Evolution of Phase Composition and Antibacterial Activity of Zr–C Thin Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Coating Deposition
2.2. Structural and Compositional Characterisation
2.3. Mechanical Evaluation
2.4. Microbiological Tests
2.5. The Wettability of Coating Surface and Corrosion Behavior
3. Results
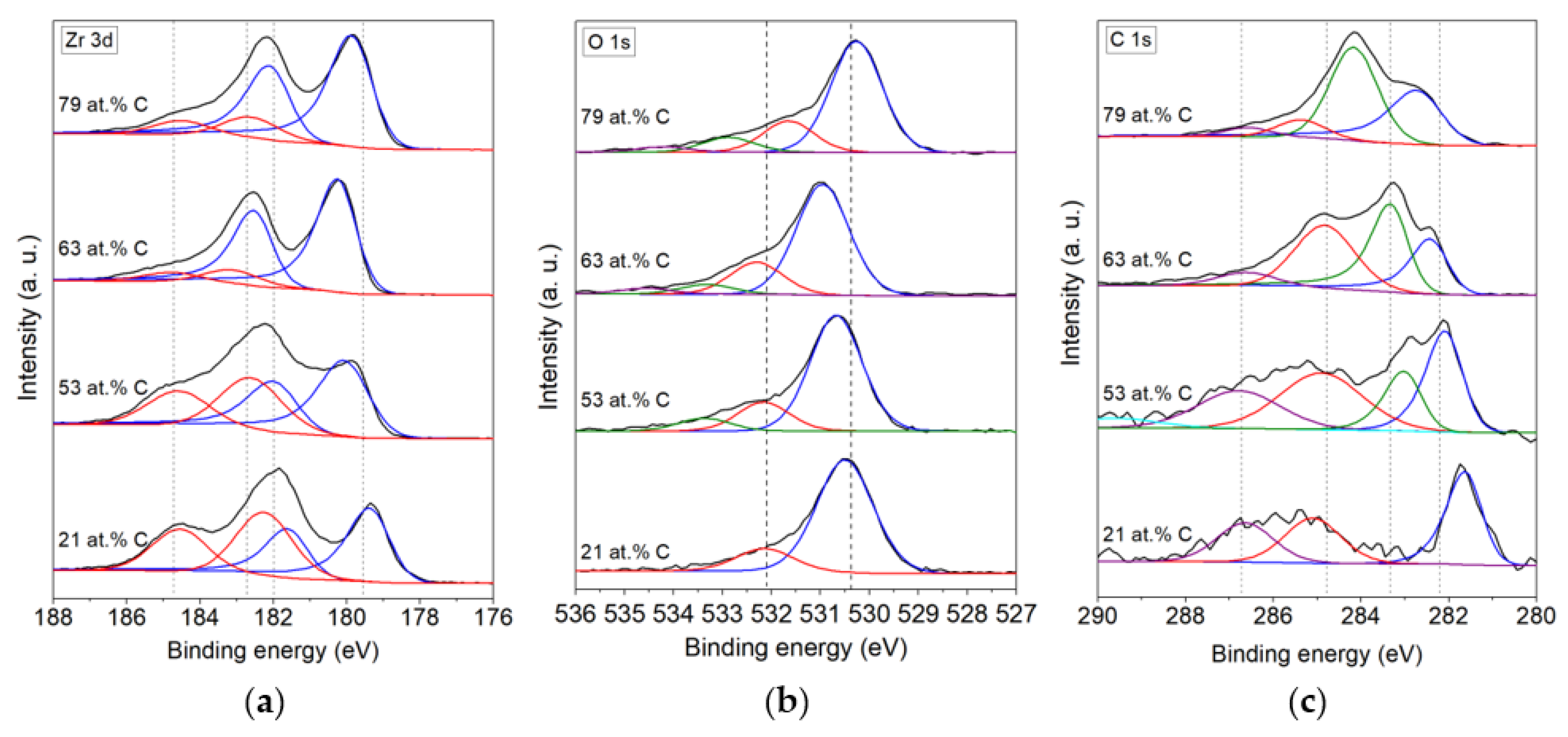
3.1. Chemical Composition
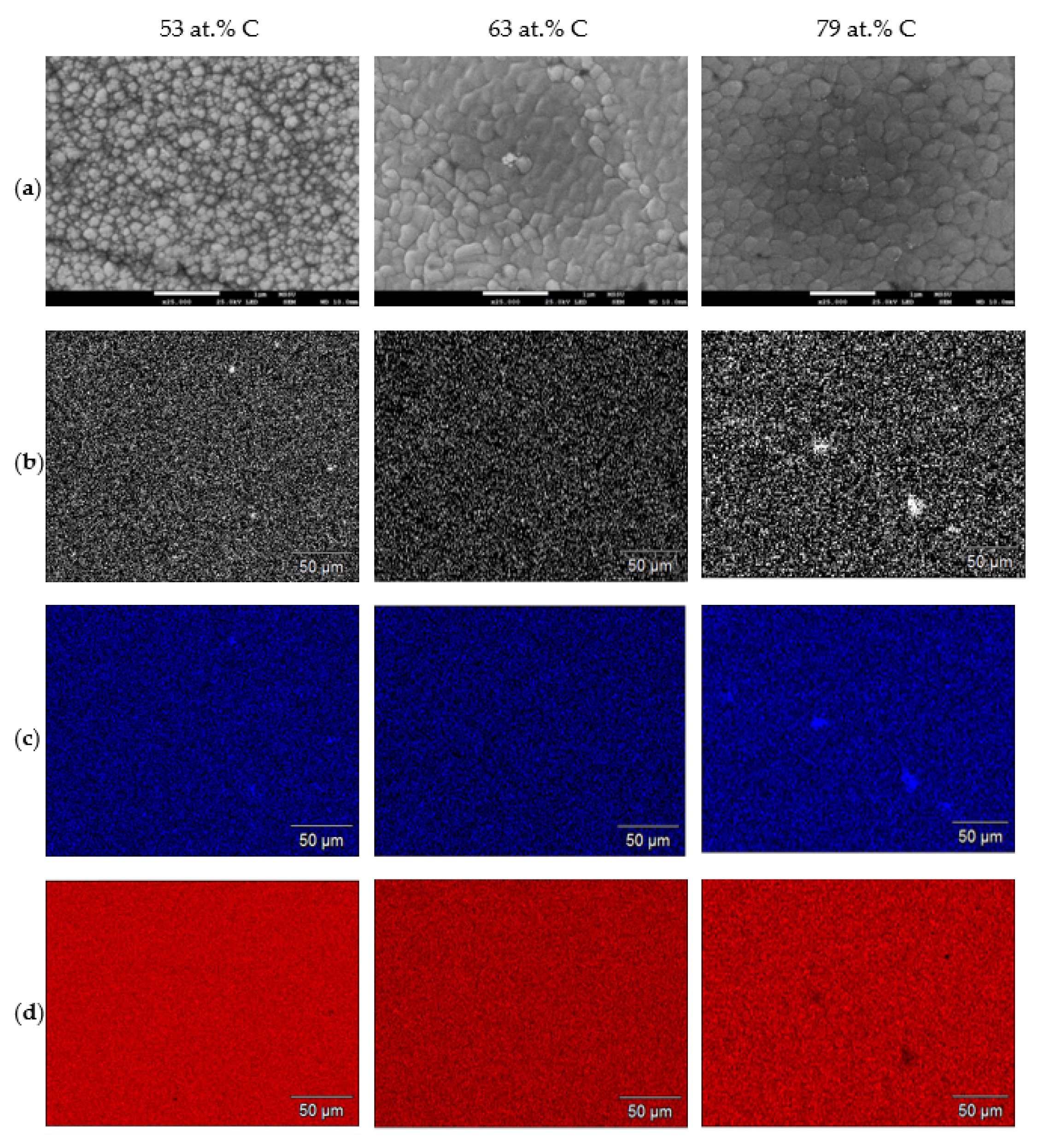
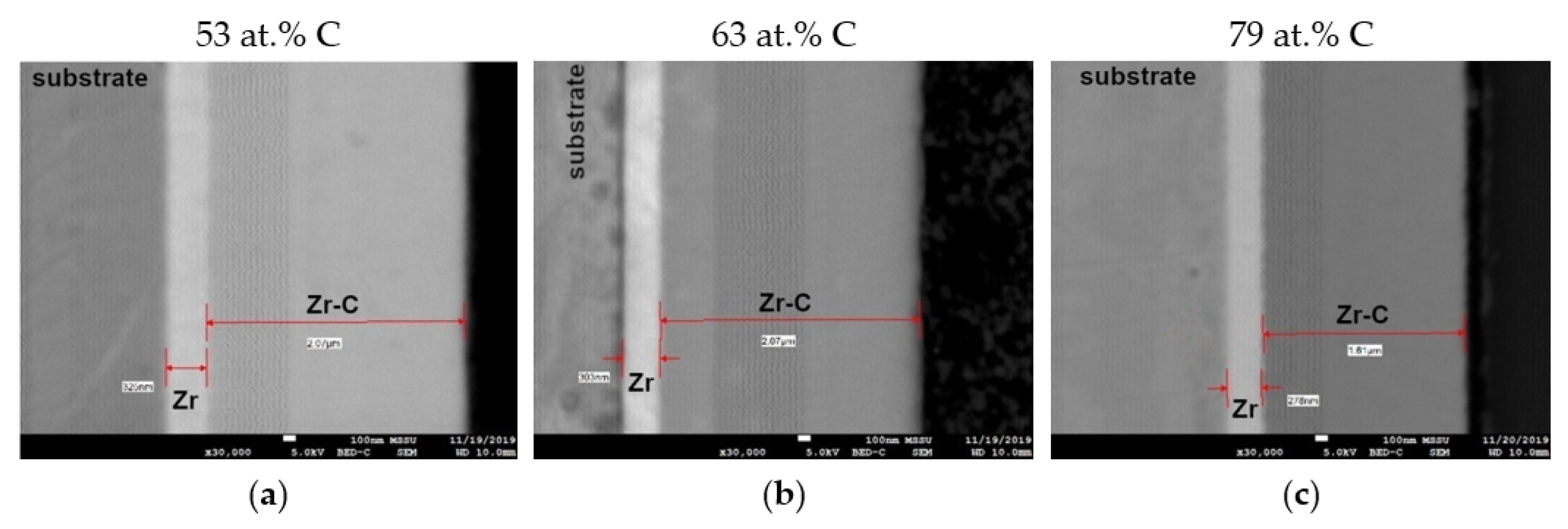
3.2. Coating Morphology
3.3. Phase Composition
3.3.1. X-ray Diffraction
3.3.2. Raman Spectroscopy
3.4. Mechanical Properties
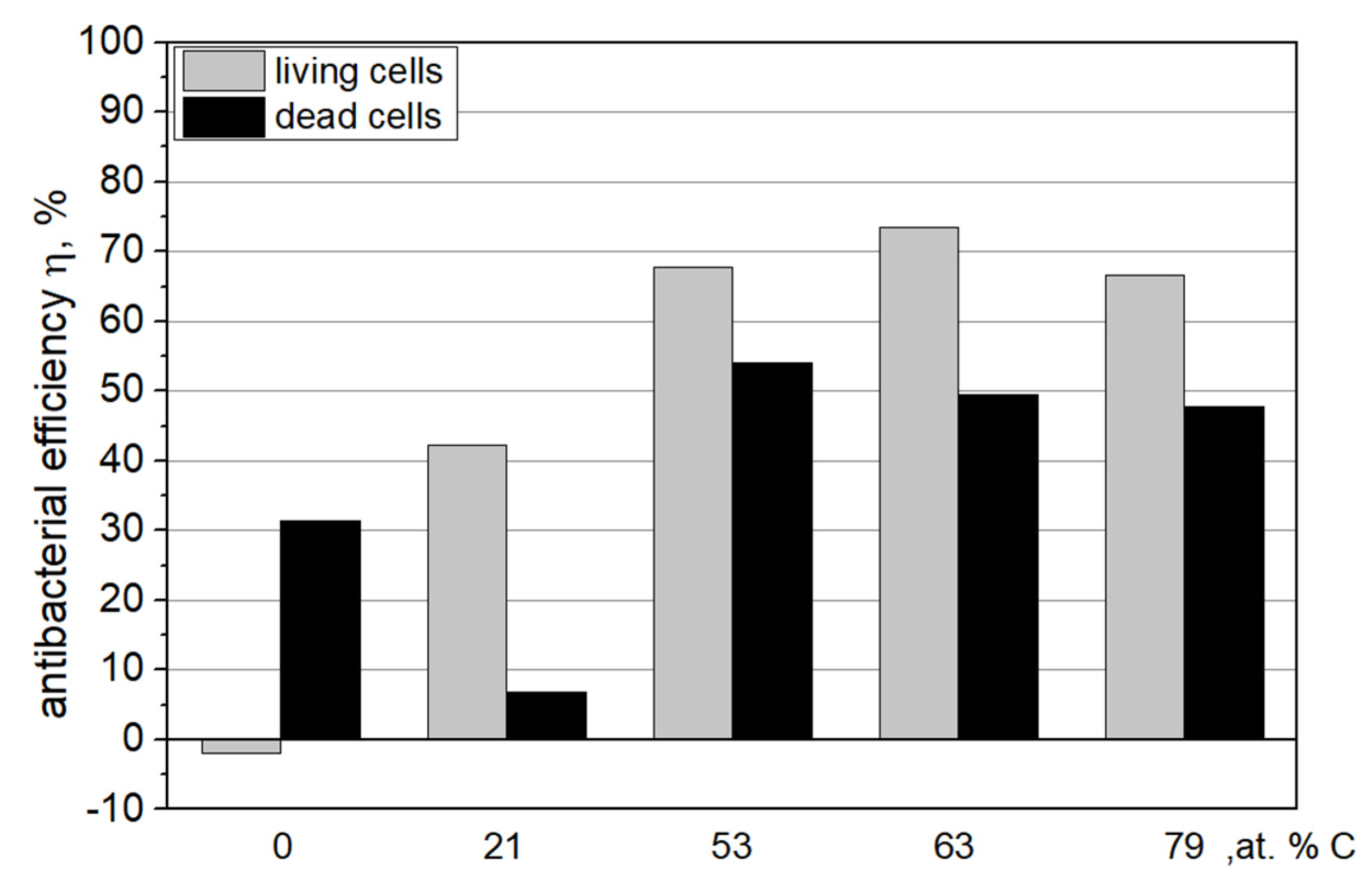
3.5. Antibacterial Properties
3.5.1. Static Zone of Inhibition
3.5.2. Numbers of Live and Dead Cells
3.5.3. Contact Angle
3.6. Corrosion Properties
4. Discussion
5. Conclusions
- It has been shown that as the concentration of carbon atoms in the coating increases, the content of the phase of carbon unbound to zirconium increases. Raman spectroscopy showed that carbon phase has an amorphous structure with a predominance of bonds with sp2 hybridization.
- Coatings with carbon concentrations close to 50 at.% show the highest hardness and Young’s modulus. At higher and lower concentrations, there is a sharp decrease in these values.
- Considering the contact angle as a measure of hydrophobicity and at the same time as an indirect assessment of potential antibacterial properties, it can be generally stated that surfaces coated with Zr–C coatings have better antibacterial properties than surfaces of uncoated 304L steel.
- There is a rapid increase in antibacterial properties when the carbon concentration in Zr–C coatings exceeds 50 at.%. Above this carbon concentration in the coatings, practically no significant differences occur in the bacteriostatic tests as well as resistance to bacterial adhesion and colonization.
- Corrosion tests showed the high anti-pitting potential of Zr–C coatings in the environment of artificial saliva.
