Enhancing the Pore Properties and Adsorption Performance of Cocoa Pod Husk (CPH)-Derived Biochars via Post-Acid Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pyrolysis and Post-Acid Treatment Experiments
2.3. Characterization of Resulting Biochar
2.4. Adsorption Experiments
3. Results and Discussion
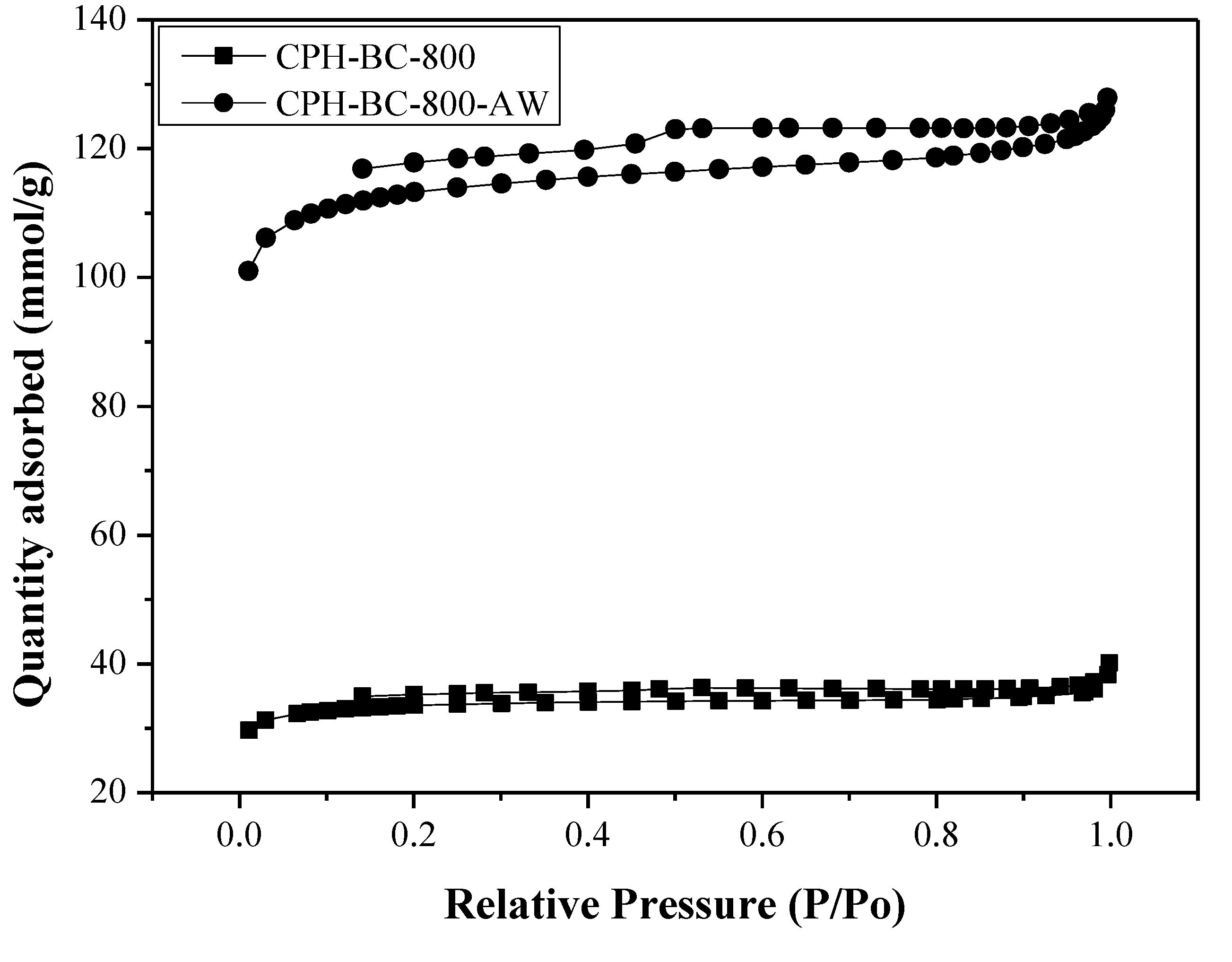
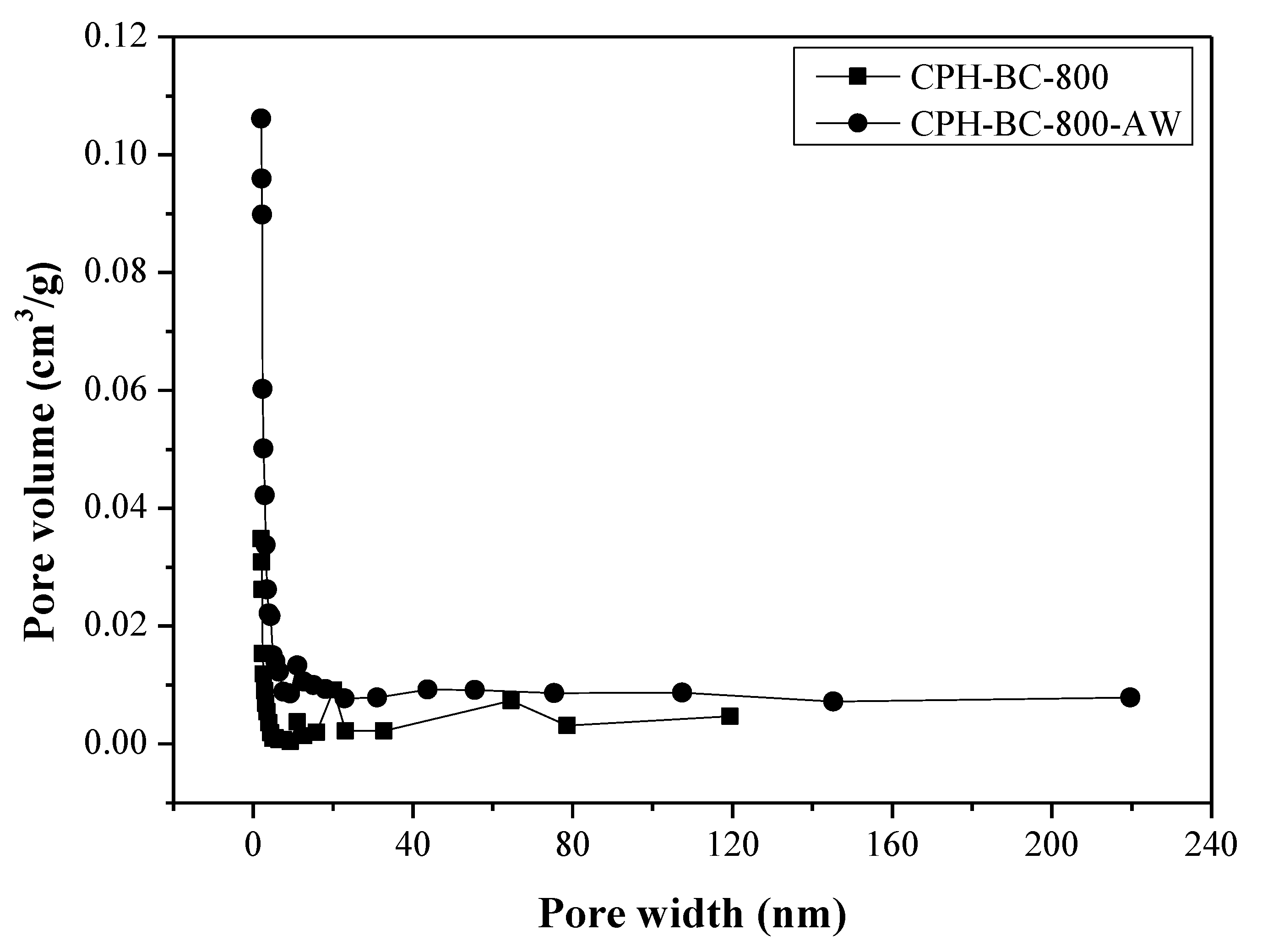
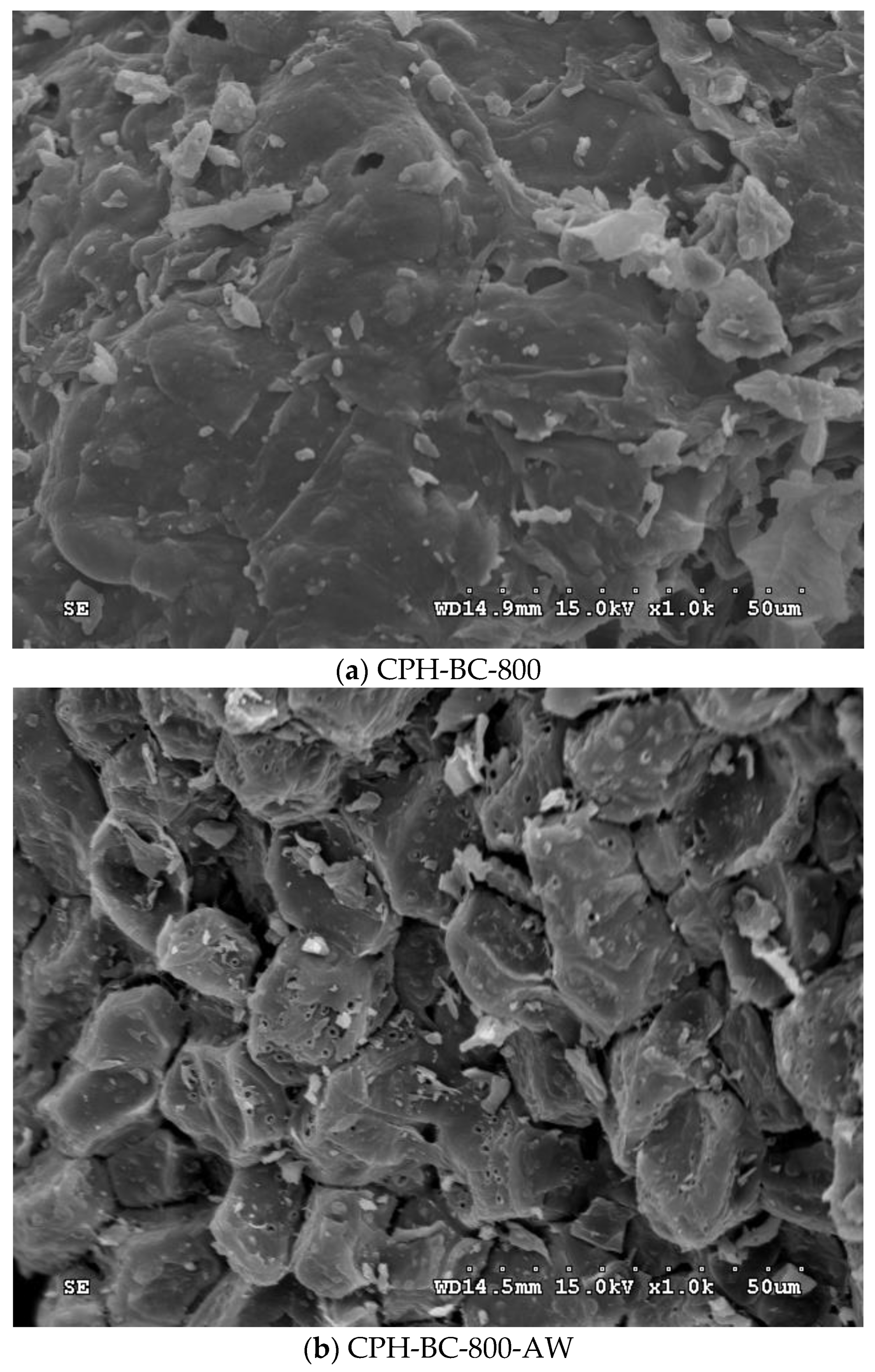
3.1. Pore Properties of Resulting Biochars
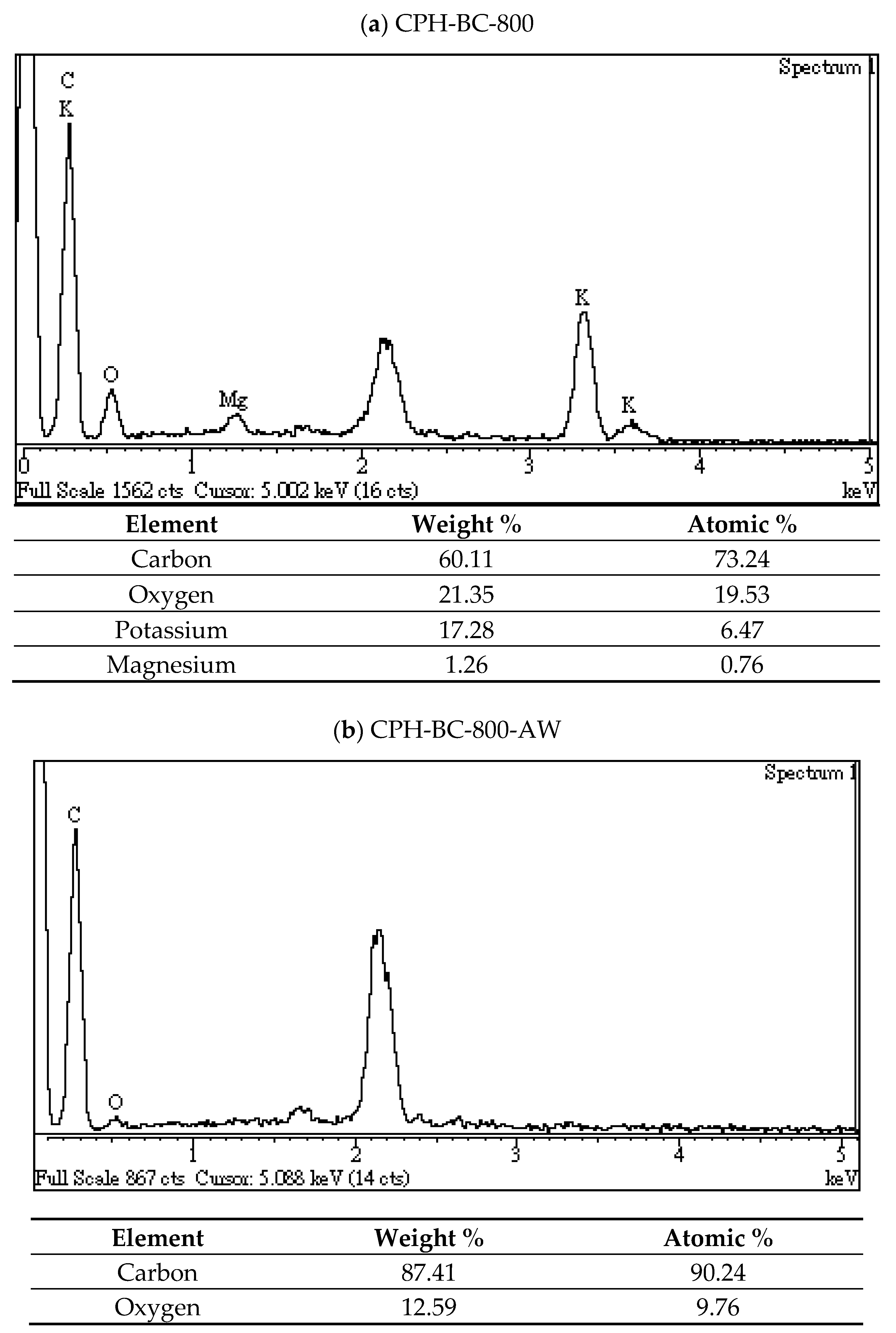
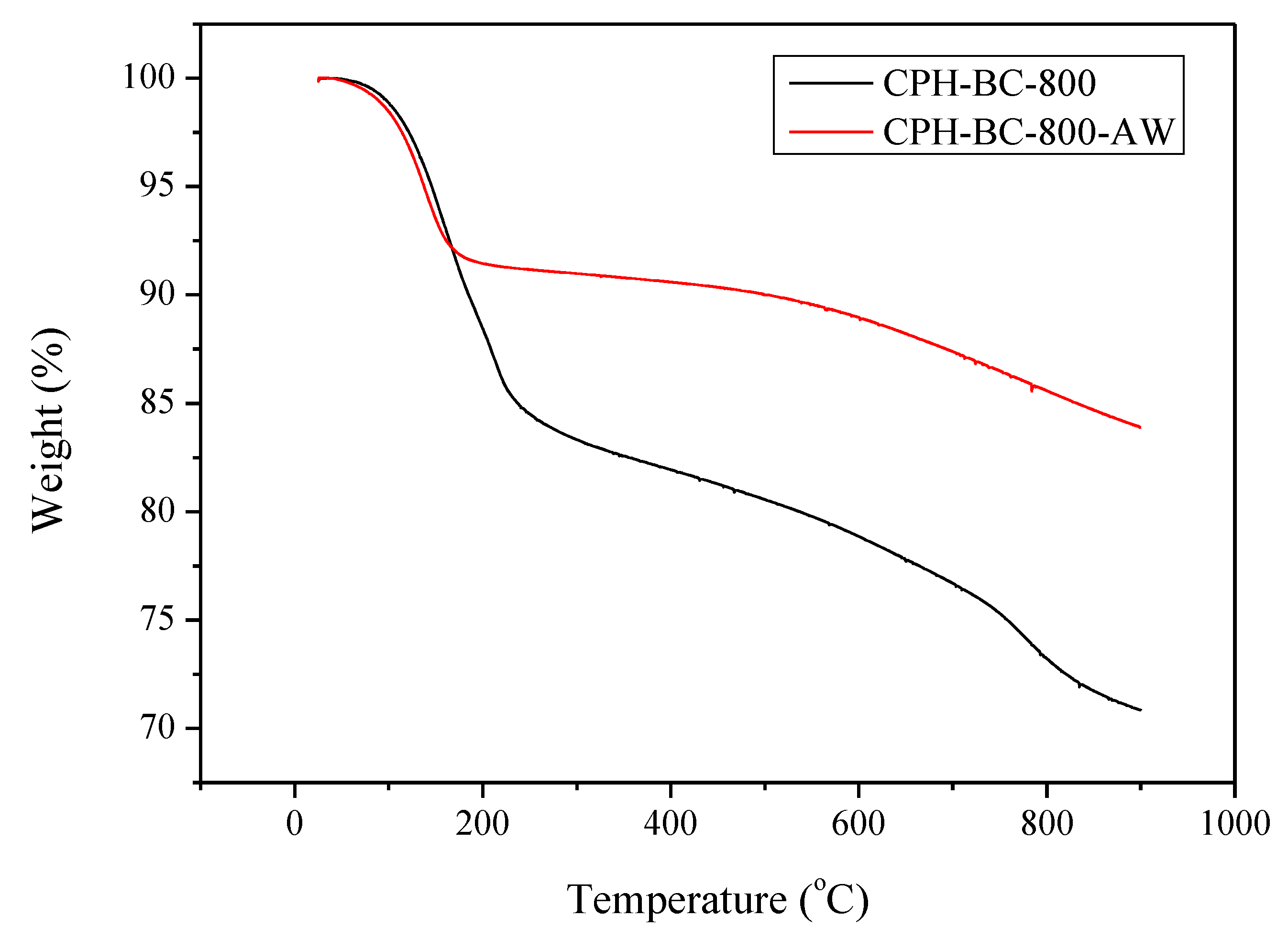
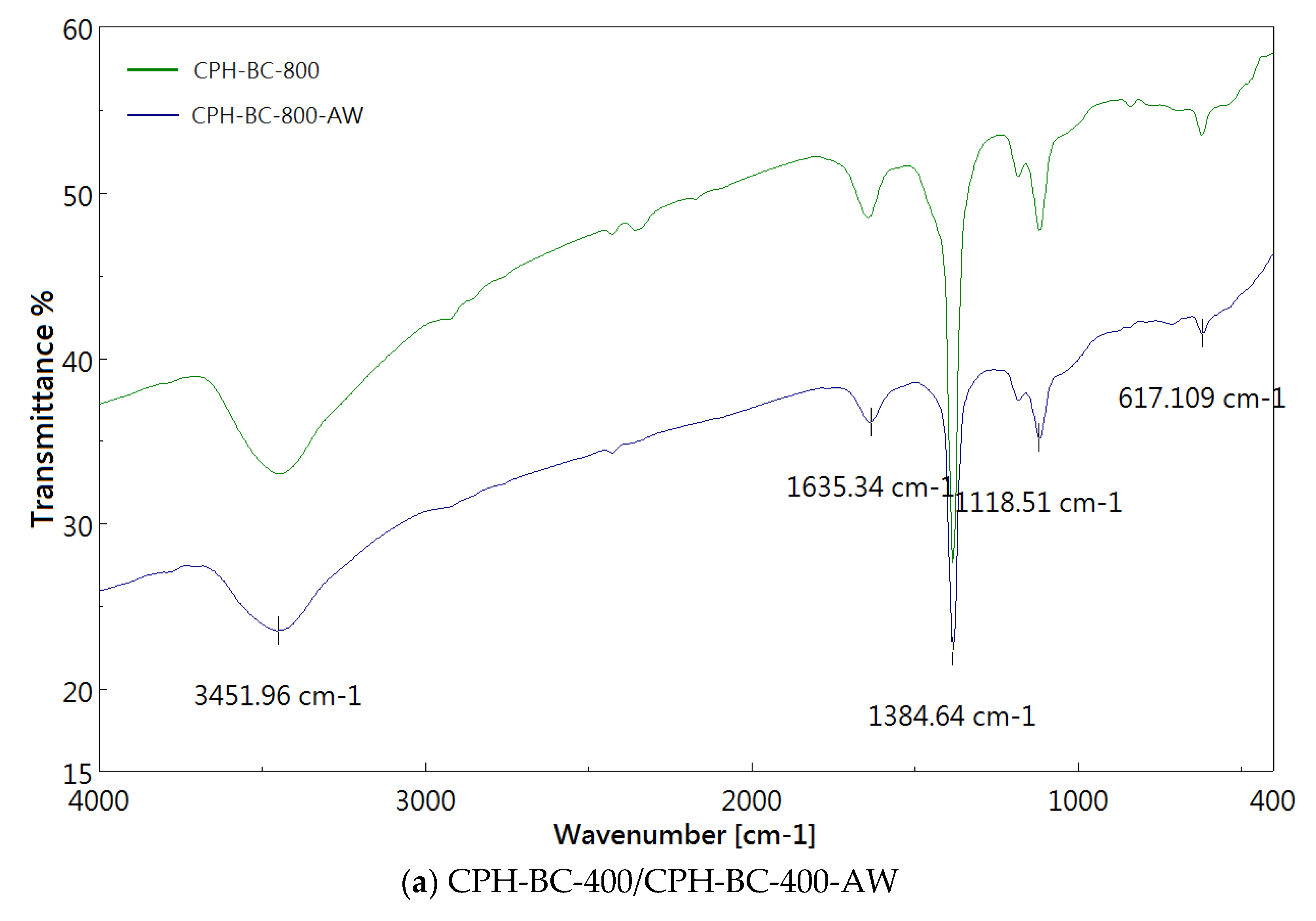
3.2. Chemical Characterization of Resulting Biochar
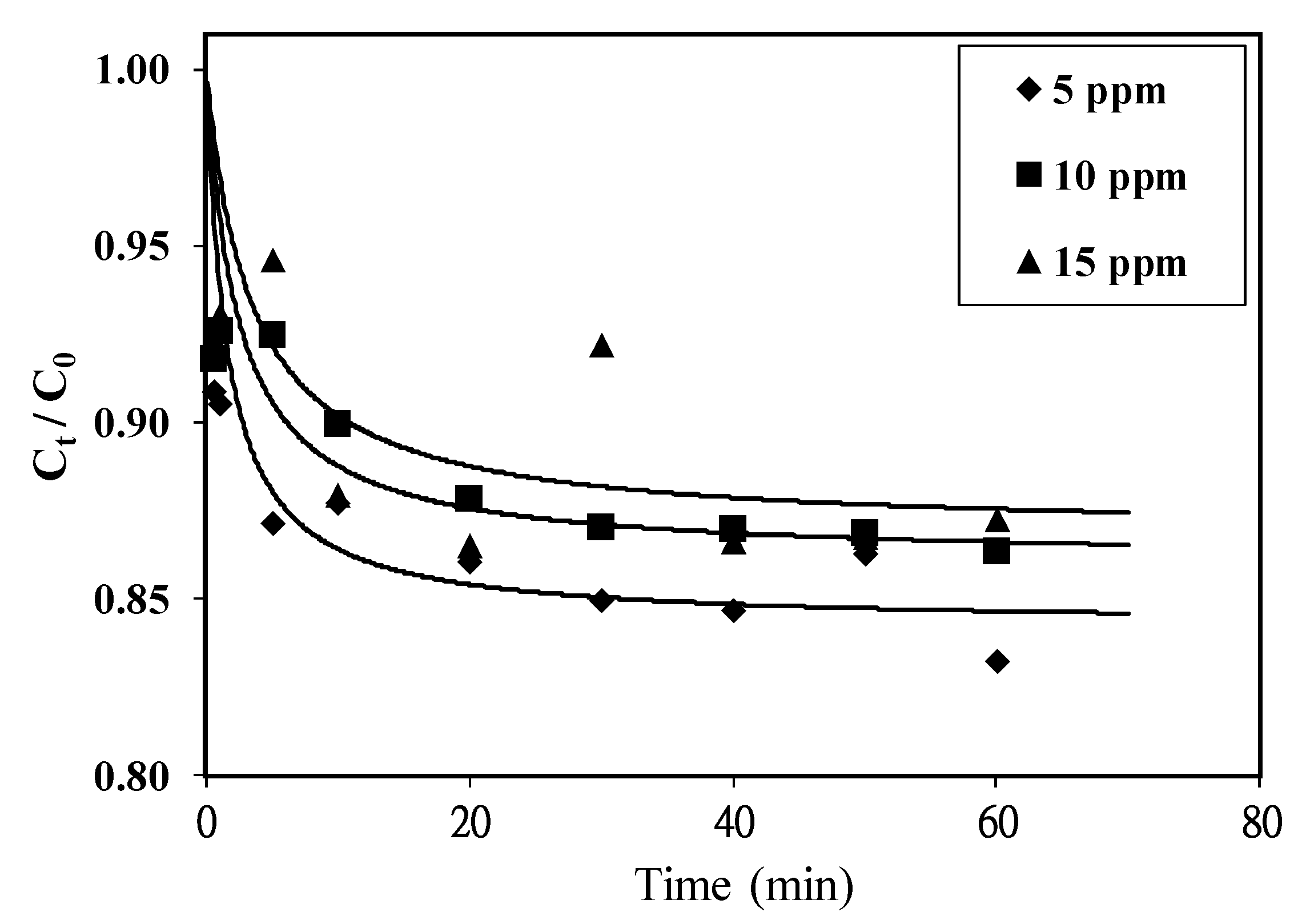
3.3. Adsorption Performance of Resulting Biochar
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The International Cocoa Organization (ICCO). Annual Report-2014–2015; ICCO: Abidjan, Côte d’Ivoire. Available online: www.icco.org/about-us/international-cocoa-agreements/cat_view/1-annual-report.html (accessed on 29 June 2019).
- Vriesmann, L.C.; Teofilo, R.F.; Petkowicz, C.L.O. Extraction and characterization of pectin from cacao pod husks (Theobroma cacao L.) with citric acid. LWT Food Sci. Technol. 2012, 49, 108–116. [Google Scholar] [CrossRef]
