Stabilization/Solidification of Strontium Using Magnesium Silicate Hydrate Cement
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
3. Results
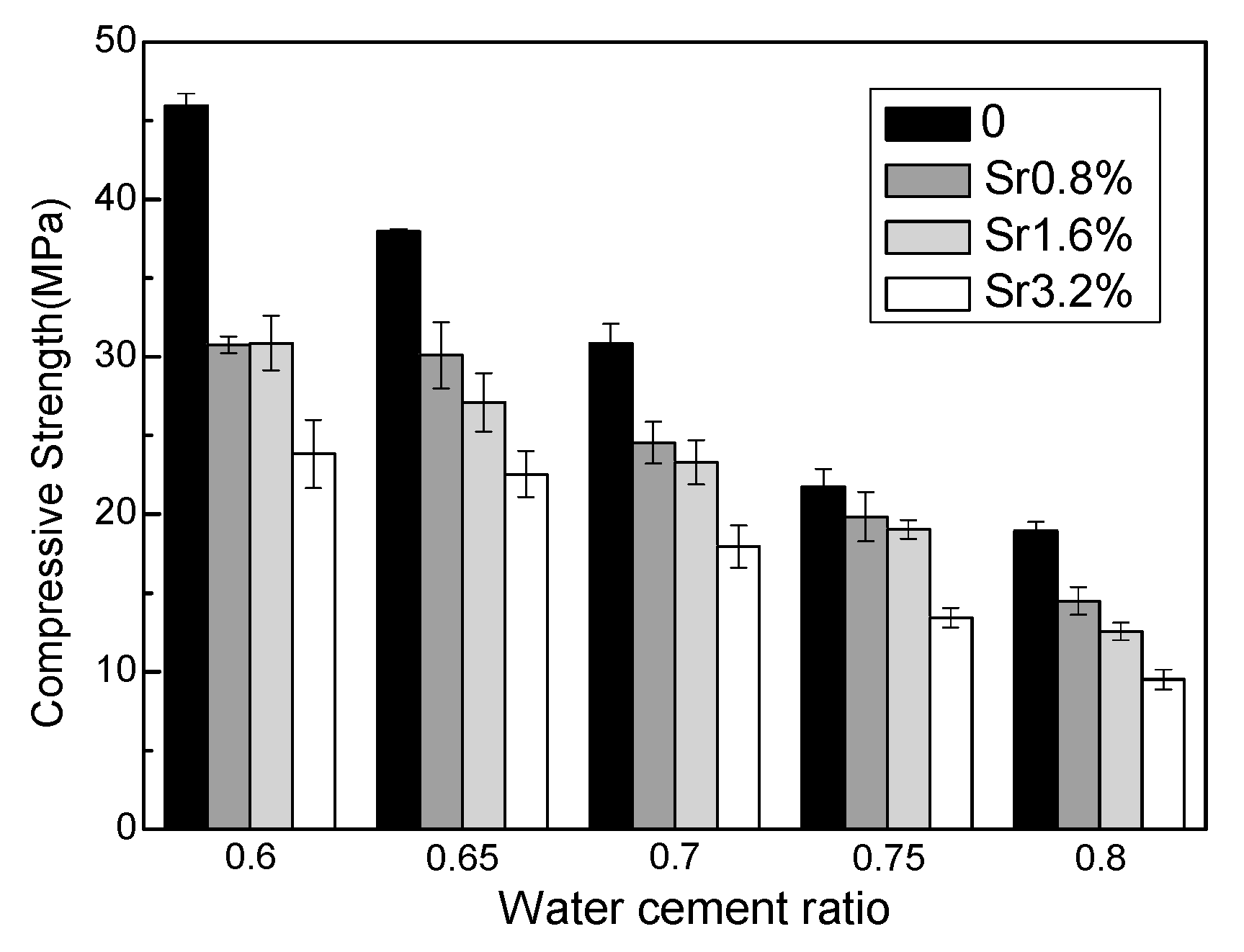
3.1. Compressive Strength
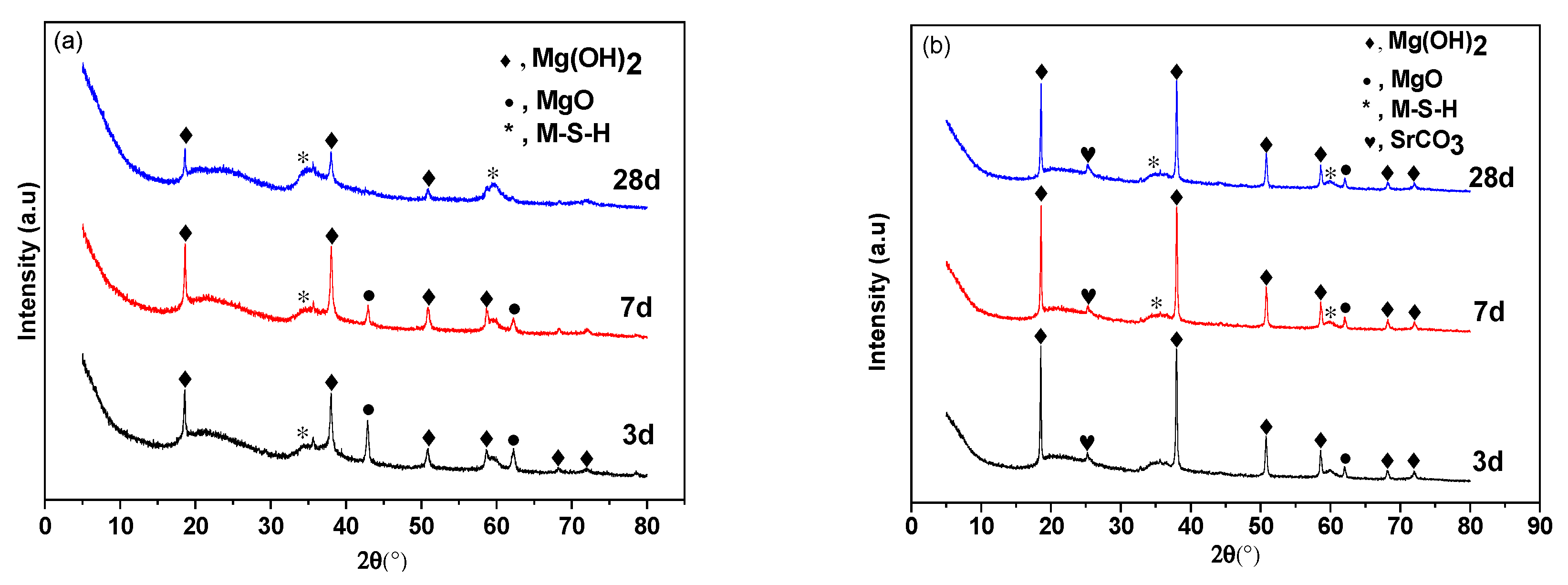
3.2. Crystalline Phase Analysis
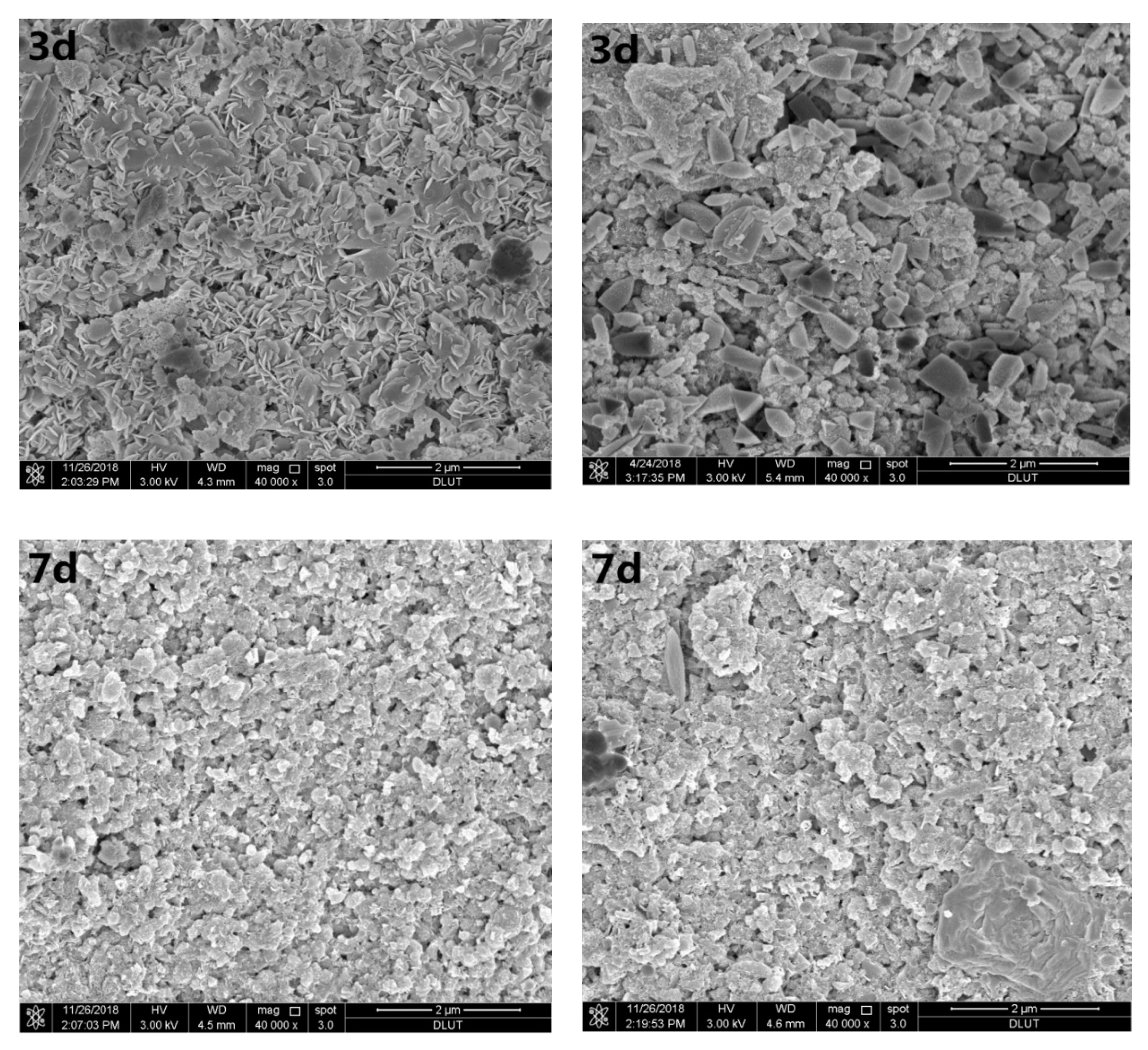