- The wide possibility of shaping the mechanical properties of Zr–C coatings in combination with relatively good antibacterial properties after exceeding 50 at.% C concentration in coatings and high protective potential of these coatings makes them a good candidate for orthodontic applications.
Author Contributions
Funding
Conflicts of Interest
References
- Sharan, D. The problem of corrosion in orthopaedic implant materials. Orthop. Update (India) 1999, 9, 1–5. [Google Scholar]
- Shih, C.C.; Shih, C.M.; Su, Y.Y.; Su, L.H.J.; Chang, M.S.; Lin, S.J. Effect of surface oxide properties on corrosion resistance of 316L stainless steel for biomedical applications. Corros. Sci. 2004, 46, 427–441. [Google Scholar] [CrossRef]
- Becker, W.; Becker, B.E.; Newman, M.G.; Nyman, S. Clinical and microbiologic findings that may contribute to dental implant failure. Int. J. Oral Maxillofac. Implant. 1990, 5, 1–17. [Google Scholar]
- Ding, M.H.; Zhang, H.S.; Zhang, C.; Jin, X. Characterization of ZrC coatings deposited on biomedical 316L stainless steel by magnetron sputtering method. Surf. Coat. Tech. 2013, 224, 34–41. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, L.; Ogino, A.; Nagatsu, M. Investigation into the antibacterial property of carbon films. Diam. Relat. Mater. 2008, 17, 1416–1419. [Google Scholar] [CrossRef]
- Yao, S.H.; Su, Y.L.; Lai, Y.C. Antibacterial and tribological performance of carbonitride coatings doped with W, Ti, Zr, or Cr deposited on AISI 316L stainless steel. Materials 2017, 10, 1189. [Google Scholar] [CrossRef]
- Größner-Schreiber, B.; Herzog, M.; Hedderich, J.; Dück, A.; Hannig, M.; Griepentrog, M. Focal adhesion contact formation by fibroblasts cultured on surface-modified dental implants: An in vitro study. Clin. Oral Implant. Res. 2006, 17, 736–745. [Google Scholar] [CrossRef]
- Groessner-Schreiber, B.; Hannig, M.; Dück, A.; Griepentrog, M.; Wenderoth, D.F. Do different implant surfaces exposed in the oral cavity of humans show different biofilm compositions and activities? Eur. J. Oral Sci. 2004, 112, 516–522. [Google Scholar] [CrossRef]
