Antidiabetic and Antilipidemic Activity of Root Extracts of Salacia oblonga against Streptozotocin-Induced Diabetes in Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.2.1. Plant Extraction
2.2.2. Phytochemical Analysis
Test for Alkaloids
Test for Flavonoids
Test for Phenols
Test for Saponins
Test for Cardiac Glycosides
Test for Steroids
Test for Terpenoids
Determination of Total Phenolic Content
Determination of Total Flavonoid Content
2.3. In Vitro Antidiabetic Activity of S. oblonga Root Extracts
2.3.1. α-Glucosidase Inhibition Activity
2.3.2. α-Amylase Inhibition Activity
2.4. In Vivo Antidiabetic and Antilipidemic Activities
2.4.1. Animals
2.4.2. Acute Toxicity Study
2.5. Experimental Design
- Group I: Normal control rats (NC)
- Group II: STZ-induced diabetic control (DC)
- Group III: STZ-induced diabetic group which were administered 2 mg/kg bw acarbose (STD)
- Group IV: STZ-induced diabetic group administered orally 50 mg/kg bw of SREE (A)
- Group V: STZ-induced diabetic group administered orally 100 mg/kg bw of SREE (B)
- Group VI: STZ-induced diabetic group administered orally 200 mg/kg bw of SREE (C)
- Group VII: STZ-induced diabetic group administered orally 400 mg/kg bw of SREE (D)
2.6. Estimation of Blood Glucose, Lipid, Bilirubin, and Creatinine Levels
2.7. Statistical Analysis
3. Results
3.1. Phytochemical Analysis of S. oblonga Root Extracts
3.2. In Vitro Antidiabetic Activity of S. oblonga Root Extracts
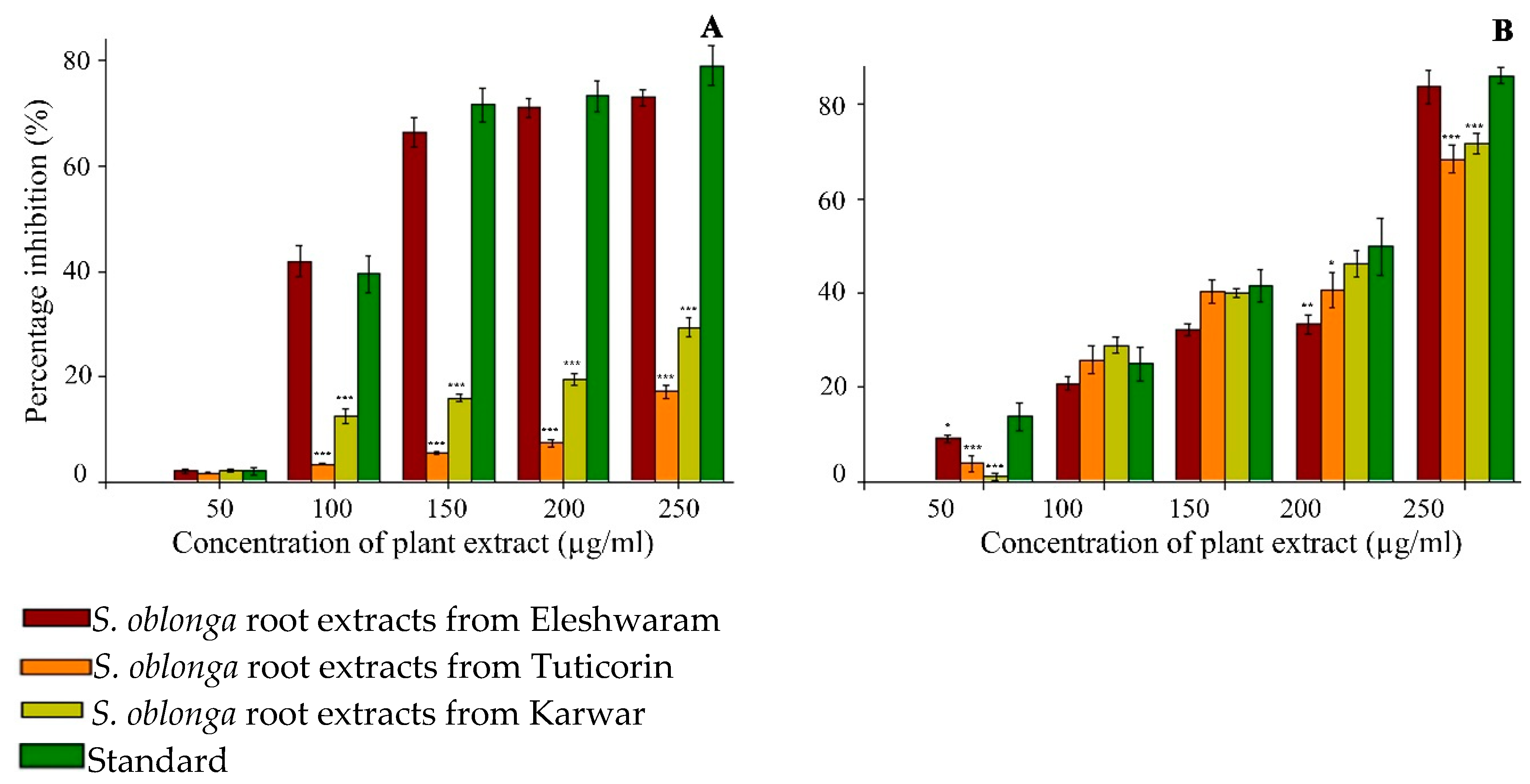
3.2.1. α-Glucosidase Inhibition Activity
3.2.2. α-Amylase Inhibition Activity
3.2.3. Acute Toxicity Studies
3.2.4. Effect of SREE on Blood Glucose Levels in STZ-Induced Diabetic Rats
3.2.5. Effect of SREE on Lipid Levels in STZ-Induced Diabetic Rats
3.2.6. Effect of SREE on Creatinine and Total Bilirubin Levels in STZ-Induced Diabetic Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pandurangan, M.; Kim, D.H. Therapeutic potential of cyanobacteria against streptozotocin-induced diabetic rats. 3 Biotech 2016, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Chinsembu, K.C. Diabetes mellitus and nature’s pharmacy of putative antidiabetic plants. J. Herb. Med. 2019, 15, 100230. [Google Scholar] [CrossRef]
- Odeyemi, S.; Dewar, J. In Vitro Antidiabetic Activity Affecting Glucose Uptake in HepG2 Cells Following Their Exposure to Extracts of Lauridia tetragona (Lf) RH Archer. Processes 2020, 8, 33. [Google Scholar] [CrossRef]
- Bouskila, M.; Pajvani, U.B.; Scherer, P.E. Adiponectin: A relevant player in PPARgamma-agonist-mediated improvements in hepatic insulin sensitivity? Int. J. Obes. (Lond.) 2005, 29 (Suppl. 1), S17–S23. [Google Scholar] [CrossRef]
