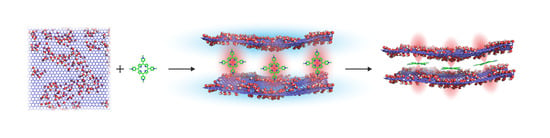
Size-Selected Graphene Oxide Loaded with Photosensitizer (TMPyP) for Targeting Photodynamic Therapy In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Size-Selected Graphene Oxide and Conjugation of Graphene Oxide with TMPyP (GO/TMPyP)
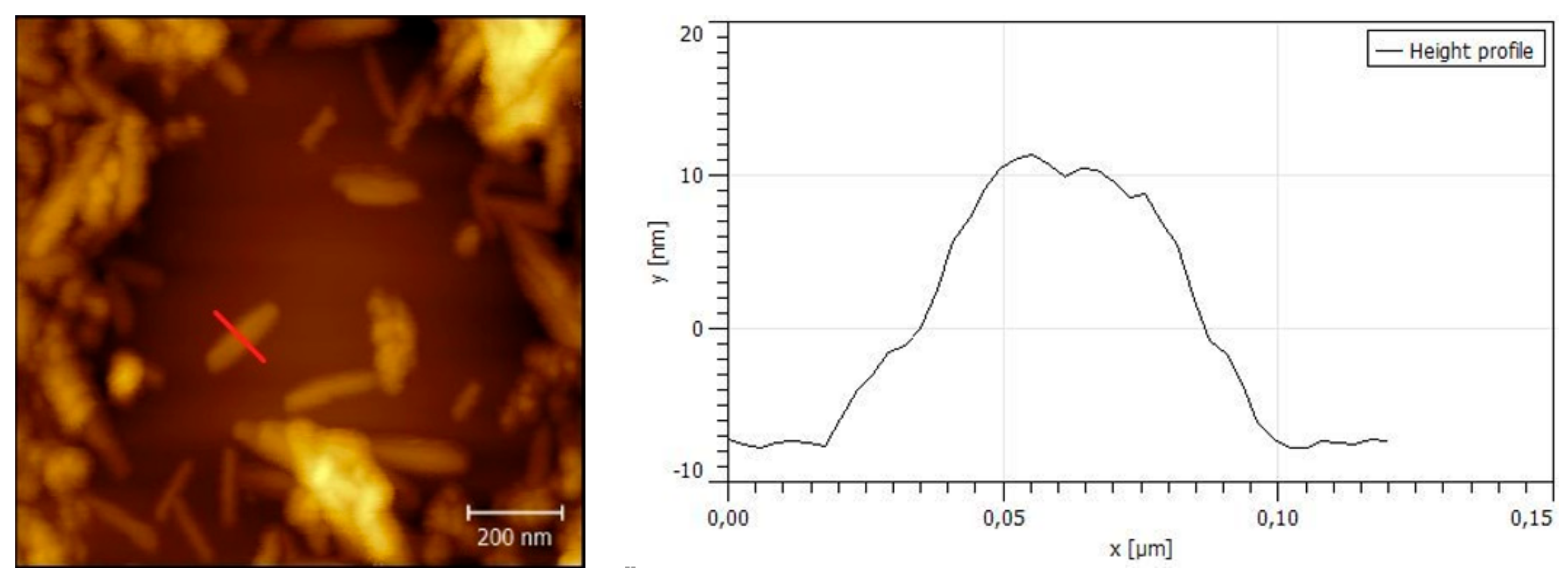
2.2. Characterization of GO and GO/TMPyP Was Carried out by Fluorescence Spectroscopy, AFM (Atomic Force Microscopy), and DLS (Dynamic Light Scattering)
2.3. In Vitro Cell Experiments
2.3.1. Cell and PDT Treatment
2.3.2. Microscopy Measurement of Cellular Uptake before and after PDT Treatment
2.3.3. MTT Test without and after PDT Treatment
2.3.4. Measurement of Reactive Oxygen Species Kinetic Production
2.3.5. Comet Assay
2.3.6. Statistical Analysis
3. Results
3.1. Preparation of Size-Selected Graphene Oxide
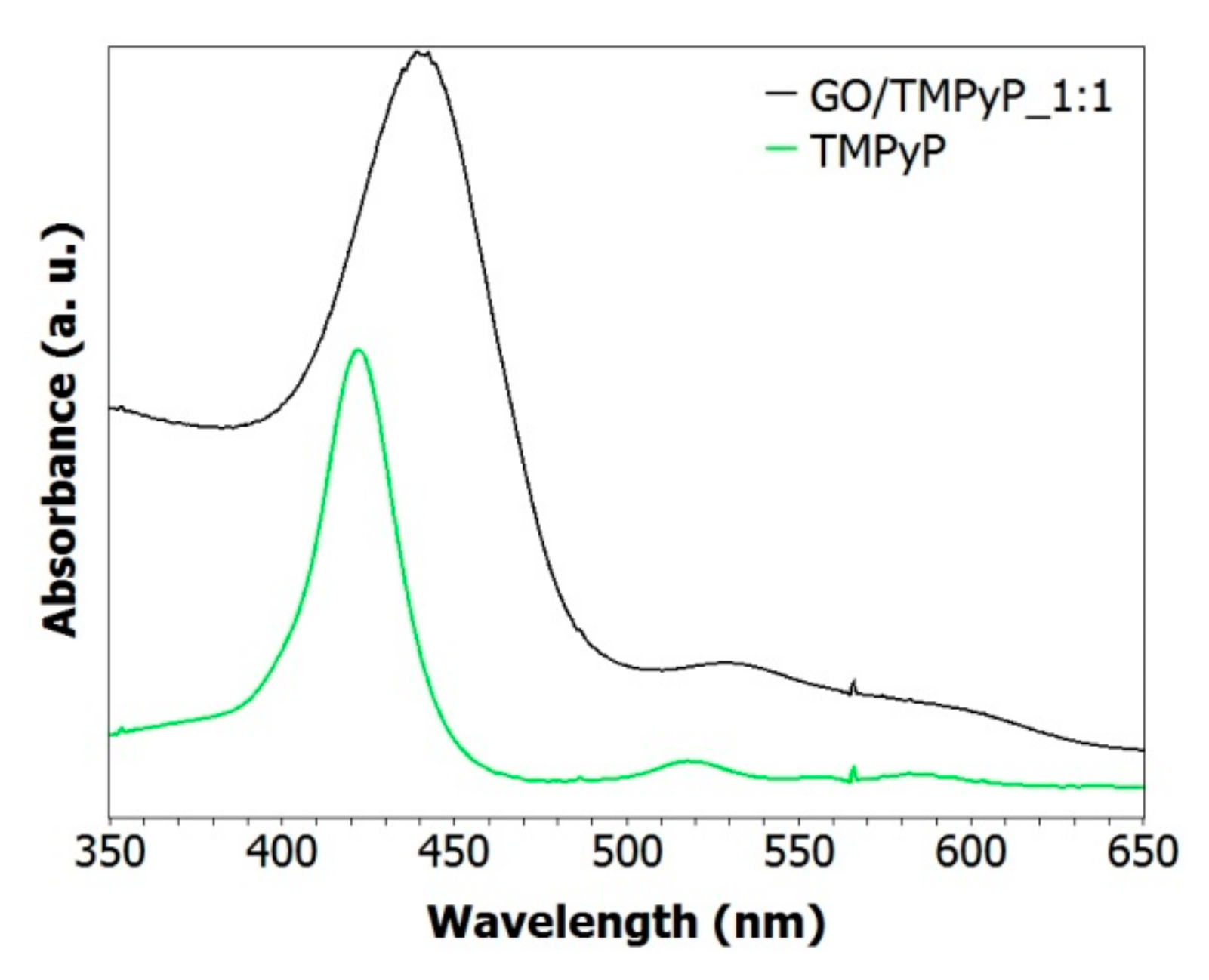
3.2. Characterization of GO/TMPyP Nanocarrier: (UV–Vis, DLS, Steady-State, and Time-Resolved Fluorescence Spectroscopy)
3.3. In Vitro Cell Experiments
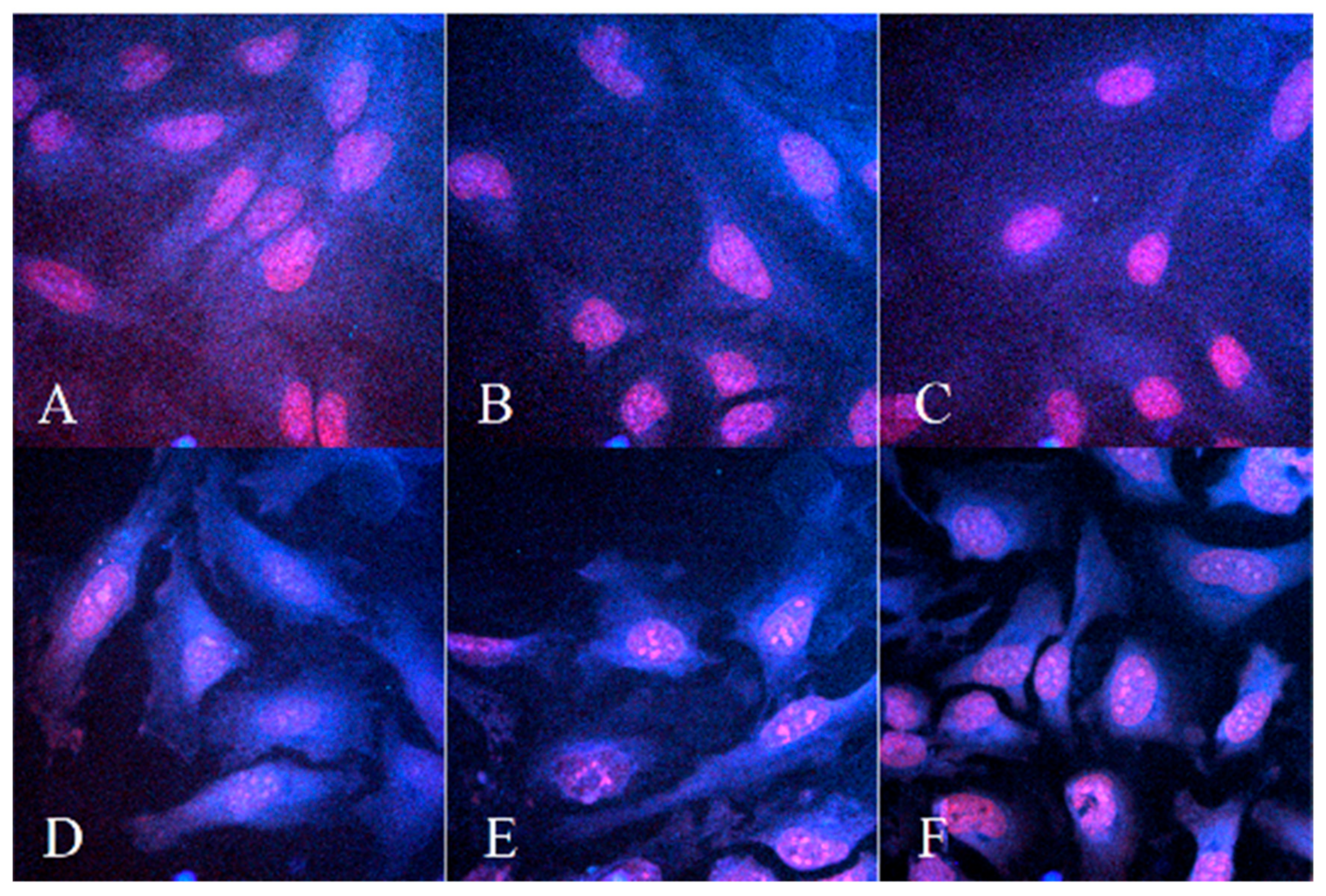
3.3.1. Microscopy Measurement of Cellular Uptake before and after PDT Treatment
3.3.2. MTT Test without and after Photodynamic Treatments
3.3.3. Measurement of Reactive Oxygen Species Kinetic Production
3.3.4. Comet Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO Cancer Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 12 September 2018).
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer Statistics 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Center, M.M.; DeSantis, C.; Ward, E.M. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xu, H.; Meyers, A.D.; Musani, A.I.; Wang, L.; Tagg, R. Photodynamic therapy for treatment of solid tumors—Potential and technical challenges. Technol. Cancer Res. Treat. 2008, 7, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C.; Patterson, M.S. The physics, biophysics and technology of photodynamic therapy. Phys. Med. Biol. 2008, 53, 61–109. [Google Scholar] [CrossRef]