- Oddoye, E.O.K.; Agyente-Badu, C.K.; Gyedu-Akoto, E. Cocoa and its by-products: Identification and utilization. In Chocolate in Health and Nutrition; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Springer: New York, NY, USA, 2013; pp. 23–37. [Google Scholar]
- Figueira, A.; Janick, J.; BeMiller, J.N. New products from Theobroma cacao: Seed pulp and pod gum. In New Crops; Janick., J., Simon, J.E., Eds.; Wiley: New York, NY, USA, 1993; pp. 475–478. [Google Scholar]
- Okiyama, D.C.G.; Navarro, S.L.B.; Rodrigues, C.E.C. Cocoa shell and its compounds: Applications in the food industry. Trends Food Sci. Technol. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- Syamsiro, M.; Saptoadi, H.; Tambunan, B.H.; Pambudi, N.A. A preliminary study on use of cocoa pod husk as a renewable source of energy in Indonesia. Energy Sustain. Dev. 2012, 16, 74–77. [Google Scholar] [CrossRef]
- Martinez-Angel, J.D.; Villamizar-Gallardo, R.A.; Ortiz-Rodriguez, O.O. Characterization and evaluation of cocoa (Theobroma cacao L.) pod husk as a renewable energy source. Agrociencia 2015, 49, 329–345. [Google Scholar]
- El-Shekeil, Y.A.; Sapuan, S.M.; Algrafi, M.W. Effect of fiber loading on mechanical and morphological properties of cocoa pod husk fibers reinforced thermoplastic polyurethane composites. Mater. Des. 2014, 64, 330–333. [Google Scholar] [CrossRef]
