Metabolomic Profiling and Antioxidant, Anticancer and Antimicrobial Activities of Hyphaene thebaica
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Phytochemical Analysis
2.2.1. Determination of Carbohydrates
2.2.2. Determination of Anthocyanins
2.2.3. Determination of Secondary Metabolites
Determination of Saponins
Determination of Total Phenolics Content
Determination of Flavonoids
Determination of Flavonols
Determination of Total Condensed Tannins
2.3. Determination of Total Antioxidant Capacity (TAC)
2.4. 2,2-Diphenyl-1-Picryl-Hydrazyl (DPPH) Free Radical Scavenging Activity Assay
2.5. Analysis of Polyphenolics Using High-Performance Liquid Chromatography (HPLC)
2.6. Assay of the In Vitro Anticancer Activity of H. thebaica Extracts
2.7. Assay of the Antimicrobial Activities of H. thebaica Extracts
2.8. Statistical Analysis
3. Results
3.1. Phytochemical Composition and Antioxidant Activity of H. thebaica
3.2. Profiling of Polyphenols in H. thebaica Using HPLC
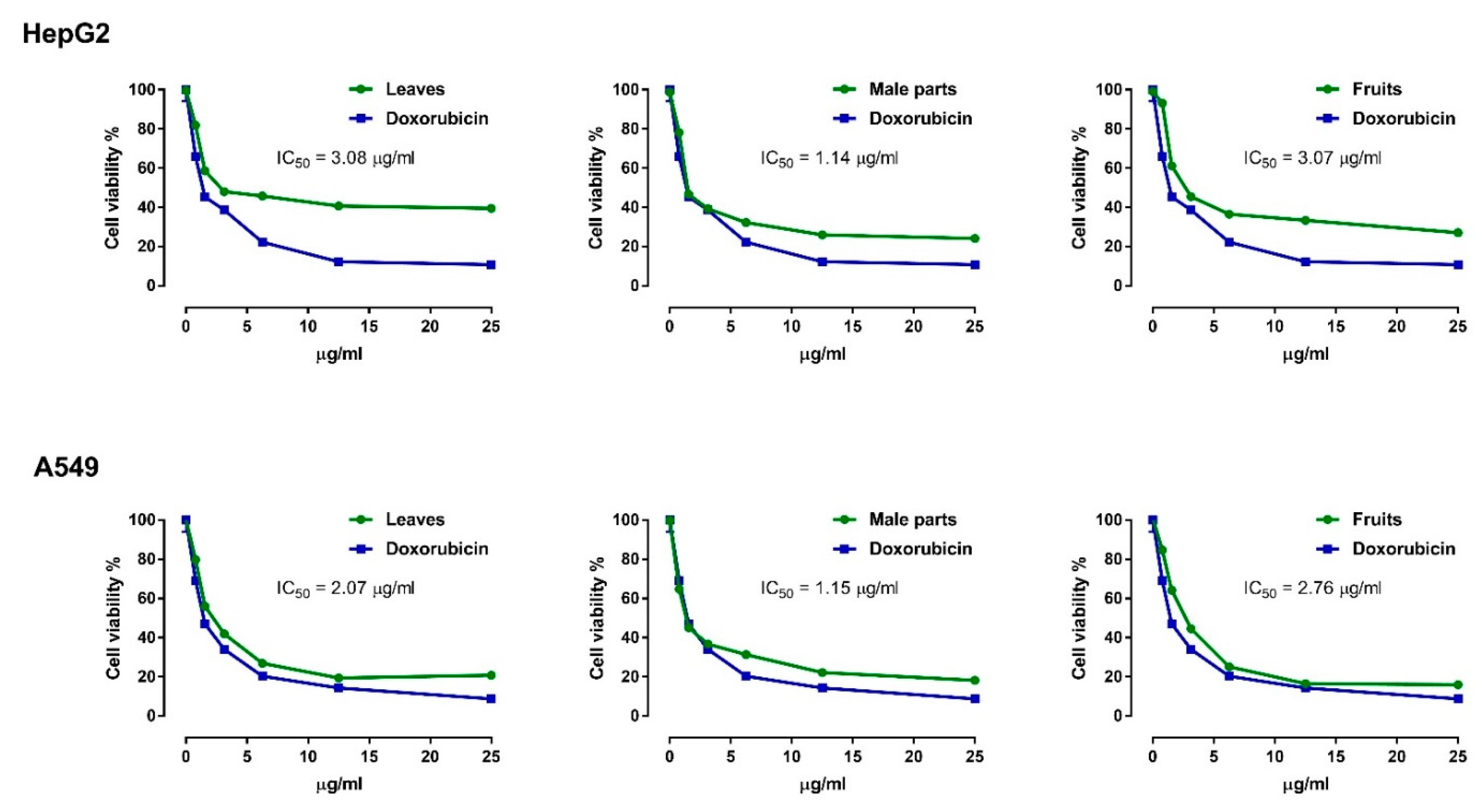
3.3. Anticancer Activity of H. thebaica
3.4. Antimicrobial Activity of H. thebaica
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahmoud, A.M.; Alexander, M.Y.; Tutar, Y.; Wilkinson, F.L.; Venditti, A. Oxidative stress in metabolic disorders and drug-induced injury: The potential role of nrf2 and ppars activators. Oxidative Med. Cell. Longev. 2017, 2017, 2508909. [Google Scholar] [CrossRef]
- Lindenschmidt, R.C.; Tryka, A.F.; Goad, M.E.; Witschi, H.P. The effects of dietary butylated hydroxytoluene on liver and colon tumor development in mice. Toxicology 1986, 38, 151–160. [Google Scholar] [CrossRef]
- Kruse, M.; Strandberg, M.; Strandberg, B. Ecological Effects of Allelopathic Plants—A Review; Technical Report No. 31; National Environmental Research Institute: Silkeborg, Denmark, 2000; p. 66. [Google Scholar]
- Olajuyigbe, O.O.; Afolayan, A.J. Phytochemical assessment and antioxidant activities of alcoholic and aqueous extracts of acacia mearnsii de wild. Int. J. Pharmacol. 2011, 7, 856–861. [Google Scholar]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Langley-Evans, S.C. Antioxidant potential of green and black tea determined using the ferric reducing power (frap) assay. Int. J. Food Sci. Nutr. 2000, 51, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Kamel, E.M.; Mahmoud, A.M.; Ahmed, S.A.; Lamsabhi, A.M. A phytochemical and computational study on flavonoids isolated from trifolium resupinatum l. and their novel hepatoprotective activity. Food Funct. 2016, 7, 2094–2106. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.; Coupar, I.M.; Ng, K. Antioxidant activity of hot water extract from the fruit of the doum palm, hyphaene thebaica. Food Chem. 2006, 98, 317–328. [Google Scholar] [CrossRef]