- Muzio, G.; Miola, M.; Perero, S.; Oraldi, M.; Maggiora, M.; Ferraris, S.; Vernè, E.; Festa, V.; Festa, F.; Canuto, R.A.; et al. Polypropylene prostheses coated with silver nanoclusters/silica coating obtained by sputtering: Biocompatibility and antibacterial properties. Surf. Coat. Tech. 2017, 319, 326–334. [Google Scholar] [CrossRef]
- Marciano, F.R.; Bonetti, L.F.; Mangolin, J.F.; Da-Silva, N.S.; Corat, E.J.; Trava-Airoldi, V.J. Investigation into the antibacterial property and bacterial adhesion of diamond-like carbon films. Vacuum 2011, 85, 662–666. [Google Scholar] [CrossRef]
- Lai, C.H.; Chang, Y.Y.; Huang, H.L.; Kao, H.Y. Characterization and antibacterial performance of ZrCN/amorphous carbon coatings deposited on titanium implants. Thin Solid Films 2011, 520, 1525–1531. [Google Scholar] [CrossRef]
- Janvier, G.; Baquey, C.; Roth, C.; Benillan, N.; Belisle, S.; Hardy, J.F. Extracorporeal circulation, hemocompatibility, and biomaterials. Ann. Thorac. Surg. 1996, 62, 1926–1934. [Google Scholar] [CrossRef]
- Huang, N.; Yang, P.; Leng, Y.X.; Chen, J.Y.; Sun, H.; Wang, J.; Wang, G.J.; Ding, P.D.; Xi, T.F.; Leng, Y. Hemocompatibility of titanium oxide films. Biomaterials 2003, 24, 2177–2187. [Google Scholar] [CrossRef]
- Liu, C.; Lin, G.; Yang, D.; Qi, M. In vitro corrosion behavior of multilayered Ti/TiN coating on biomedical AISI 316L stainless steel. Surf. Coat. Tech. 2006, 200, 4011–4016. [Google Scholar] [CrossRef]
- Hauert, R. An overview on the tribological behavior of diamond-like carbon in technical and medical applications. Tribol. Int. 2004, 37, 991–1003. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.A.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants–a review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Gilewicz, A.; Mydłowska, K.; Ratajski, J.; Szparaga, Ł.; Bartosik, P.; Kochmański, P.; Jędrzejewski, R. Structural, mechanical and tribological properties of ZrC thin films deposited by magnetron sputtering. Vacuum 2019, 169, 108909. [Google Scholar] [CrossRef]