- Chun, K.S.; Husseinsyah, S.; Osman, H. Utilization of cocoa pod husk as filler in polypropylene biocomposites: Effect of maleated polypropylene. J. Thermoplast. Compos. 2015, 28, 1507–1521. [Google Scholar] [CrossRef]
- Bello, O.S.; Ahmad, M.A. Adsorptive removal of a synthetic textile dye using cocoa pod husk. Toxicol. Environ. Chem. 2011, 93, 1298–1308. [Google Scholar] [CrossRef]
- Mylsamy, S.; Theivarasu, C. Adsorption of reactive dye using low cost adsorbent: Cocoa (Theobroma cacao) shell. World J. Appl. Environ. Chem. 2012, 1, 22–29. [Google Scholar]
- Njoku, V.O.; Ayuk, A.A.; Oguzie, E.E.; Ejike, E.N. Biosorption of Cd (II) from aqueous solution by cocoa pod husk biomass: Equilibrium, kinetic, and thermodynamic studies. Sep. Sci. Technol. 2012, 47, 753–761. [Google Scholar] [CrossRef]
- Pua, F.L.; Sajab, M.S.; Chia, C.H.; Zakaria, S.; Rahman, I.A.; Salit, M.S. Alkaline-treated cocoa pod husks as adsorbent for removing methylene blue from aqueous solutions. J. Environ. Chem. Eng. 2013, 1, 460–465. [Google Scholar] [CrossRef]
- Ahmad, F.; Daud, W.M.A.W.; Ahmad, M.A.; Radzi, R. Using cocoa (Theobroma cacao) shell-based activated carbon to remove 4-nitrophenol from aqueous solution: Kinetics and equilibrium studies. Chem. Eng. J. 2011, 178, 461–467. [Google Scholar]