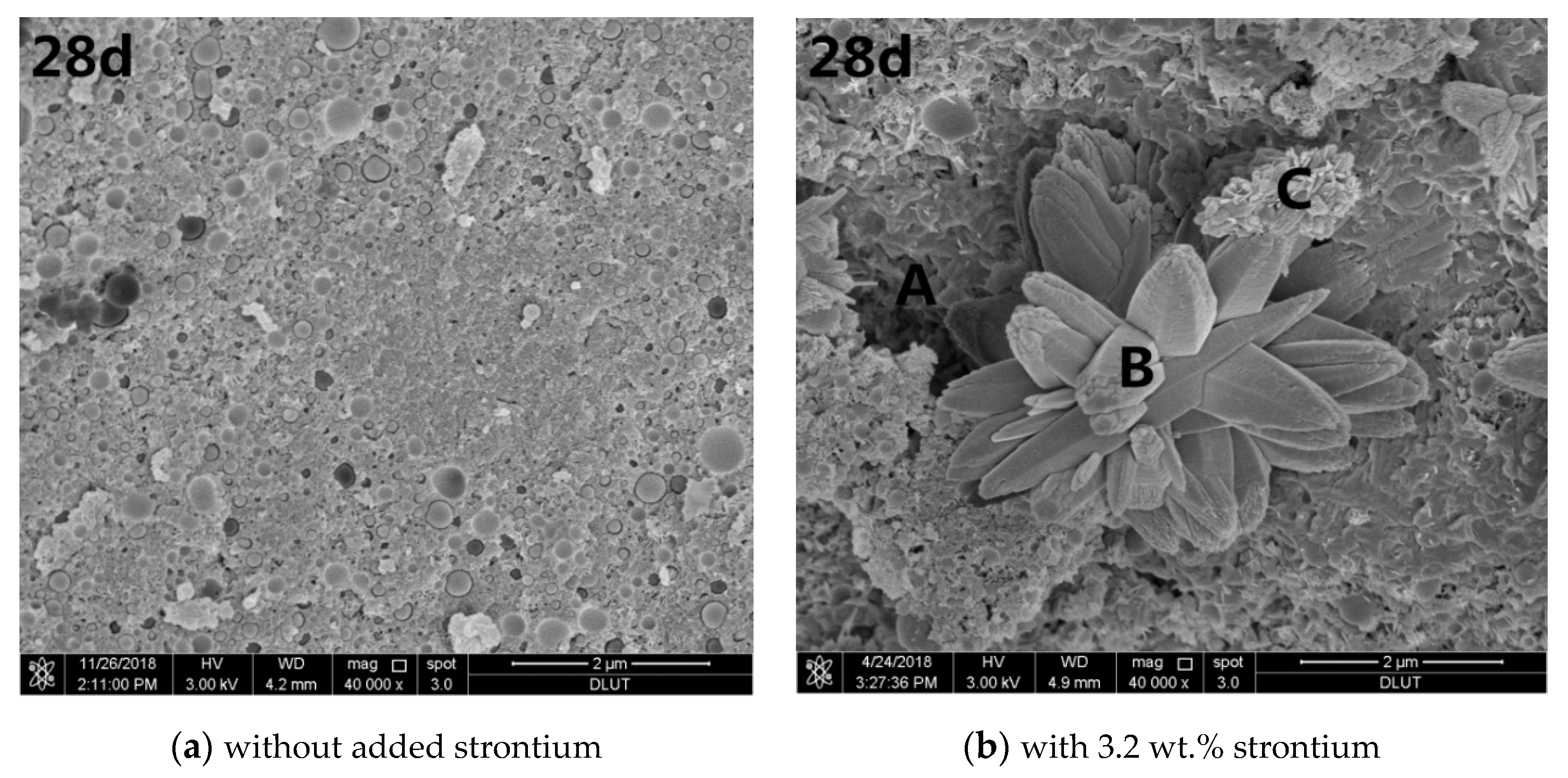
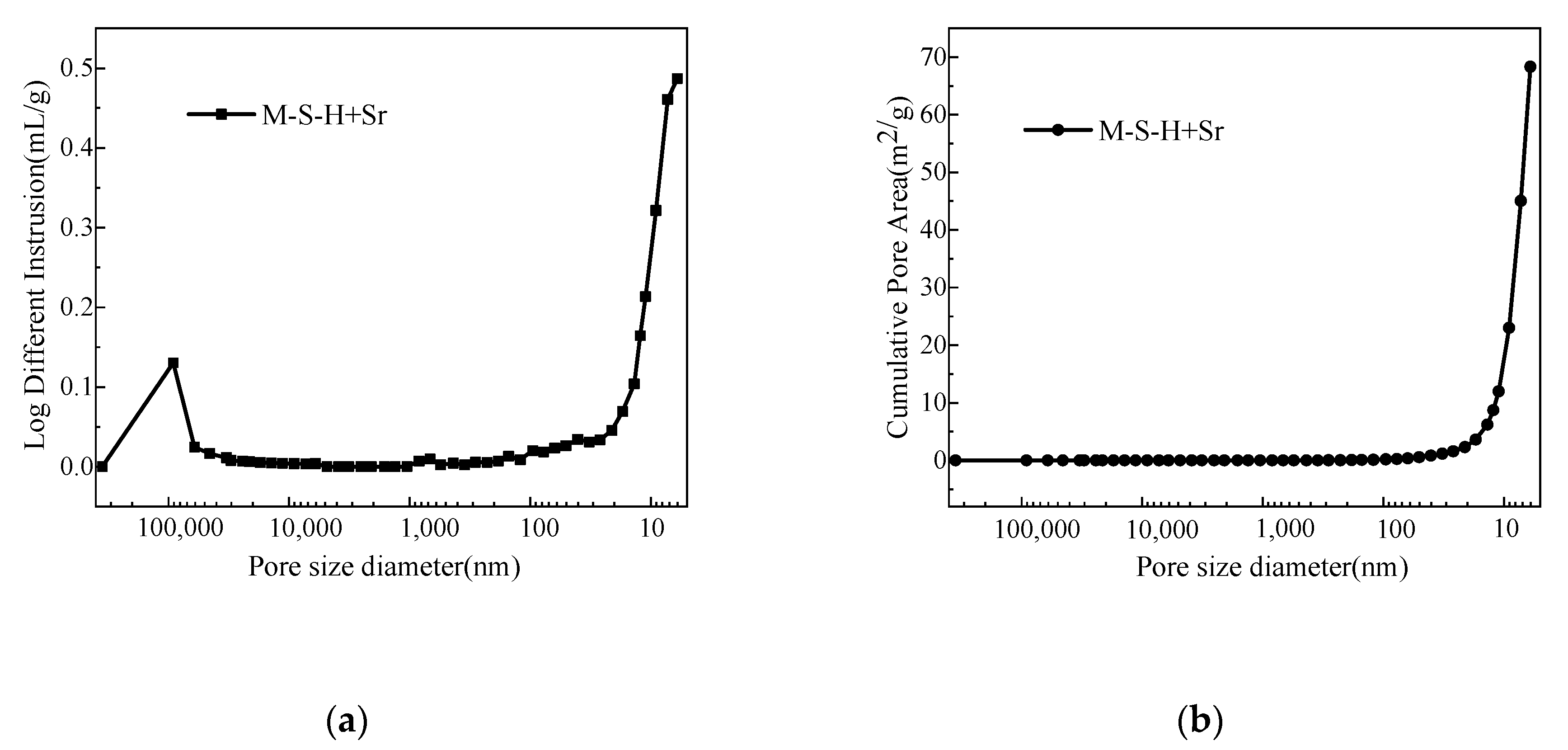
3.3. Microstructural Analysis
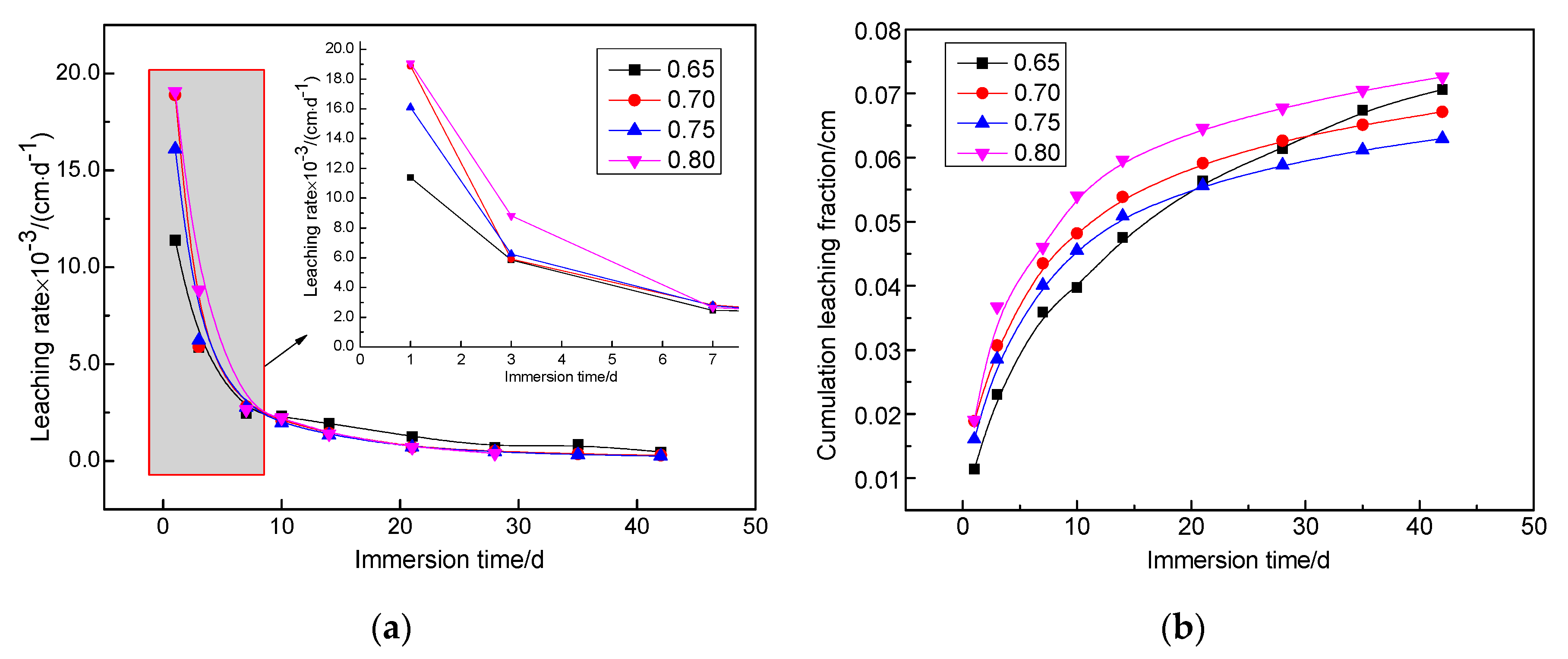