- Salib, J.Y.; Michael, H.N.; Eskande, E.F. Anti-diabetic properties of flavonoid compounds isolated from hyphaene thebaica epicarp on alloxan induced diabetic rats. Pharmacogn. Res. 2013, 5, 22–29. [Google Scholar] [CrossRef]
- AbdEl-Moniem, M.; Mustafa, H.N.; Megahed, H.A.; Agaibyi, M.H.; Hegazy, G.A.; El-Dabaa, M.A. The ameliorative potential of hyphaene thebaica on streptozotocin-induced diabetic nephropathy. Folia Morphol. 2015, 74, 447–457. [Google Scholar] [CrossRef][Green Version]
- Tohamy, A.A.; Mohammed, R.S.; Abdalla, M.S.; Ibrahim, A.K.; Mahran, K.F.; Ahmed, K.A. The effect of lupinus albus and hyphaene thebaica on chromosomal aberrations and histopathological changes of liver and pancreas in streptozotocin-induced diabetic rats. Egypt. J. Hosp. Med. 2013, 53, 763–769. [Google Scholar] [CrossRef]
- Hetta, M.H.; Yassin, N.Z. Comparative studies on hypocholesterolemic effect of different fractions of hyphaene thebaica (doum) in experimental animals. Die Pharm. 2006, 61, 230–232. [Google Scholar]
- Aboshora, W.; Lianfu, Z.; Dahir, M.; Qingran, M.; Qingrui, S.; Jing, L.; Al-Haj, N.Q.M.; Ammar, A.F.; Lianfu, Z.; Aboshora, W.; et al. Effect of extraction method and solvent power on polyphenol and flavonoid levels in hyphaene thebaica l mart (arecaceae) (doum) fruit, and its antioxidant and antibacterial activities. Trop. J. Pharm. Res. 2014, 13, 2057–2063. [Google Scholar] [CrossRef]
- Morris, D.L. Quantitative determination of carbohydrates with dreywood’s anthrone reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: London, UK, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Kumaran, A.; Joel Karunakaran, R. In vitro antioxidant activities of methanol extracts of five phyllanthus species from india. LWT Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Kim, K.-H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine b colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Abdel-Farid, I.B.; Sheded, M.G.; Mohamed, E.A. Metabolomic profiling and antioxidant activity of some acacia species. Saudi J. Biol. Sci. 2014, 21, 400–408. [Google Scholar] [CrossRef]