- Szparaga, Ł.; Mydłowska, K.; Gilewicz, A.; Ratajski, J. Mechanical and anti-wear properties of multi-module Cr/CrN coatings. Int. J. Surf. Sci. Eng. 2019, 13, 37–49. [Google Scholar] [CrossRef]
- Lewin, E.; Wilhelmsson, O.; Jansson, U. Nanocomposite nc-TiC∕a-C thin films for electrical contact applications. J. Appl. Phys. 2006, 100, 054303. [Google Scholar] [CrossRef]
- Chen, C.S.; Liu, C.P. Diffusion barrier properties of amorphous ZrCN films for copper metallization. J. Non-Cryst. Solids 2005, 351, 3725–3729. [Google Scholar] [CrossRef]
- Sasaki, M.; Kozukue, Y.; Hashimoto, K.; Takayama, K.; Nakamura, I.; Takano, I.; Sawada, Y. Properties of carbon films with a dose of titanium or zirconium prepared by magnetron sputtering. Surf. Coat. Tech. 2005, 196, 236–240. [Google Scholar] [CrossRef]
- Martínez-Martínez, D.; López-Cartes, C.; Fernández, A.; Sánchez-López, J.C. Influence of the microstructure on the mechanical and tribological behavior of TiC/aC nanocomposite coatings. Thin Solid Films 2009, 517, 1662–1671. [Google Scholar] [CrossRef]
- Liu, B.; Liu, C.; Shao, Y.; Zhu, J.; Yang, B.; Tang, C. Deposition of ZrC-coated particle for HTR with ZrCl4 powder. Nucl. Eng. Des. 2012, 251, 349–353. [Google Scholar] [CrossRef]
- Jansson, U.; Lewin, E. Sputter deposition of transition-metal carbide films—A critical review from a chemical perspective. Thin Solid Films 2013, 536, 1–24. [Google Scholar] [CrossRef]
- Andersson, M.; Urbonaite, S.; Lewin, E.; Jansson, U. Magnetron sputtering of Zr–Si–C thin films. Thin Solid Films 2012, 520, 6375–6381. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Liu, X.L.; Zhang, H.F. Properties of Zr–ZrC–ZrC/DLC gradient films on TiNi alloy by the PIIID technique combined with PECVD. Surf. Coat. Tech. 2008, 202, 3011–3016. [Google Scholar] [CrossRef]