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef]
- Dabelea, D.; Snell-Bergeon, J.K.; Hartsfield, C.L.; Bischoff, K.J.; Hamman, R.F.; McDuffie, R.S. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care 2005, 28, 579–584. [Google Scholar] [CrossRef]
- Sohn, E.; Kim, J.; Kim, C.-S.; Jo, K.; Lee, Y.M.; Kim, J.S. Root of Polygonum cuspidatum extract reduces progression of diabetes-induced mesangial cell dysfunction via inhibition of platelet-derived growth factor-BB (PDGF-BB) and interaction with its receptor in streptozotocin-induced diabetic rats. BMC Complement Altern. Med. 2014, 14, 1–9. [Google Scholar] [CrossRef]
- Boden, G. Endoplasmic reticulum stress: Another link between obesity and insulin resistance/inflammation? Diabetes 2009, 58, 518–519. [Google Scholar] [CrossRef]
- Almdal, T.P.; Vilstrup, H. Effects of streptozotocin-induced diabetes and diet on nitrogen loss from organs and on the capacity of urea synthesis in rats. Diabetologia 1987, 30, 952–956. [Google Scholar] [CrossRef]
- Rehman, G.; Khan, S.A.; Hamayun, M. Studies on diabetic nephropathy and secondary diseases in type 2 diabetes. Int. J. Diabetes Dev. Ctries. 2005, 25, 25–29. [Google Scholar] [CrossRef]
- Deepak, K. Nageswara Rao Reddy n, Surekha C Role of Antidiabetic Compounds on Glucose Metabolism–A Special Focus on Medicinal Plant: Salacia sps. Med. Chem. 2014, 4, 373–381. [Google Scholar]
- Matsuda, H.; Murakami, T.; Yashiro, K.; Yamahara, J.; Yoshikawa, M. Antidiabetic principles of natural medicines. IV. Aldose reductase and qlpha-glucosidase inhibitors from the roots of Salacia oblonga Wall. (Celastraceae): Structure of a new friedelane-type triterpene, kotalagenin 16-acetate. Chem. Pharm. Bull. (Tokyo) 1999, 47, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Karunanayake, E.; Welihinda, J.; Sirimanne, S.; Adorai, G.S. Oral hypoglycaemic activity of some medicinal plants of Sri Lanka. J. Ethnopharmacol. 1984, 11, 223–231. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Yashiro, K.; Matsuda, H. Kotalanol, a potent alpha-glucosidase inhibitor with thiosugar sulfonium sulfate structure, from antidiabetic ayurvedic medicine Salacia reticulata. Chem. Pharm. Bull. (Tokyo) 1998, 46, 1339–1340. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, K.; Augusti, K.T.; Vijayammal, P.L. Anti-peroxidative and hypoglycaemic activity of salacia oblonga extract in diabetic rats. Pharm. Biol. 2000, 38, 101–105. [Google Scholar] [CrossRef]
- Li, Y.; Peng, G.; Li, Q.; Wen, S.; Huang, T.H.; Roufogalis, B.D.; Yamahara, J. Salacia oblonga improves cardiac fibrosis and inhibits postprandial hyperglycemia in obese Zucker rats. Life Sci. 2004, 75, 1735–1746. [Google Scholar] [CrossRef]