- Mansour, R.B.; Jilani, I.B.H.; Bouaziz, M.; Gargouri, B.; Elloumi, N.; Attia, H.; Ghrabi-Gammar, Z.; Lassoued, S. Phenolic contents and antioxidant activity of ethanolic extract of capparis spinosa. Cytotechnology 2016, 68, 135–142. [Google Scholar] [CrossRef]
- Kicel, A.; Olszewska, M.A. Evaluation of antioxidant activity, and quantitative estimation of flavonoids, saponins and phenols in crude extract and dry fractions of medicago lupulina aerial parts. Nat. Prod. Commun. 2015, 10, 483–486. [Google Scholar] [PubMed]
- Diaconeasa, Z.; Leopold, L.; Rugina, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Milbradt, R. Skin anti-inflammatory activity of apigenin-7-glucoside in rats. Arzneim. Forsch. 1993, 43, 370–372. [Google Scholar]
- Abdel-Farid, I.B.; Mahalel, U.A.; Jahangir, M.; Elgebaly, H.A.; El-Naggar, S.A. Metabolomic profiling and antioxidant activity of opophytum forsskalii. Aljouf Sci. Eng. J. 2016, 286, 1–6. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Hirun, S.; Chuen, T.L.K.; Goldsmith, C.D.; Murchie, S.; Bowyer, M.C.; Phillips, P.A.; Scarlett, C.J. Antioxidant and anticancer capacity of saponin-enriched carica papaya leaf extracts. Int. J. Food Sci. Technol. 2015, 50, 169–177. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, C.; Guigas, C.; Ma, Y.; Corrales, M.; Tauscher, B.; Hu, X. Composition, antimicrobial activity, and antiproliferative capacity of anthocyanin extracts of purple corn (Zea mays L.) from China. Eur. Food Res. Technol. 2009, 228, 759–765. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Abdella, E.M.; El-Derby, A.M. Protective effects of turbinaria ornata and padina pavonia against azoxymethane-induced colon carcinogenesis through modulation of ppar gamma, nf-kappab and oxidative stress. Phytother. Res. 2015, 29, 737–748. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Mohammed, H.M.; Khadrawy, S.M.; Galaly, S.R. Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of nrf2/are/ho-1, ppargamma and tgf-beta1/smad3 signaling, and amelioration of oxidative stress and inflammation. Chem. Biol. Interact. 2017, 277, 146–158. [Google Scholar] [CrossRef]
- Nepka, C.; Asprodini, E.; Kouretas, D. Tannins, xenobiotic metabolism and cancer chemoprevention in experimental animals. Eur. J. Drug Metab. Pharmacokinet. 1999, 24, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Nagesh, P.K.B.; Chowdhury, P.; Hatami, E.; Jain, S.; Dan, N.; Kashyap, V.K.; Chauhan, S.C.; Jaggi, M.; Yallapu, M.M. Tannic acid inhibits lipid metabolism and induce ros in prostate cancer cells. Sci. Rep. 2020, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Hatami, E.; Nagesh, P.K.B.; Chowdhury, P.; Shetty, A.B.; Tripathi, M.K.; Chauhan, S.; Jaggi, M.; Yallapu, M. Abstract 1871: Tannic acid: A natural anticancer agent for non-small cell lung cancer. Cancer Res. 2019, 79, 1871. [Google Scholar]