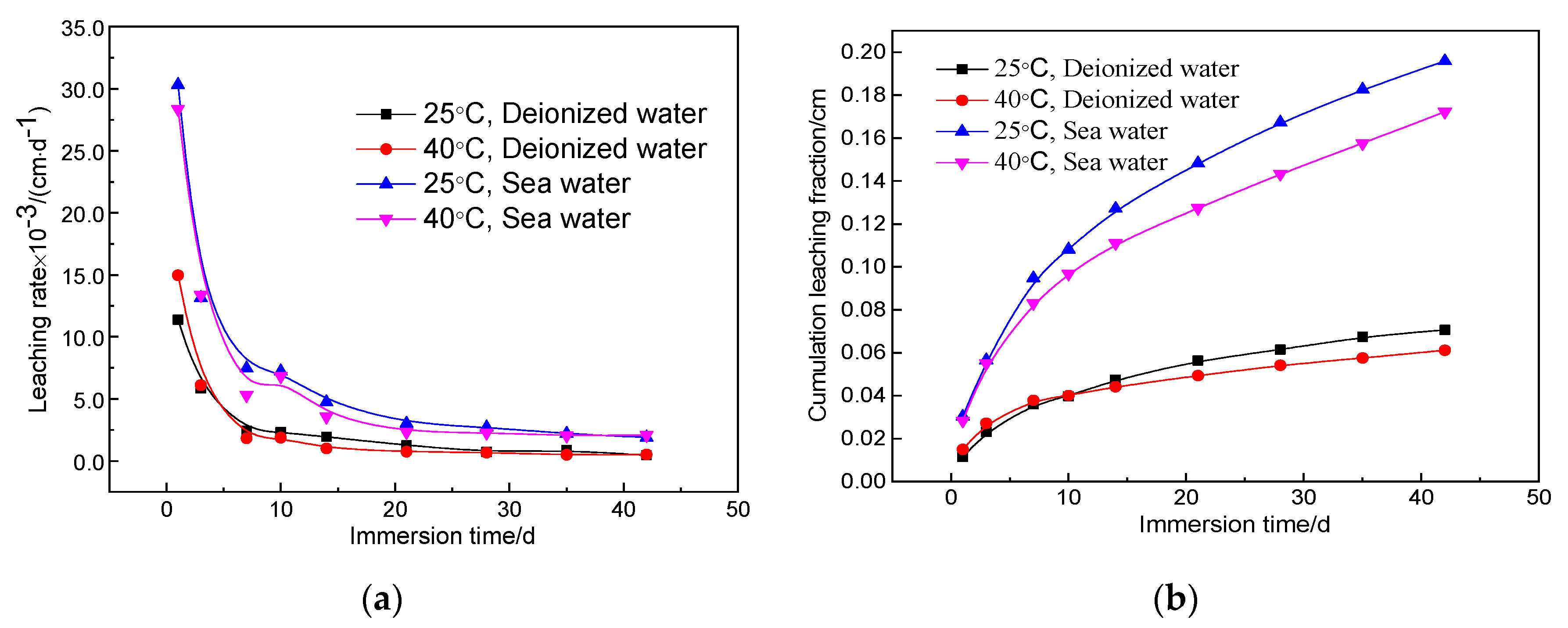
3.4. Leach Test Results
4. Discussion
5. Conclusions
- (1)
- Incorporating the Sr nuclide reduced the working performance of the magnesium silicate cement and inhibited the development of compressive strength owing to the inhibition of hydration process.