- Chu, C.L.; Ji, H.L.; Yin, L.H.; Pu, Y.P.; Lin, P.H.; Chu, P.K. Fabrication, properties, and cytocompatibility of ZrC film on electropolished NiTi shape memory alloy. Mat. Sci. Eng. C 2011, 31, 423–427. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Huang, H.L.; Lai, C.H.; Hsu, J.T.; Shieh, T.M.; Wu, A.Y.J.; Chen, C.L. Analyses of antibacterial activity and cell compatibility of titanium coated with a Zr–C–N film. PLoS ONE 2013, 8, e56771. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Long, Y.; Javed, A.; Chen, J.; Chen, Z.K.; Xiong, X. Phase composition, microstructure and mechanical properties of ZrC coatings produced by chemical vapor deposition. Ceram. Int. 2014, 40, 707–713. [Google Scholar] [CrossRef]
- Balaceanu, M.; Braic, M.; Braic, V.; Vladescu, A.; Negrila, C.C. Surface chemistry of plasma deposited ZrC hard coatings. J. Optoelectron. Adv. Mater. 2005, 7, 2557–2560. [Google Scholar]
- Lewin, E.; Persson, P.A.; Lattemann, M.; Stüber, M.; Gorgoi, M.; Sandell, A.; Ziebert, C.; Schäfers, F.; Braun, W.; Halbritter, J.; et al. On the origin of a third spectral component of C1s XPS-spectra for nc-TiC/a-C nanocomposite thin films. Surf. Coat. Technol. 2008, 202, 3563–3570. [Google Scholar]
- Meng, Q.N.; Wen, M.; Mao, F.; Nedfors, N.; Jansson, U.; Zheng, W.T. Deposition and characterization of reactive magnetron sputtered zirconium carbide films. Surf. Coat. Technol. 2013, 232, 876–883. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Capano, M.A.; Laube, S.J.P.; Donley, M.S.; Zabinski, J.S. Design of a Ti/TiC/DLC functionally gradient coating based on studies of structural transitions in Ti–C thin films. Thin Solid Films 1997, 298, 107–115. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond–like carbon, and nanodiamond. Philos. Trans. R. Soc. A 2004, 362, 2477–2512. [Google Scholar] [CrossRef]
- Quirynen, M.; Marechal, M.; Busscher, H.J.; Weerkamp, A.H.; Arends, J.; Darius, P.L.; van Steenberghe, D. The influence of surface free-energy on planimetric plaque growth in man. J. Dent. Res. 1989, 68, 796–799. [Google Scholar] [CrossRef]