- Chandra, Y.P.; Viswanathswamy, A.H.M. Chemo preventive effect of rutin against n-nitrosodiethylamine-induced and phenobarbital-promoted hepatocellular carcinoma in wistar rats. Indian J. Pharm. Educ. Res. 2018, 52, 78–86. [Google Scholar] [CrossRef]
- Yamagata, K.; Izawa, Y.; Onodera, D.; Tagami, M. Chlorogenic acid regulates apoptosis and stem cell marker-related gene expression in a549 human lung cancer cells. Mol. Cell. Biochem. 2018, 441, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Gull, T.; Sultana, B.; Bhatti, I.A.; Jamil, A. Antibacterial potential of capparis spinosa and capparis decidua extracts. Int. J. Agric. Biol. 2015, 17, 727–733. [Google Scholar] [CrossRef]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as antimicrobial agents of natural plant origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.F.R.; Froufe, H.J.C.; Abreu, R.M.V.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, sar analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Nayaka, H.B.; Londonkar, R.L.; Umesh, M.K.; Tukappa, A. Antibacterial attributes of apigenin, isolated from Portulaca oleracea L. Int. J. Bacteriol. Int. J. Bacteriol. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]

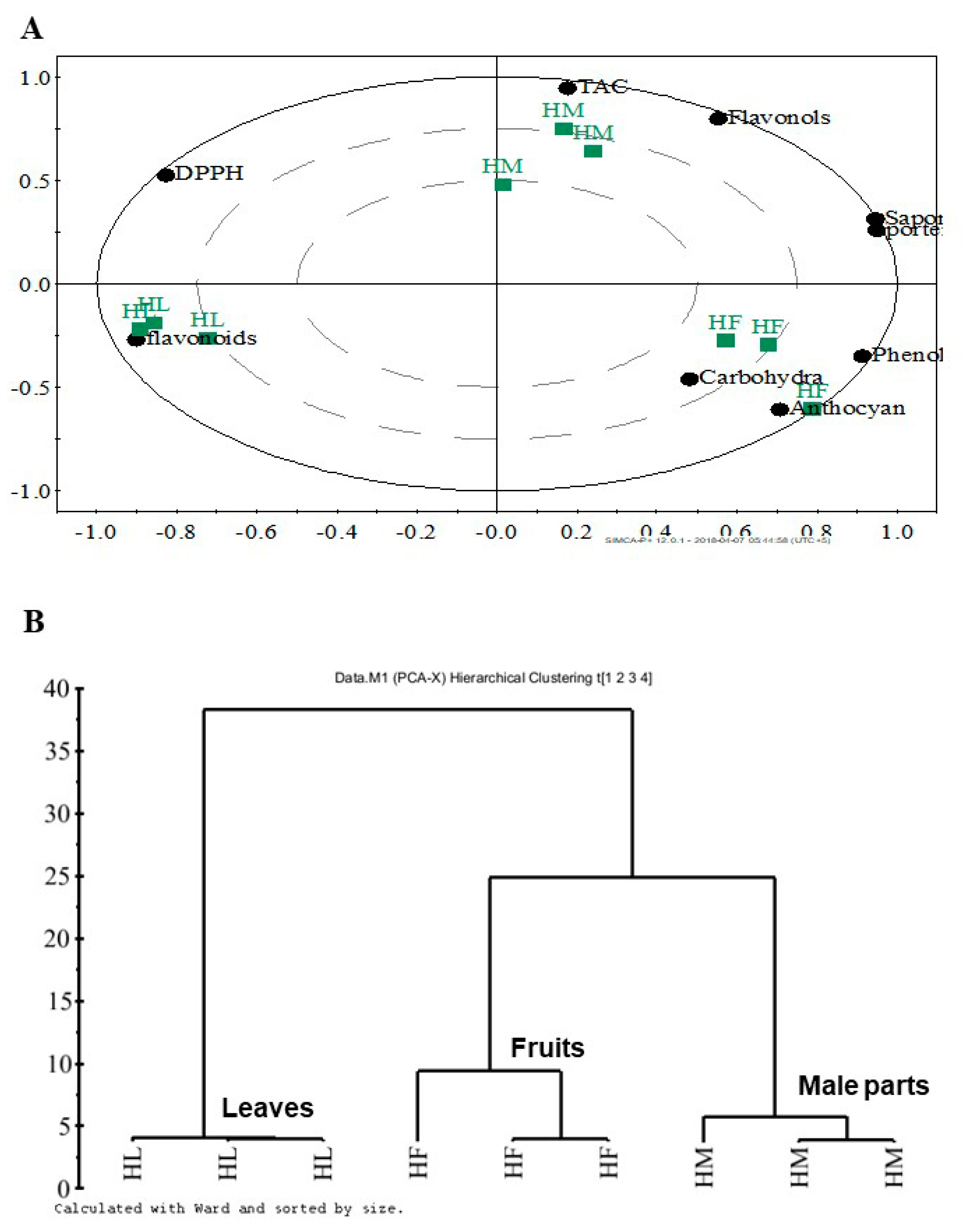

| Leaves | Male parts | Fruits | |
|---|---|---|---|
| Carbohydrates (mg/g DW) | 135.4 ± 0.18 | 124.4 ± 0.5 | 173.77 ± 0.4 |
| Saponins (mg saponins equivalent/g) | 17.5 ± 0.1 a | 18.6 ± 0.1 b | 18.65 ± 0.04 b |
| Phenolics (mg/gallic acid equivalent/g) | 20.6 ± 2.9 a | 36.04 ± 0.06 b | 72.3 ± 2.9 c |
| Flavonoids (mg quercetin equivalent/g) | 32.7 ± 2.9 a | 22.1 ± 2.4 b | 20.4 ± 2.0 b |
| Flavonols (mg quercetin equivalent/g) | 61.29 ± 3.51 a | 61.23 ± 3.56 a | 38.2 ± 4.5 b |
| Anthocyanin (μmol/g) | 0.11 ± 0.05 a | 0.12 ± 0.09 a | 0.33 ± 0.06 b |
| Tannins (mg catechols equivalent/g) | 0.57 ± 0.03 a | 3.85 ± 0.3 b | 3.44 ± 0.05 b |
| Correlation Coefficient (r) | Probability (p) | |
|---|---|---|
| Carbohydrates | 0.904 | 0.03 |
| Saponins | - | - |
| Phenolics | 0.957 | 0.003 |
| Flavonoids | - | - |
| Flavonols | 0.935 | 0.006 |