- (2)
- The leaching rate and cumulative leaching fraction of the solidified body after immersion for 42 days in the standard environment (4.64 × 10−4 cm/d) and high temperature seawater (1.89 × 10−3 cm/d) were one order of magnitude lower than the limit value in the National Standard.
- (3)
- The presence of Sr affected the hydration reactions of the magnesium silicate cement and was encapsulated the interior of the matrix in the form of a strontium carbonate precipitate. This was beneficial to ensure low long-term leaching performance of the solidified body.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IAEA. Classification of Radioactive Waste; Safety Series GSG-1; IAEA: Vienna, Austria, 2009. [Google Scholar]
- Hashimoto, S.; Matsuura, T.; Nanko, K.; Linkov, I.; Shaw, G.; Kaneko, S. Predicted spatio-temporal dynamics of radio cesium deposited onto forests following the Fukushima nuclear accident. Sci. Rep. 2013, 3, 2564. [Google Scholar] [CrossRef] [PubMed]
- Koarashi, J.; Atarashi-Andoh, M.; Amano, H.; Matsunaga, T. Vertical distributions of global fallout 137Cs and 14C in a Japanese forest soil profile and their implications for the fate and migration processes of Fukushima-derived 137Cs. J. Radioanal. Nucl. Chem. 2017, 311, 473–481. [Google Scholar] [CrossRef]
- Ota, M.; Nagai, H.; Koarashi, J. Modeling dynamics of 137Cs in forest surface environments: Application to a contaminated forest site near Fukushima and assessment of potential impacts of soil organic matter interactions. Sci. Total Environ. 2016, 551, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Buesseler, K.O.; Jayne, S.R.; Fisher, N.S.; Rypina, I.I.; Baumann, H.; Baumann, Z.; Breier, C.F.; Casacuberta, E.M.; Masqué, N.P.; Garcia-Orellana, J.; et al. 90Sr and 89Sr in seawater off Japan as a consequence of the Fukushima Dai-ichi nuclear accident. Bioresources 2013, 10, 3649–3659. [Google Scholar] [CrossRef]
- Kubota, T.; Shibahara, Y.; Fukutani, S.; Fujii, T.; Ohta, T.; Kowatari, M.; Mizuno, S.; Takamiya, K.; Yamana, H. Cherenkov counting of 90Sr and 90Y in bark and leaf samples collected around Fukushima Daiichi Nuclear Power Plant. J. Radioanal. Nucl. Chem. 2015, 303, 39–46. [Google Scholar] [CrossRef][Green Version]
- Steinhauser, G.; Schauer, V.; Shozugawa, K. Concentration of Strontium-90 at selected hot spots in Japan. PLoS ONE 2013, 8, e57760. [Google Scholar] [CrossRef] [PubMed]
- Toth, F.L.; Rogner, H.H. Oil and nuclear power: Past, present, and future. Energy Econ. 2006, 28, 1–25. [Google Scholar] [CrossRef]
- Halim, C.E.; Amal, R.; Beydoun, D.; Scott, J.A.; Low, G. Low, Implications of the structure of cementitious wastes containing Pb (II), Cd (II), As (V), and Cr (VI) on the leaching of metals. Cem. Concr. Res. 2004, 34, 1093–1102. [Google Scholar] [CrossRef]
- Shi, C.J.; Fernández-Jiménez, A. Stabilization/solidification of hazardous and radioactive wastes with alkali-activated cements. J. Hazard. Mater. 2006, 137, 1656–1663. [Google Scholar] [CrossRef]
- Qian, G.G.; Sun, D.D.; Tay, J.H. New aluminium-rich alkali slag matrix with clay minerals for immobilizing simulated radioactive Sr and Cs waste. J. Nucl. Mater. 2001, 299, 199–204. [Google Scholar] [CrossRef]
- Guangren, Q.; Yuxiang, L.; Facheng, Y.; Rongming, S. Improvement of metakaolin on radioactive Sr and Cs immobilization of alkali-activated slag matrix. J. Hazard. Mater. 2002, 92, 289–300. [Google Scholar] [CrossRef]
- Plecas, I.; Pavlovic, R.; Paclovic, S. Leaching behavior of 60Co and 137Cs from spent ion exchange resins in cement–bentonite clay matrix. J. Nucl. Mater. 2004, 327, 171–174. [Google Scholar] [CrossRef]
- Wei, M.L. Research on Mechanism and Long-Term Performance of a New Phosphate-Based Binder for Stabilization of Soils Contaminated with High Levels of Zinc and Lead. Ph.D. Thesis, Southeast University, Nanjing, China, 2017. [Google Scholar]