- Bruinsma, G.M.; Van der Mei, H.C.; Busscher, H.J. Bacterial adhesion to surface hydrophilic and hydrophobic contact lenses. Biomaterials 2001, 22, 3217–3224. [Google Scholar] [CrossRef]
- Wang, J.; Huang, N.; Pan, C.J.; Kwok, S.C.H.; Yang, P.; Leng, Y.X.; Chen, J.Y.; Sun, H.; Wan, G.J.; Liu, Z.Y.; et al. Bacterial repellence from polyethylene terephthalate surface modified by acetylene plasma immersion ion implantation–deposition. Surf. Coat. Tech. 2004, 186, 299–304. [Google Scholar] [CrossRef]
- Liao, T.T.; Zhang, T.F.; Li, S.S.; Deng, Q.Y.; Wu, B.J.; Zhang, Y.Z.; Zhou, Y.J.; Guo, Y.B.; Leng, Y.X.; Huang, N. Biological responses of diamond-like carbon (DLC) films with different structures in biomedical application. Mat. Sci. Eng. C 2016, 69, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Gottenbos, B.; Van der Mei, H.C.; Busscher, H.J. Models for studying initial adhesion and surface growth in biofilm formation on surfaces. Method Enzymol. 1999, 310, 523–534. [Google Scholar]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Hardt, J.I.; Quick, K.L.; Kim-Han, J.S.; Erlanger, B.F.; Huang, T.T.; Epstein, C.J.; Dugan, L.L. A biologically effective fullerene (C60) derivative with superoxide dismutase mimetic properties. Free Radic. Bio. Med. 2004, 37, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.J.; Ashcroft, J.M.; Chen, D.; Min, G.; Kim, C.H.; Murkhejee, B.; Larabell, C.; Keasling, J.D.; Chen, F.F. Charge-associated effects of fullerene derivatives on microbial structural integrity and central metabolism. Nano Lett. 2007, 7, 754–760. [Google Scholar] [CrossRef] [PubMed]









| Compound | KCl | NaCl | CaCl2 × 2H2O | NaH2PO4 × 2H2O | Na2S × 9H2O | Urea |
|---|---|---|---|---|---|---|
| Concentration (g/L) | 0.400 | 0.400 | 0.906 | 0.690 | 0.005 | 1.000 |
| C2H2 Volumetric Flow Rate, sccm | C, at.% | Zr, at.% | O, H and Others, at.% |