- Malina, L.; Tomankova, K.B.; Malohlava, J.; Jiravova, J.; Manisova, B.; Zapletalova, J.; Kolarova, H. The in vitro cytotoxicity of metal-complexes of porphyrin sensitizer intended for photodynamic therapy. Toxicol. Vitro 2016, 34, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.A.; Evans, D.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B Biol. 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumor immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Tapajos, E.C.C.; Longo, J.P.; Simioni, A.R.; Lacava, Z.G.M.; Santos, M.F.M.A.; Morais, P.C.; Tedesco, A.C.; Azevedo, R.B. In vitro photodynamic therapy on human oral keratinocytes using chloroaluminum-phthalocyanine. Oral Onkol. 2008, 44, 1073–1079. [Google Scholar] [CrossRef]
- Pizova, K.; Bajgar, R.; Fillerova, R.; Kriegova, E.; Cenklova, V.; Langova, K.; Konecny, P.; Kolarova, H. C-MYC and C-FOS expression changes and cellular aspects of the photodynamic reaction with photosensitizers TMPyP and ClAlPcS2. J. Photochem. Photobiol. B Biol. 2015, 142, 186–196. [Google Scholar] [CrossRef]
- Tomankova, K.; Polakova, K.; Pizova, K.; Binder, S.; Havrdova, M.; Kolarova, M.; Kriegova, E.; Zapletalova, J.; Malina, L.; Malohlava, J.; et al. In vitro cytotoxicity analysis of doxorubicin loaded/superparamagnetic iron oxide colloidal nanoassemblies on MCF7 and NIH3T3 cell lines. Int. J. Nanomed. 2015, 10, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Koo, O.M.; Rubinstein, I.; Onyuksel, H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomed. NBM 2005, 3, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 1–18. [Google Scholar] [CrossRef]
- Min, Y.; Caster, J.M.; Eblan, M.J.; Wang, A.Z. Clinical translation of nanomedicine. Chem. Rev. 2015, 115, 11147–11190. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Tucek, J.; Sofer, Z.; Bousa, D.; Pumera, M.; Hola, K.; Mala, A.; Polakova, K.; Havrdova, M.; Cepe, K.; Tomanec, O.; et al. Air-stable superparamagnetic metal nanoparticles entrapped in graphene oxide matrix. Nat. Commun. 2016, 7, 12879. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, S.; Li, Y.; Wang, M.; Shi, P.; Huang, X. Covalent functionalization of graphene oxide with biocompatible poly(ethylene glycol) for delivery of paclitaxel. ACS Appl. Mater. Interfaces 2014, 6, 17268–17276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, X.; Zhang, F.; Long, G.; Zhang, T.; Leng, K.; Zhang, Y.; Huang, Y.; Ma, Y.; Zhang, M.; et al. Controlling the Effective Surface Area and Pore Size Distribution of sp2 Carbon Materials and Their Impact on the Capacitance Performance of These Materials. J. Am. Chem. Soc. 2013, 135, 5921–5929. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, L.; Bai, H.; Hong, W.; Li, C.; Shi, G. Chemically Converted Graphene Induced Molecular Flattening of 5,10,15,20- tetrakis(1-methyl-4-pyridinio)porphyrin and Its Application for Optical Detection of Cadmium(II) Ions. J. Am. Chem. Soc. 2009, 131, 13490–13497. [Google Scholar] [CrossRef]
- Özçakır, E.; Eskizeybek, V. A facile and effective method for size sorting of large flake graphene oxide. In Proceedings of the World Congress on Recent Advances in Nanotechnology (RAN’16), Prague, Czech Republic, 1−2 April 2016. [Google Scholar] [CrossRef]
- Tomankova, K.; Kejlova, K.; Binder, S.; Daskova, A.; Zapletalova, J.; Bendova, H.; Kolarova, H.; Jirova, D. In vitro cytotoxicity and phototoxicity study of cosmetics colorants. Toxicol. Vitro 2011, 25, 1242–1250. [Google Scholar] [CrossRef]
- Wychowaniec, J.K.; Iliut, M.; Zhou, M.; Moffat, J.; Elsawy, M.A.; Pinheiro, W.A.; Hoyland, J.A.; Miller, A.F.; Vijayaraghavan, A.; Saiani, A. Designing Peptide/Graphene Hybrid Hydrogels through Fine-Tuning of Molecular Interactions. Biomacromolecules 2018, 19, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Bornhütter, T.; Pohl, J.; Fischer, C.; Saltsman, I.; Mahammed, A.; Gross, Z.; Röder, B. Development of Singlet Oxygen Luminescence Kinetics during the Photodynamic Inactivation of Green Algae. Molecules 2016, 21, 485. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.; Liu, Z. Photothermally Enhanced Photodynamic Therapy Delivered by Nano-Graphene Oxide. ASC Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef]
- Mixhalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Trase, I.; Ren, M.; Duval, K.; Guo, X.; Chen, Z. Design of Nnaoparticle-Based carrieres for targeted drug delivery. J. Nanomater. 2016, 2016, 1087250. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.T.; Roy, I.; Swihart, M.T.; Prasad, P.N. Multifunctional nanoparticles as biocompatible targeted probes for human cancer diagnosis and therapy. J. Mater. Chem. 2009, 19, 4655–4672. [Google Scholar] [CrossRef] [PubMed]
- Azzazy, H.M.E.; Mansour, M.M.H.; Kazmierczak, S.C. From diagnostics to therapy: Prospects of quantum dots. Clin. Biochem. 2007, 40, 917–927. [Google Scholar] [CrossRef]
- Prasad, P.N. Nanophotonics; Wiley-Interscience: New York, NY, USA, 2004. [Google Scholar]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Xu, X.L.; Lin, F.W.; Du, Y.; Zhang, X.; Wu, J.; Xu, Z.K. Graphene Oxide Nanofiltration Membranes Stabilized by Cationic Porphyrin for High Salt Rejection. ACS Appl. Mater. Interfaces 2016, 8, 12588–12593. [Google Scholar] [CrossRef]
- Mosinger, J.; Slavětínská, L.; Lang, K.; Coufal, K.; Kubát, P. Cyclodextrin carriers of positivelycharged porphyrin sensitizers. Org. Biomol. Chem. 2009, 7, 3797–3804. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wu, F.; Wang, K.; Zheng, Y.; Li, Z.; Zhang, X.; Wong, W.K. Photocytotoxicity, cellular uptake and subcellular localization of amidinophenylporphyrins as potential photodynamic therapeutic agents: An in vitro cell study. Bioorg. Med. Chem. Lett. 2015, 25, 4513–4517. [Google Scholar] [CrossRef] [PubMed]
- Hanakova, A.; Bogdanova, K.; Tomankova, K.; Pizova, K.; Malohlava, J.; Binder, S.; Bajgar, R.; Langova, K.; Kolar, M.; Mosinger, J.; et al. The application of antimicrobial photodynamic therapy on S. aureus and E. coli using porphyrin photosensitizers bound to cyclodextrin. Microbiol. Res. 2014, 169, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Lüpertz, R.; Wätjen, W.; Kahl, R.; Chovolou, Y. Dose- and time-dependent effects of doxorubicin on cytotoxicity, cell cycle and apoptotic cell death in human colon cancer cells. Toxicology 2010, 271, 115–121. [Google Scholar] [CrossRef]
