | 0.1971 |
| Interaction | ||||
| β12 | −3.690 | 0.0586 | 4.728 | 0.5581 |
| β13 | −1.404 | 0.3958 | −12.586 | 0.1559 |
| β23 | −0.510 | 0.7496 | 8.994 | 0.2864 |
| Quadratic | ||||
| β11 | −0.734 | 0.6604 | −21.222 * | 0.0425 |
| β22 | 5.956 * | 0.0128 | −0.015 | 0.9986 |
| β33 | 2.734 | 0.1429 | 1.146 | 0.8896 |
| Responses | Predicted Value | Actual Result | Conventional Extraction |
|---|---|---|---|
| Extraction time (min) | 76 | 76 | 150 * |
| Temperature (°C) | 50 | 50 | 40.7 * |
| Carotenoid yield (mg/100 g DW) | 278.5 ± 6.8 a | 269.1 ± 12.2 a | 271.1 ± 8.5 a* |
| Antioxidant capacity (µM TE/100 g DW) | 837.4 ± 36.0 b | 822.3 ± 32.2 b | 737.3 ± 23.8 c* |
| Individual Carotenoid | Retention Time (min) | Percentage (%) |
|---|---|---|
| β-carotene | 1.89 | 46.1 |
| Lycopene | 2.55 | 28.1 |
| Lutein | 3.12 | 11.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuyen, H.V.; Roach, P.D.; Golding, J.B.; Parks, S.E.; Nguyen, M.H. Ultrasound-Assisted Extraction of GAC Peel: An Optimization of Extraction Conditions for Recovering Carotenoids and Antioxidant Capacity. Processes 2020, 8, 8. https://doi.org/10.3390/pr8010008
Chuyen HV, Roach PD, Golding JB, Parks SE, Nguyen MH. Ultrasound-Assisted Extraction of GAC Peel: An Optimization of Extraction Conditions for Recovering Carotenoids and Antioxidant Capacity. Processes. 2020; 8(1):8. https://doi.org/10.3390/pr8010008
Chicago/Turabian StyleChuyen, Hoang V., Paul D. Roach, John B. Golding, Sophie E. Parks, and Minh H. Nguyen. 2020. "Ultrasound-Assisted Extraction of GAC Peel: An Optimization of Extraction Conditions for Recovering Carotenoids and Antioxidant Capacity" Processes 8, no. 1: 8. https://doi.org/10.3390/pr8010008
APA StyleChuyen, H. V., Roach, P. D., Golding, J. B., Parks, S. E., & Nguyen, M. H. (2020). Ultrasound-Assisted Extraction of GAC Peel: An Optimization of Extraction Conditions for Recovering Carotenoids and Antioxidant Capacity. Processes, 8(1), 8. https://doi.org/10.3390/pr8010008