| Anthocyanin | - | - |
| Tannins | - | - |
| Leaves | Male Parts | Fruits | |
|---|---|---|---|
| Protocatechuic acid | 0.072 | 0.463 | ND |
| p-hydroxybenzoic acid | 0.126 | 0.275 | ND |
| Catechins | 0.56 | ND | ND |
| Chlorogenic acid | 0.085 | 0.08 | 0.152 |
| Caffeic acid | 0.078 | 0.132 | ND |
| Syringic acid | 0.037 | 0.049 | ND |
| Vanillic acid | 0.555 | 1.081 | ND |
| Ferulic acid | 0.034 | 0.03 | ND |
| Sinapic acid | 0.052 | 0.065 | ND |
| Rutin | 2.695 | 0.324 | 0.073 |
| p-coumaric acid | 0.116 | 0.137 | 0.062 |
| Apigenin-7-glucoside | 5.428 | ND | 0.169 |
| Rosmarinic acid | 0.054 | 0.544 | ND |
| Chrysin | ND | 0.083 | ND |
| Zone of Inhibition (mm) | |||||
|---|---|---|---|---|---|
| Control | Leaves | Male Parts | Fruits | ||
| Gram +ve bacteria | Bacillus subtilis | 28.0 ± 2.0 | 12.3 ± 1.1 | 10.0 ± 0.1 | 10.7 ± 0.5 |
| Staphylococcus aureus | 31.0 ± 6.5 | 13.3 ± 1.5 | 12.3 ± 0.5 | 12.3 ± 1.1 | |
| Gram −ve bacteria | Pseudomonas aeruginosa | 32.4 ± 1.1 | 13.3 ± 0.5 | 10.3 ± 0.5 | 11.3 ± 0.5 |
| Escherichia coli | 29.0 ± 1.7 | 13.7 ± 0.5 | 10.0 ± 0.0 | 12.3 ± 1.0 | |
| Fungi | Aspergillus flavus | 14.6 ± 1.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Candida albicans | 16.3 ± 1.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 9.0 ± 0.0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, G.A.; Abdel-Farid, I.B.; Elgebaly, H.A.; Mahalel, U.A.; Sheded, M.G.; Bin-Jumah, M.; Mahmoud, A.M. Metabolomic Profiling and Antioxidant, Anticancer and Antimicrobial Activities of Hyphaene thebaica. Processes 2020, 8, 266. https://doi.org/10.3390/pr8030266
Taha GA, Abdel-Farid IB, Elgebaly HA, Mahalel UA, Sheded MG, Bin-Jumah M, Mahmoud AM. Metabolomic Profiling and Antioxidant, Anticancer and Antimicrobial Activities of Hyphaene thebaica. Processes. 2020; 8(3):266. https://doi.org/10.3390/pr8030266
Chicago/Turabian StyleTaha, Ghada A., Ibrahim B. Abdel-Farid, Hassan A. Elgebaly, Usama A. Mahalel, Mohamed G. Sheded, May Bin-Jumah, and Ayman M. Mahmoud. 2020. "Metabolomic Profiling and Antioxidant, Anticancer and Antimicrobial Activities of Hyphaene thebaica" Processes 8, no. 3: 266. https://doi.org/10.3390/pr8030266
APA StyleTaha, G. A., Abdel-Farid, I. B., Elgebaly, H. A., Mahalel, U. A., Sheded, M. G., Bin-Jumah, M., & Mahmoud, A. M. (2020). Metabolomic Profiling and Antioxidant, Anticancer and Antimicrobial Activities of Hyphaene thebaica. Processes, 8(3), 266. https://doi.org/10.3390/pr8030266







