Flow and Diffusion Characteristics of Typical Halon Extinguishing Agent Substitute under Different Release Pressures
Abstract
:1. Introduction
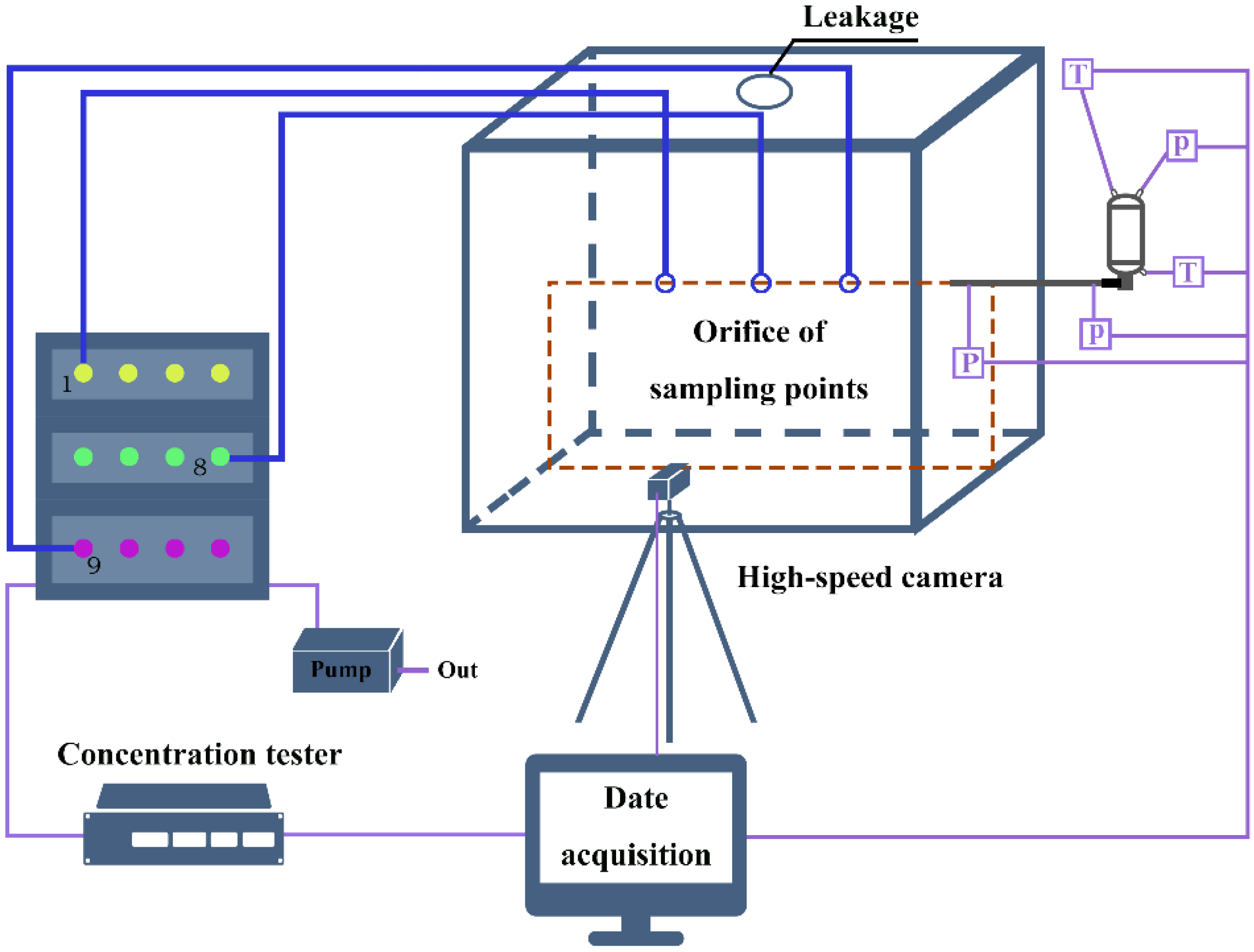
2. Experiment Apparatus
3. Fire Extinguishing Agent Properties and Experimental Conditions