- Csík, G.; Egyeki, M.; Herényi, L.; Majer, Z.; Tóth, K. Role of structure-proteins in the porphyrin–DNA interaction. J. Photochem. Photobiol. B Biol. 2009, 96, 207–215. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, M.-C.; Zheng, M.; Jin, S.F.; Tang, D.T.; Chen, J.; Ma, Y.-A.; Lin, J.-Q.; Wang, X.-H.; Liu, H.J. G-quadruplex DNA interactions, docking and cell photocytotoxicity research of porphyrin dyes. Dyes Pigment. 2016, 128, 41–48. [Google Scholar] [CrossRef]





| Treatment | IC50 (µM) TMPyP | IC50 (µM) GO/TMPyP |
|---|---|---|
| No irradiation | / | / |
| 740 nm 30 J/cm2 + 414 nm 1 J/cm2 | 8.224 | 5.2275 |
| 414 nm/cm2 1 J/cm2 | 8.285 | / |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartoň Tománková, K.; Opletalová, A.; Poláková, K.; Kalytchuk, S.; Jiravová, J.; Malohlava, J.; Malina, L.; Kolářová, H. Size-Selected Graphene Oxide Loaded with Photosensitizer (TMPyP) for Targeting Photodynamic Therapy In Vitro. Processes 2020, 8, 251. https://doi.org/10.3390/pr8020251
Bartoň Tománková K, Opletalová A, Poláková K, Kalytchuk S, Jiravová J, Malohlava J, Malina L, Kolářová H. Size-Selected Graphene Oxide Loaded with Photosensitizer (TMPyP) for Targeting Photodynamic Therapy In Vitro. Processes. 2020; 8(2):251. https://doi.org/10.3390/pr8020251
Chicago/Turabian StyleBartoň Tománková, Kateřina, Ariana Opletalová, Kateřina Poláková, Sergii Kalytchuk, Jana Jiravová, Jakub Malohlava, Lukáš Malina, and Hana Kolářová. 2020. "Size-Selected Graphene Oxide Loaded with Photosensitizer (TMPyP) for Targeting Photodynamic Therapy In Vitro" Processes 8, no. 2: 251. https://doi.org/10.3390/pr8020251
APA StyleBartoň Tománková, K., Opletalová, A., Poláková, K., Kalytchuk, S., Jiravová, J., Malohlava, J., Malina, L., & Kolářová, H. (2020). Size-Selected Graphene Oxide Loaded with Photosensitizer (TMPyP) for Targeting Photodynamic Therapy In Vitro. Processes, 8(2), 251. https://doi.org/10.3390/pr8020251