- Collene, A.L.; Hertzler, S.R.; Williams, J.A.; Wolf, B.W. Effects of a nutritional supplement containing Salacia oblonga extract and insulinogenic amino acids on postprandial glycemia, insulinemia, and breath hydrogen responses in healthy adults. Nutrition 2005, 21, 848–854. [Google Scholar] [CrossRef]
- Surekha, C. Salacia as an ayurvedic medicine with multiple targets in diabetes and obesity. Ann. Phytomed. 2015, 4, 46–53. [Google Scholar]
- Deepak, K.G.; Suneetha, G.; Surekha, C. A simple and effective method for vegetative propagation of an endangered medicinal plant Salacia oblonga Wall. J. Nat. Med. 2016, 70, 115–119. [Google Scholar] [CrossRef]
- Liu, W.; Yin, D.; Li, N.; Hou, X.; Wang, D.; Li, D.; Liu, J. Influence of Environmental Factors on the Active Substance Production and Antioxidant Activity in Potentilla fruticosa L. and Its Quality Assessment. Sci. Rep. 2016, 6, 28591. [Google Scholar] [CrossRef]
- Penuelas, J.; Llusià, J. Effects of carbon dioxide, water supply, and seasonality on terpene content and emission by Rosmarinus officinalis. J. Chem. Ecol. 1997, 23, 979–993. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Yin, D.; Zhao, X. Influence of ecological factors on the production of active substances in the anti-cancer plant Sinopodophyllum hexandrum (Royle) TS Ying. PLoS ONE 2015, 10, e0122981. [Google Scholar]
- Dong, J.; Ma, X.; Wei, Q.; Peng, S.; Zhang, S. Effects of growing location on the contents of secondary metabolites in the leaves of four selected superior clones of Eucommia ulmoides. Ind. Crops Prod. 2011, 34, 1607–1614. [Google Scholar] [CrossRef]
- Swarna, J.; Ravindhran, R. Pharmacognostical and phytochemical evaluation of Talinum triangulare (Jacq.) Willd. Int. J. Pharm. Pharm. Sci. 2013, 5, 1–8. [Google Scholar]
- Nagarani, G.; Abirami, A.; Siddhuraju, P. A comparative study on antioxidant potentials, inhibitory activities against key enzymes related to metabolic syndrome, and anti-inflammatory activity of leaf extract from different Momordica species. Food Sci. Hum. Wellness 2014, 3, 36–46. [Google Scholar] [CrossRef]
- Kushwaha, P.S.; Singh, A.K.; Keshari, A.K.; Maity, S.; Saha, S. An Updated Review on the Phytochemistry, Pharmacology, and Clinical Trials of Salacia oblonga. Pharmacogn. Rev. 2016, 10, 109–114. [Google Scholar] [PubMed]
- Bhat, B.M.; Raghuveer, C.V.; D’Souza, V.; Manjrekar, P.A. Antidiabetic and hypolipidemic effect of salacia oblonga in streptozotocin induced diabetic rats. J. Clin. Diagn. Res. 2012, 6, 1685–1687. [Google Scholar]