- Zhang, H.; He, P.J.; Shao, L.M. Fate of heavy metals during municipal solid waste incineration in Shanghai. J. Hazard. Mater. 2008, 156, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wahbi, A.; Ma, L.; Li, B.; Yang, Y. Immobilization of Zn, Cu, and Pb in contaminated soils using phosphate rock and phosphoric acid. J. Hazard. Mater. 2009, 164, 555–564. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Qian, J.; Qin, J.; Wang, H.; Wang, Q.; Ye, Z. Effect of early hydration temperature on hydration product and strength development of magnesium phosphate cement (MPC). Cem. Concr. Res. 2015, 78, 179–189. [Google Scholar] [CrossRef]
- Lai, Z.; Lai, X.; Shi, J.; Lu, Z. Effect of Zn2+ on the early hydration behavior of potassium phosphate based magnesium phosphate cement. Constr. Build. Mater. 2016, 129, 70–78. [Google Scholar] [CrossRef]
- Lai, Z.Y.; Qian, J.S.; Liang, P. Study on the curing properties of 90Sr by magnesium phosphate cement. Acta Sci. Circumstantiae 2011, 31, 2792–2797. [Google Scholar] [CrossRef]
- Qiao, F.; Chau, C.K.; Li, Z. Property evaluation of magnesium phosphate cement mortar as patch repair material. Constr. Build. Mater. 2010, 24, 695–700. [Google Scholar] [CrossRef]
- Fei, J.; Al-Tabbaa, A. Strength and hydration products of reactive MgO–silica pastes. Cem. Concr. Compos. 2014, 52, 27–33. [Google Scholar] [CrossRef]
- Fei, J.; Gu, K.; Al-Tabbaa, A. Strength and drying shrinkage of reactive MgO modified alkali-activated slag paste. Constr. Build. Mater. 2014, 51, 395–404. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, T.; Hu, J.; Tang, Y.; Niu, Y.; Wei, J.; Yu, Q. Characterization of reaction products and reaction process of MgO-SiO2-H2O system at room temperature. Constr. Build. Mater. 2014, 61, 252–259. [Google Scholar] [CrossRef]
- Wei, J.X. Study on MgO-SiO2-H2O Cementitious System and Its Mechanism of Hydration and Hardenin; China Academy of Building Materials Science: Beijing, China, 2004. [Google Scholar]
- Wei, J.X.; Chen, Y.M.; Li, Y.X. The reaction mechanism between MgO and microsilica at room temperature. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2006, 21, 88–91. [Google Scholar] [CrossRef]
- Zhang, T.T.; Cheesemana, C.R.; Vandeperre, L.J. Development of low pH cement systems forming magnesium silicate hydrate (M-S-H). Cem. Concr. Res. 2011, 41, 439–442. [Google Scholar] [CrossRef]
- Cole, W.F.; Demediuk, T. X-Ray, thermal, and Dehydration studies on Magnesium oxychlorides. Aust. J. Chem. 1955, 8, 234–251. [Google Scholar] [CrossRef]
- Sevim, A.M.; Hojiyev, R.; Gül, A.; Çelik, M.S. An investigation of the kinetics and thermodynamics of the adsorption of a cationic cobalt porphyrazine onto sepiolite. Dyes Pigments 2011, 88, 25–38. [Google Scholar] [CrossRef]
- Alexandre-Franco, M.; Albarrán-Liso, A.; Gómez-Serrano, V. An identification study of vermiculites and micas: Adsorption of metal ions in aqueous solution. Fuel Process. Technol. 2011, 92, 200–205. [Google Scholar] [CrossRef]
- Franco, F. Adsorption of Methylene Blue on magnesium silicate: Kinetics, equilibria and comparison with other adsorbents. J. Environ. Sci. 2010, 22, 467–473. [Google Scholar] [CrossRef]
- Fouad, H.K.; Bishay, A.F. Uranium uptake from acidic solutions using synthetic titanium and magnesium based adsorbents. J. Radioanal. Nucl. Chem. 2010, 283, 765–772. [Google Scholar] [CrossRef]
- Zhang, T.T.; Vandeperre, L.J.; Cheeseman, C. Magnesium-silicate-hydrate cements for encapsulating problematic aluminium containing wastes. J. Sustain. Cem.-Based Mater. 2012, 1, 34–45. [Google Scholar] [CrossRef]
- Zhang, T.T.; Vandeperre, L.J.; Christopher, R. Formation of magnesium silicate hydrate (M-S-H) cement pastes using sodium hexametaphosphate. Cem. Concr. Res. 2014, 65, 8–14. [Google Scholar] [CrossRef]
- Jia, Y. The Effect of Na-HMP and CaO on the Reaction Mechanism of MgO-SiO2-H2O System; Dalian University of Technology: Dalian, China, 2017. [Google Scholar]
- GB/T 17671-1999. Determinations for Isotopes of Lead, Strontium and Neodymium in Rock Samples. Available online: https://books.google.com.br/books?id=ghrVAwAAQBAJ&pg=PA477&lpg=PA477&dq=GB/T+17671-19992011 (accessed on 10 December 2019).