- Tsai, C.H.; Tsai, W.T.; Liu, S.C.; Lin, Y.Q. Thermochemical characterization of biochar from cocoa pod husk prepared at low pyrolysis temperature. Biomass Convers. Biorefin. 2018, 8, 237–243. [Google Scholar] [CrossRef]
- Tsai, W.T.; Huang, P.C. Characterization of acid-leaching cocoa pod husk (CPH) and its resulting activated carbon. Biomass Convers. Biorefin. 2018, 8, 521–528. [Google Scholar] [CrossRef]
- Adjin-Tetteh, M.; Asiedu, N.; Dodoo-Arhin, D.; Karam, A.; Amaniampong, P.N. Thermochemical conversion and characterization of cocoa pod husks a potential agricultural waste from Ghana. Ind. Crops Prod. 2018, 119, 304–312. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction, 2nd ed.; Academic Press: London, UK, 2013. [Google Scholar]
- Mukherjee, A.; Lal, R. Biochar impacts on soil physical properties and greenhouse gas emissions. Agronomy 2013, 3, 313–339. [Google Scholar] [CrossRef]
- Biochar Use in Soils. Available online: https://biochar-international.org/soil-health/ (accessed on 29 June 2019).
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction. In Biochar for Environmental Management, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Routledge: New York, NY, USA, 2015; pp. 1–13. [Google Scholar]
- Titiloye, J.O.; Baker, M.S.A.; Odetoye, T.E. Thermochemical characterisation of agricultural wastes from West Africa. Ind. Crops Prod. 2013, 47, 199–203. [Google Scholar] [CrossRef]