|---|---|---|---|
| 0 | ~0.0 | 91.8 | 8.2 |
| 1.5 | 20.7 | 71.6 | 7.7 |
| 3.5 | 52.9 | 43.7 | 3.4 |
| 4.5 | 63.3 | 33.0 | 3.7 |
| 6.5 | 79.0 | 18.9 | 2.1 |
| Zr (3d) | O (1s) | C (1s) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of Coating | Phase | at.% | B.E. (eV) | Phase | at.% | B.E. (eV) | Phase | at.% | B.E. (eV) |
| 21 at.% C | ZrC | 45 | 179.4 (3d5/2) | ZrO2 | 83.3 | 530.5 | ZrC | 39.72 | 281.6 |
| 181.6 (3d3/2) | O ads. | 16.7 | 532.2 | C-C | 32.16 | 285.1 | |||
| ZrO2 | 55 | 182.3 (3d5/2) | C-O-C, C-OH | 28.12 | 286.7 | ||||
| 184.5 (3d3/2) | |||||||||
| 53 at.% C | ZrC | 52.5 | 180.1 (3d3/2) | ZrO2 | 73.6 | 530.7 | ZrC | 24.64 | 282.1 |
| 182.0 (3d5/2) | O ads. | 18.3 | 532.2 | ZrC * | 14.6 | 283 | |||
| ZrO2 | 47.5 | 182.6 (3d3/2) | 8.1 | 533.4 | C-C | 33.18 | 284.9 | ||
| 184.6 (3d5/2) | C-O-C, C-OH | 27.57 | 286.8 | ||||||
| 289.6 | |||||||||
| 63 at.% C | ZrC | 84.2 | 180.3 (3d5/2) | ZrO2 | 70.1 | 531 | ZrC | 19.95 | 282.43 |
| 182.5 (3d3/2) | O ads. | 20.2 | 532.3 | ZrC * | 31.94 | 283.34 | |||
| ZrO2 | 15.8 | 183.1 (3d5/2) | 6.1 | 533.3 | C-C | 39.55 | 284.83 | ||
| 184.8 (3d3/2) | 3.6 | 534.6 | C-O-C, C-OH | 8.56 | 286.61 | ||||
| 79 at.% C | ZrC | 79.2 | 179.9 (3d5/2) | ZrO2 | 68.5 | 530.3 | ZrC | 31.7 | 282.7 |
| 182.1 (3d3/2) | O ads. | 19.2 | 531.7 | ZrC * | 53.2 | 284.2 | |||
| ZrO2 | 20.8 | 182.6 (3d5/2) | 9.1 | 532.9 | C-C | 10 | 285.4 | ||
| 184.5 (3d3/2) | 3.2 | 534.2 | C-O-C, C-OH | 5.1 | 286.6 | ||||
| C (at.%) | I(D)/I(G) | G Peak Position (cm−1) |
|---|---|---|
| 53 | 3.08 ± 0.13 | 1577.1 ± 0.42 |
| 63 | 4.5 ± 0.08 | 1575.4 ± 0.35 |
| 79 | 3.91 ± 0.09 | 1575.1 ± 0.33 |
| Sample | Contact Angle After 30 s | Contact Angle After 300 s |
|---|---|---|
| (°) | (°) | |
| 304L | 87.0 ± 0.7 | 80.0 ± 0.8 |
| 304L/Zr | 69.1 ± 0.3 | 56.0 ± 1.7 |
| 304L/Zr–C-21 at.% C | 73.2 ± 1.4 | 59.8 ± 1.3 |
| 304L/Zr–C-53 at.% C | 94.3 ± 0.3 | 86.7 ± 0.5 |
| 304L/Zr–C-63 at.% C | 99.2 ± 0.4 | 92.7 ± 0.8 |
| 304L/Zr–C-79 at.% C | 97.1 ± 0.4 | 89.5 ± 1.7 |