- GB/T 7023-2011. Standard Test Method for Leachability of Low and Intermediate Level Solidified Radioactive Waste Forms. Available online: https://www.chinesestandard.net/PDF/English.aspx/GBT7023-2011 (accessed on 10 December 2019).
- GB 14569.1-2011. Performance Requirements for Low and Intermediate Level Radioactive Waste Form—Cemented Waste Form. Available online: http://english.mee.gov.cn/Resources/standards/Radioactivity/radiation/201111/t20111101_219413.shtml (accessed on 10 December 2019).
- Li, Z.H. Reaction Mechanisms and Application Study of MgO-SiO2-H2O Cementitious System; South China University of Technology: Guangzhou, China, 2015. [Google Scholar]
- Lai, Z.Y. Study on Solidification of Low and Medium Radioactive Waste with Magnesium Phosphate Cement; Chongqing University: Chongqing, China, 2012. [Google Scholar]
- Brew, D.R.M.; Glasser, F.P. Synthesis and characterisation of magnesium silicate hydrate gels. Cem. Concr. Res. 2005, 35, 85–98. [Google Scholar] [CrossRef]
| Chemical Composition (wt.%) | MgO | CaO | SiO2 | Fe2O3 | Al2O3 | K2O | MnO | SO3 |
|---|---|---|---|---|---|---|---|---|
| light-burned magnesia | 98.45 | 0.62 | 0.49 | 0.18 | 0.04 | − | 0.04 | − |
| silica fume | 0.45 | 0.43 | 97.34 | 0.05 | 0.12 | 0.97 | 0.04 | 0.35 |
| NaCl | MgCl2 | Na2SO4 | CaCl2 | KCl | NaHCO3 | KBr | |
|---|---|---|---|---|---|---|---|
| Quality/g | 23.50 | 4.98 | 3.92 | 1.10 | 0.66 | 0.19 | 0.09 |
| Element | A | B | C | |||
|---|---|---|---|---|---|---|
| Weight/% | Atom/% | Weight/% | Atom/% | Weight/% | Atom/% | |
| C | 5.61 | 10.50 | 17.85 | 32.30 | 14.25 | 26.32 |
| O | 32.52 | 45.72 | 40.36 | 54.82 | 38.52 | 53.43 |
| Mg | 15.25 | 14.11 | 1.39 | 1.24 | 4.63 | 4.23 |
| Si | 31.57 | 25.28 | 1.88 | 1.45 | 7.04 | 5.56 |
| Sr | 13.39 | 3.44 | 37.11 | 9.20 | 32.19 | 8.15 |
| Average Pore Diameter/nm | Median Pore Diameter/nm | Total Pore Area/m2/g | Porosity/% |
|---|---|---|---|
| 15.30 | 8.0 | 68.34 | 30.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Zou, J.; Li, Y.; Jia, Y.; Cheeseman, C.R. Stabilization/Solidification of Strontium Using Magnesium Silicate Hydrate Cement. Processes 2020, 8, 163. https://doi.org/10.3390/pr8020163
Zhang T, Zou J, Li Y, Jia Y, Cheeseman CR. Stabilization/Solidification of Strontium Using Magnesium Silicate Hydrate Cement. Processes. 2020; 8(2):163. https://doi.org/10.3390/pr8020163
Chicago/Turabian StyleZhang, Tingting, Jing Zou, Yimiao Li, Yuan Jia, and Christopher R. Cheeseman. 2020. "Stabilization/Solidification of Strontium Using Magnesium Silicate Hydrate Cement" Processes 8, no. 2: 163. https://doi.org/10.3390/pr8020163
APA StyleZhang, T., Zou, J., Li, Y., Jia, Y., & Cheeseman, C. R. (2020). Stabilization/Solidification of Strontium Using Magnesium Silicate Hydrate Cement. Processes, 8(2), 163. https://doi.org/10.3390/pr8020163