- Miles, T.R.; Rasmussen, E.M.; Gray, M. Aqueous contaminant removal and stormwater treatment using biochar. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Guo, M., He, Z., Uchimiya, S.M., Eds.; Soil Science Society of America: Madison, WI, USA, 2016; pp. 341–376. [Google Scholar]
- Steiner, C. Considerations in biochar characterization. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Guo, M., He, Z., Uchimiya, S.M., Eds.; Soil Science Society of America: Madison, WI, USA, 2016; pp. 87–100. [Google Scholar]
- Tsai, T.W.; Hsu, C.H.; Lin, Y.Q. Highly porous and nutrients-rich biochar derived from dairy cattle manure and its potential for removal of cationic compound from water. Agriculture 2019, 9, 114. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area, and Porosity; Academic Press: London, UK, 1982. [Google Scholar]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Smith, J.M. Chemical Engineering Kinetics, 3rd ed.; McGraw-Hill: New York, NY, USA, 1981. [Google Scholar]
- Suzuki, M. Adsorption Engineering; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Mukome, F.N.D.; Parikh, S.J. Chemical, physical, and surface characterization of biochar. In Biochar: Production, Characterization, and Applications; Ok, Y.S., Uchimiya, S.M., Chang, S.X., Bolan, N., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 67–96. [Google Scholar]