| Sample | Ecorr | icorr | -bc | Rpol | Eb | Protective Efficiency [%] |
|---|---|---|---|---|---|---|
| (V) | (A/cm2) | (V/dec) | (Wcm2) | (V) | ||
| 304L | 0.191 ± 0.019 | (827 ± 40) × 10−9 | 0.187 ± 0.012 | (98 ± 3) × 103 | 0.350 ± 0.022 | |
| 304L/Zr | 0.430 ± 0.049 | (7.80 ± 1.73 × 10−9 | 0.097 ± 0.011 | (4.45 ± 0.86) × 106 | 1.175 ± 0.189 | 99.1 |
| 304L/Zr–C-21 at.% C | 0.162 ± 0.016 | (27.76 ± 7.25) × 10−9 | 0.115 ± 0.031 | (1.93 ± 0.52) × 106 | 1.403 ± 0.033 | 96.6 |
| 304L/Zr–C-53 at.% C | 0.161 ± 0.010 | (20.41 ± 0.81) × 10−9 | 0.115 ± 0.004 | (1.82 ± 0.55) × 106 | 1.219 ± 0.154 | 97.5 |
| 304L/Zr–C-63 at.% C | 0.157 ± 0.007 | (41.29 ± 5.07) × 10−9 | 0.161 ± 0.017 | (1.11 ± 0.09) × 106 | - | 95.0 |
| 304L/Zr–C-79 at.% C | 0.085 ± 0.016 | (19.12 ± 2.00) × 10−9 | 0.137 ± 0.009 | (2.18 ± 0.22) × 106 | - | 97.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mydłowska, K.; Czerwińska, E.; Gilewicz, A.; Dobruchowska, E.; Jakubczyk, E.; Szparaga, Ł.; Ceynowa, P.; Ratajski, J. Evolution of Phase Composition and Antibacterial Activity of Zr–C Thin Films. Processes 2020, 8, 260. https://doi.org/10.3390/pr8030260
Mydłowska K, Czerwińska E, Gilewicz A, Dobruchowska E, Jakubczyk E, Szparaga Ł, Ceynowa P, Ratajski J. Evolution of Phase Composition and Antibacterial Activity of Zr–C Thin Films. Processes. 2020; 8(3):260. https://doi.org/10.3390/pr8030260
Chicago/Turabian StyleMydłowska, Katarzyna, Ewa Czerwińska, Adam Gilewicz, Ewa Dobruchowska, Ewa Jakubczyk, Łukasz Szparaga, Przemysław Ceynowa, and Jerzy Ratajski. 2020. "Evolution of Phase Composition and Antibacterial Activity of Zr–C Thin Films" Processes 8, no. 3: 260. https://doi.org/10.3390/pr8030260
APA StyleMydłowska, K., Czerwińska, E., Gilewicz, A., Dobruchowska, E., Jakubczyk, E., Szparaga, Ł., Ceynowa, P., & Ratajski, J. (2020). Evolution of Phase Composition and Antibacterial Activity of Zr–C Thin Films. Processes, 8(3), 260. https://doi.org/10.3390/pr8030260