- Leach, M.J.; Lauche, R.; Zhang, A.L.; Cramer, H.; Adams, J.; Langhorst, J.; Dobos, G. Characteristics of herbal medicine users among internal medicine patients: A cross-sectional analysis. J. Herb. Med. 2017, 10, 59–63. [Google Scholar] [CrossRef]
- El-Demerdash, F.; Yousef, M.I.; El-Naga, N.A. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem. Toxicol. 2005, 43, 57–63. [Google Scholar] [CrossRef]
| Phytochemical | SREE | SRET | SREK |
|---|---|---|---|
| Phenols | ++ | ++ | + |
| Flavonoids | + | + | + |
| Alkaloids | ++ | + | + |
| Saponins | ++ | ++ | + |
| Glycosides | − | − | − |
| Steroids | + | ++ | + |
| Terpenoids | + | ++ | + |
| Plant Extract (250 µg/mL) | Total Phenols (µg GAE/250 µg Extract) | Total Flavonoids (µg QE/250 µg Extract) |
|---|---|---|
| SREE | 78.9 ± 2.1 | 83.2 ± 3.5 |
| SRET | 42.1 ± 2.7 | 50.9 ± 3.3 |
| SREK | 38.6 ± 2.7 | 48.8 ± 3.8 |
| IC50 Values of Carbohydrate-Hydrolyzing Enzymes Inhibition Activities (µg/mL) | ||
|---|---|---|
| Plant Extract | α-Glucosidase Inhibition | α-Amylase Inhibition |
| SREE | 147.3 ± 2.3 * | 193.7 ± 1.6 * |
| SRET | 764.5 ± 4.2 * | 199.4 ± 2.3 * |
| SREK | 427.0 ± 2.3 * | 189.6 ± 2.5 * |
| Acarbose | 141.7 ± 1.8 | 169.9 ± 1.9 |
| Concentration of Random Blood Glucose (mg/dL) | |||
| 0th day | 7th day | 14th day | |
| NC | 69.7 ± 6.9 | 79.1 ± 10.3 | 80.3 ± 6.6 |
| DC | 385.2 ± 17.4 | 379.6 ± 12.2 | 369.5 ± 40.9 |
| Std | 302.4 ± 37.4 ** | 94.6 ± 26.7 *** | 90.8 ± 29.9 *** |
| A | 315.5 ± 45.1 * | 169.2 ± 34.3 *** | 122.8 ± 24.4 *** |
| B | 346.2 ± 25.7 * | 166.8 ± 29.8 *** | 150.4 ± 31.6 *** |
| C | 337.5 ± 64.6 | 123.9 ± 20.5 *** | 129.7 ± 29.3 *** |
| D | 364.4 ± 46.3 | 107.1 ± 20.7 *** | 108.4 ± 11.0 *** |
| Concentration of Postprandial Blood Glucose (mg/dL) | |||
| 0th day | 7th day | 14th day | |
| NC | 106.5 ± 14.5 | 98.1 ± 16.5 | 96.6 ± 21.0 |
| DC | 401.3 ± 15.2 | 388.6 ± 10.0 | 385.3 ± 21.6 |
| Std | 325.4 ± 22.3 * | 101.6 ± 21.0 * | 102.0 ± 27.2 * |
| A | 324.1 ± 37.2 * | 182.4 ± 21.3 * | 136.7 ± 23.2 * |
| B | 349.8 ± 20.4 * | 176.5 ± 24.1 * | 157.2 ± 33.3 * |
| C | 356.8 ± 48.9 *** | 134.5 ± 15.0 * | 137.1 ± 22.5 * |
| D | 370.7 ± 40.0 | 119.7 ± 13.5 * | 114.4 ± 10.3 * |
| Total Cholesterol (mg/dL) | HDL–Cholesterol (mg/dL) | Triglycerides (mg/dL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0th day | 7th day | 14th day | 0th day | 7th day | 14th day | 0th day | 7th day | 14th day | |
| NC | 25.9 ± 3.4 | 42.1 ± 9.1 | 44.6 ± 11.0 | 28.9 ± 3.3 | 31.6 ± 4.8 | 33.3 ± 6.6 | 132.8 ± 14.0 | 124.3 ± 27.5 | 105.1 ± 36.7 |
| DC | 44.8 ± 8.4 | 61.7 ± 8.5 | 64.1 ± 9.0 | 38.5 ± 7.5 | 36.5 ± 11.8 | 34.2 ± 6.5 | 363.2 ± 22.8 | 183.7 ± 56.8 | 162.9 ± 33.2 |
| Std | 39.8 ± 19.6 | 42.5 ± 7.6 ** | 36.3 ± 7.0 ** | 29.3 ± 3.1 | 30.7 ± 3.2 * | 33.2 ± 4.3 | 378.3 ± 32.8 | 128.1 ± 39.5 | 113.7 ± 28.2 |