- Chia, C.H.; Downie, A.; Munroe, P. Characteristics of biochar: Physical and structural properties. In Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Routledge: New York, NY, USA, 2015; pp. 89–109. [Google Scholar]
- Leng, L.; Huang, H. An overview of the effect of pyrolysis process parameters on biochar stability. Bioresour. Technol. 2018, 270, 627–642. [Google Scholar] [CrossRef]
- Masek, O.; Johnston, C.T. Thermal analysis for biochar characterisation. In Biochar: A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 283–293. [Google Scholar]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Johnston, C.T. Biochar analysis by Fourier-transform infra-red spectroscopy. In Biochar: A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 199–213. [Google Scholar]
- Ho, Y.S.; Chiang, C.C.; Hsu, Y.C. Sorption kinetics for dye removal from aqueous solution using activated clay. Sep. Sci. Technol. 2001, 36, 2473–2488. [Google Scholar] [CrossRef]
- Demirbas, E.; Kobya, M.; Sulak, M.T. Adsorption kinetics of a basic dye from aqueous solutions onto apricot stone activated carbon. Bioresour. Technol. 2008, 99, 5368–5373. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Golestani Nasab, A.; Khodadoust, S.; Rajabi, M.; Azizian, S. Application of activated carbon as adsorbents for efficient removal of methylene blue: Kinetics and equilibrium study. J. Ind. Eng. Chem. 2014, 20, 2317–2324. [Google Scholar] [CrossRef]
- Tsai, W.T.; Huang, P.C.; Lin, Y.Q. Reusing cow manure for the production of activated carbon by potassium hydroxide (KOH) activation process and its liquid-phase adsorption performance. Processes 2019, 7, 737. [Google Scholar] [CrossRef]

| Biochar Product a | SBET b (m2/g) | Vt c (cm3/g) | Dave d (Å) | ρs e (g/cm3) | ρp f (g/cm3) | εp g (g/cm3) |
|---|---|---|---|---|---|---|
| CPH-BC-400 | 0.3 | 0.0028 | 328.62 | 1.4137 | 1.4080 | 0.0040 |
| CPH-BC-400-AW | 0.9 | 0.0042 | 197.18 | 1.4641 | 1.4552 | 0.0061 |
| CPH-BC-500 | 1.3 | 0.0053 | 167.96 | 1.3791 | 1.3691 | 0.0073 |
| CPH-BC-600 | 0.4 | 0.0030 | 336.87 | 1.7042 | 1.6955 | 0.0028 |
| CPH-BC-700 | 3.4 | 0.0044 | 51.71 | 1.6338 | 1.6221 | 0.0072 |
| CPH-BC-800 | 101.0 | 0.0593 | 23.47 | 1.4545 | 1.3390 | 0.0794 |
| CPH-BC-800-AW | 342.0 | 0.1949 | 22.79 | 1.9708 | 1.4239 | 0.2775 |
| Absorption Peak (cm−1) | Possible Functional Group Assignments |
|---|---|
| 3450 | O-H stretching |
| 1630 | C=O stretching or C=C aromatic stretching |
| 1380 | O-H bending (phenolic) or symmetric C-H bending |
| 1120 | C-O symmetric stretching |
| 620 | C-H bending |