| A | 38.2 ± 15.6 | 66.6 ± 14.7 | 43.3 ± 10.4 * | 36.7 ± 14.1 | 31.22 ± 3.8 | 33.6 ± 5.4 | 320.8 ± 22.2 | 119.4 ± 37.5 | 102.0 ± 33.2 |
| B | 51.6 ± 4.0 | 43.1 ± 5.7 ** | 41.5 ± 7.2 ** | 32.1 ± 2.9 | 36.9 ± 13.2 | 33.8 ± 5.6 | 389.1 ± 32.3 | 80.1 ± 16.4 * | 82.1 ± 16.4 * |
| C | 44.8 ± 6.5 | 39.6 ± 7.6 ** | 38.6 ± 4.3 ** | 30.4 ± 7.4 | 35.8 ± 10.3 | 33.9 ± 6.7 ** | 331.2 ± 49.6 | 164.5 ± 43.7 | 79.9 ± 6.9 * |
| D | 42.8 ± 17.0 | 44.0 ± 21.0 | 34.3 ± 9.2 ** | 34.8 ± 5.7 | 37.6 ± 12.9 | 40.7 ± 7.9 | 385.3 ± 22.1 | 129.8 ± 31.0 | 75.9 ± 5.6 * |
| Creatinine (mg/dL) | Bilirubin (mg/dL) | |||||
|---|---|---|---|---|---|---|
| 0th day | 7th day | 14th day | 0th day | 7th day | 14th day | |
| NC | 0.47 ± 0.1 | 0.54 ± 0.0 | 0.54 ± 0.1 | 0.51 ± 0.03 | 0.57 ± 0.0 | 0.75 ± 0.1 *** |
| DC | 0.42 ± 0.1 | 1.04 ± 0.2 | 0.84 ± 0.2 | 0.52 ± 0.04 | 1.74 ± 0.1 | 1.16 ± 0.1 |
| Std | 0.44 ± 0.1 | 0.73 ± 0.1 * | 0.64 ± 0.1 * | 0.54 ± 0.06 | 0.84 ± 0.1 * | 0.71 ± 0.0 *** |
| A | 0.54 ± 0.1 | 0.59 ± 0.1 * | 0.55 ± 0.1 * | 0.51 ± 0.05 | 1.10 ± 0.1 | 0.84 ± 0.1 *** |
| B | 0.45 ± 0.0 | 0.81 ± 0.1 * | 0.61 ± 0.1 * | 0.53 ± 0.04 | 0.84 ± 0.1 ** | 0.8 ± 0.0 *** |
| C | 0.47 ± 0.1 | 0.60 ± 0.1 * | 0.50 ± 0.1 * | 0.49 ± 0.03 | 0.9 ± 0.2 * | 0.73 ± 0.1*** |
| D | 0.48 ± 0.1 | 0.58 ± 0.1 * | 0.55 ± 0.1 * | 0.49 ± 0.03 | 0.71 ± 0.1 *** | 0.62 ± 0.0 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deepak, K.G.K.; Challa, S.; Suhasin, G.; Nagesewara Rao Reddy, N.; Elansary, H.O.; El-Ansary, D.O. Antidiabetic and Antilipidemic Activity of Root Extracts of Salacia oblonga against Streptozotocin-Induced Diabetes in Wistar Rats. Processes 2020, 8, 301. https://doi.org/10.3390/pr8030301
Deepak KGK, Challa S, Suhasin G, Nagesewara Rao Reddy N, Elansary HO, El-Ansary DO. Antidiabetic and Antilipidemic Activity of Root Extracts of Salacia oblonga against Streptozotocin-Induced Diabetes in Wistar Rats. Processes. 2020; 8(3):301. https://doi.org/10.3390/pr8030301
Chicago/Turabian StyleDeepak, Kakara Gift Kumar, Surekha Challa, Ganta Suhasin, Neelapu Nagesewara Rao Reddy, Hosam O. Elansary, and Diaa O. El-Ansary. 2020. "Antidiabetic and Antilipidemic Activity of Root Extracts of Salacia oblonga against Streptozotocin-Induced Diabetes in Wistar Rats" Processes 8, no. 3: 301. https://doi.org/10.3390/pr8030301
APA StyleDeepak, K. G. K., Challa, S., Suhasin, G., Nagesewara Rao Reddy, N., Elansary, H. O., & El-Ansary, D. O. (2020). Antidiabetic and Antilipidemic Activity of Root Extracts of Salacia oblonga against Streptozotocin-Induced Diabetes in Wistar Rats. Processes, 8(3), 301. https://doi.org/10.3390/pr8030301