| Initial MB Concentration (mg/L) | k (g/mg. min) | qe (mg/g) | Correlation Coefficient | t1/2 (min) |
|---|---|---|---|---|
| 5 | 0.119 | 5.25 | 0.983 | 1.60 |
| 10 | 0.045 | 9.29 | 0.996 | 2.39 |
| 15 | 0.022 | 13.18 | 0.912 | 3.45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, W.-T.; Hsu, C.-H.; Lin, Y.-Q.; Tsai, C.-H.; Chen, W.-S.; Chang, Y.-T. Enhancing the Pore Properties and Adsorption Performance of Cocoa Pod Husk (CPH)-Derived Biochars via Post-Acid Treatment. Processes 2020, 8, 144. https://doi.org/10.3390/pr8020144
Tsai W-T, Hsu C-H, Lin Y-Q, Tsai C-H, Chen W-S, Chang Y-T. Enhancing the Pore Properties and Adsorption Performance of Cocoa Pod Husk (CPH)-Derived Biochars via Post-Acid Treatment. Processes. 2020; 8(2):144. https://doi.org/10.3390/pr8020144
Chicago/Turabian StyleTsai, Wen-Tien, Chien-Hung Hsu, Yu-Quan Lin, Chi-Hung Tsai, Wen-Shing Chen, and Yao-Tsung Chang. 2020. "Enhancing the Pore Properties and Adsorption Performance of Cocoa Pod Husk (CPH)-Derived Biochars via Post-Acid Treatment" Processes 8, no. 2: 144. https://doi.org/10.3390/pr8020144
APA StyleTsai, W.-T., Hsu, C.-H., Lin, Y.-Q., Tsai, C.-H., Chen, W.-S., & Chang, Y.-T. (2020). Enhancing the Pore Properties and Adsorption Performance of Cocoa Pod Husk (CPH)-Derived Biochars via Post-Acid Treatment. Processes, 8(2), 144. https://doi.org/10.3390/pr8020144