4. Results and Discussion
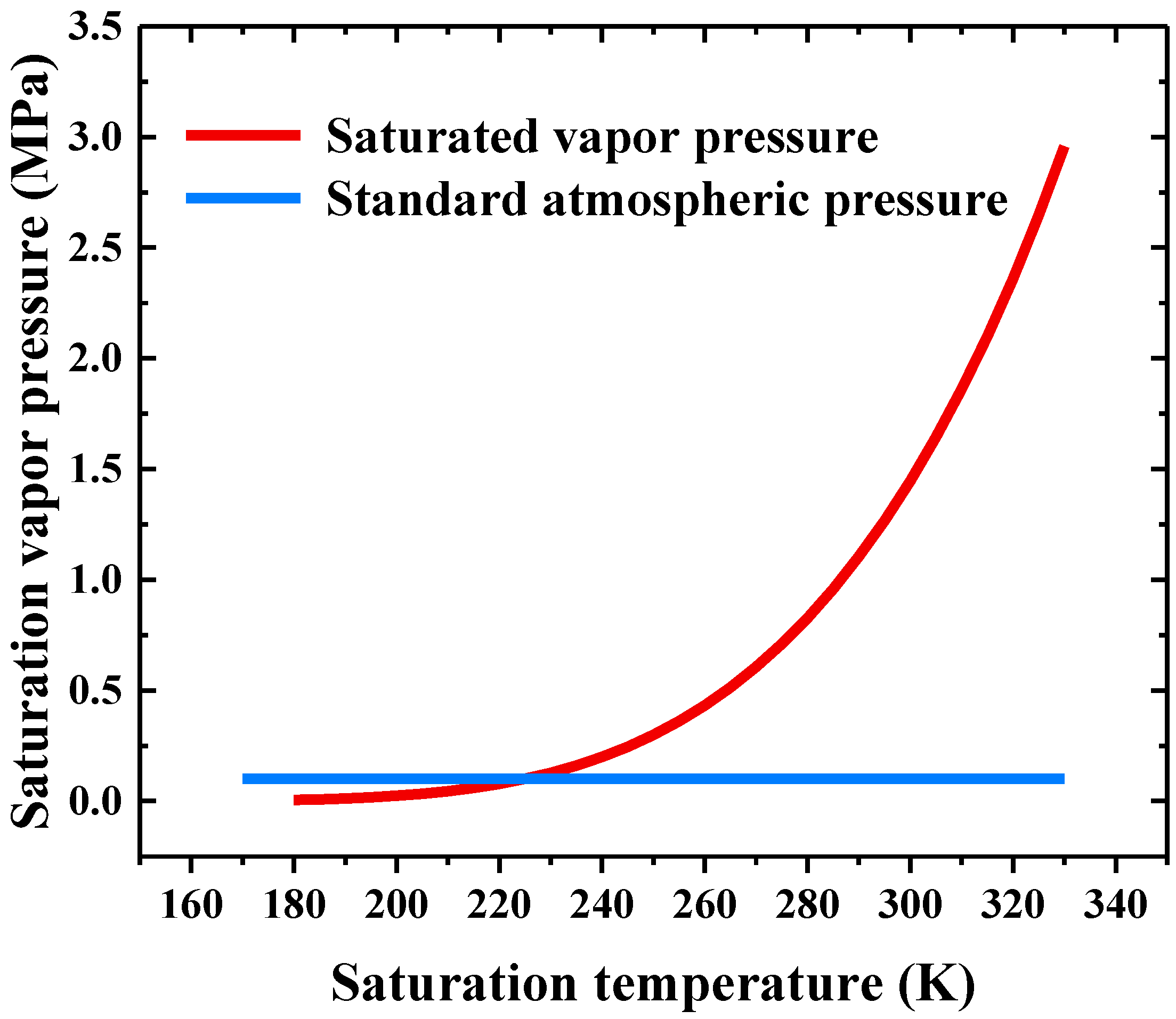
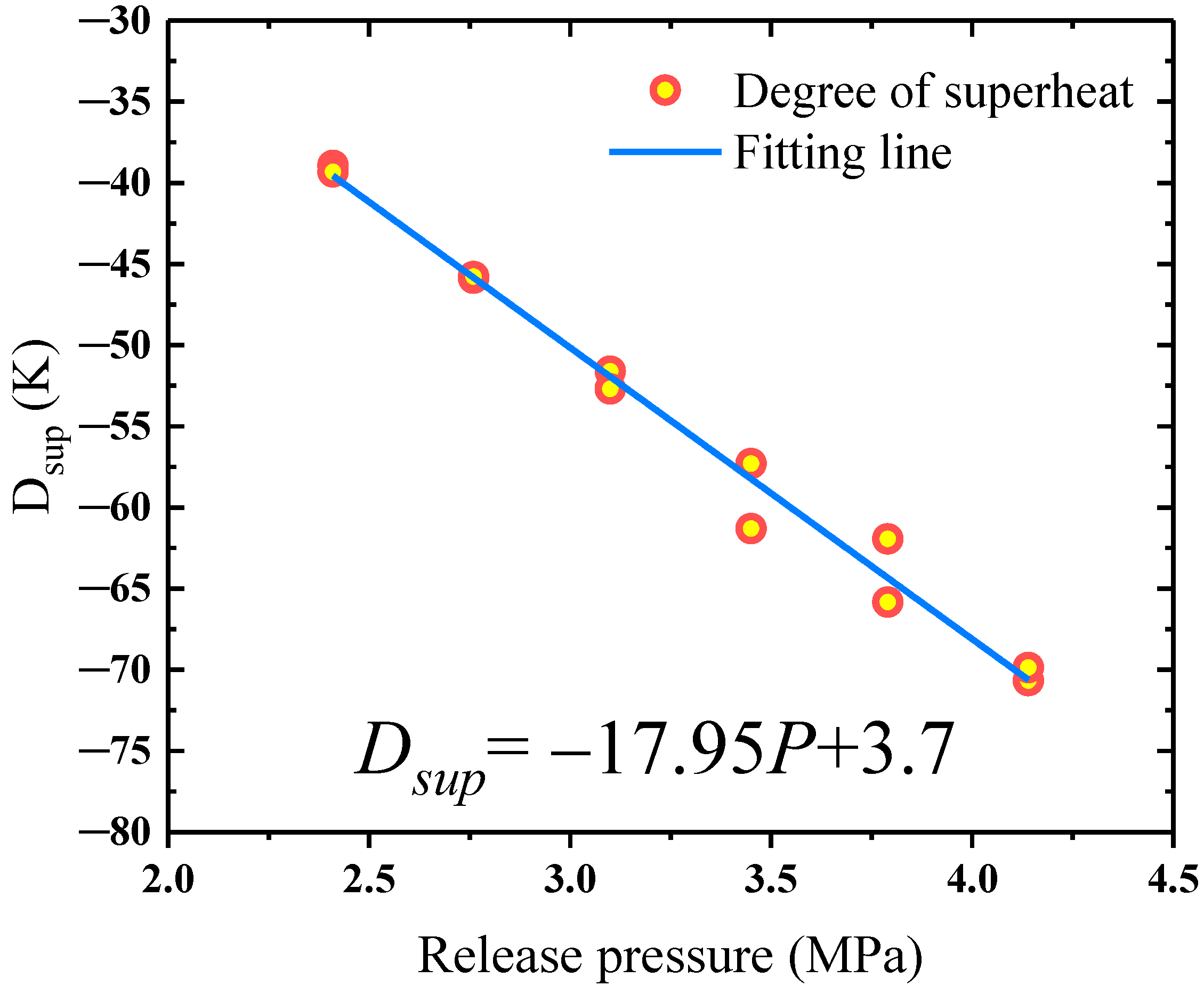
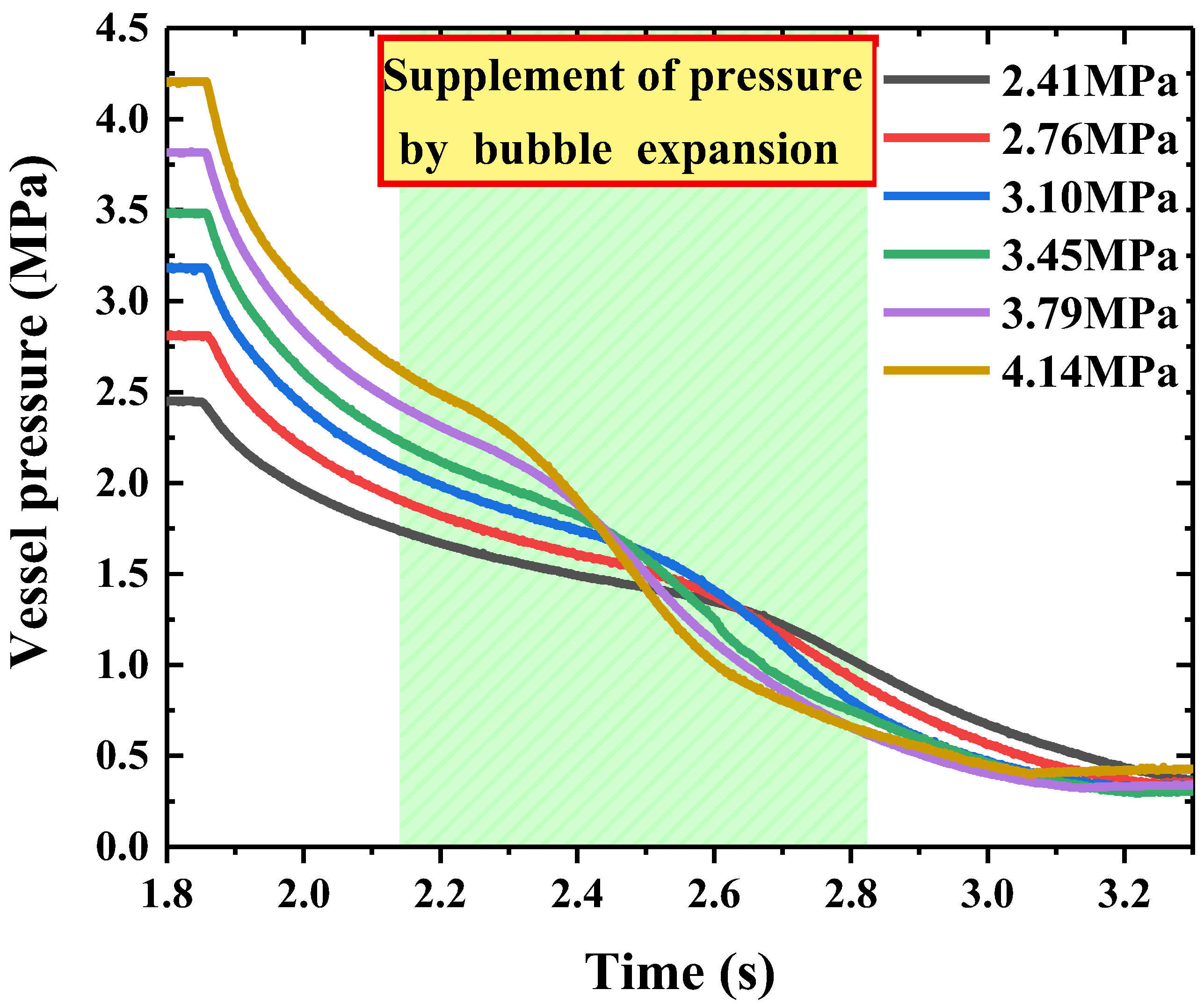
4.1. Degree of Superheat under Different Release Pressures
4.2. Injection Duration under Different Release Pressures
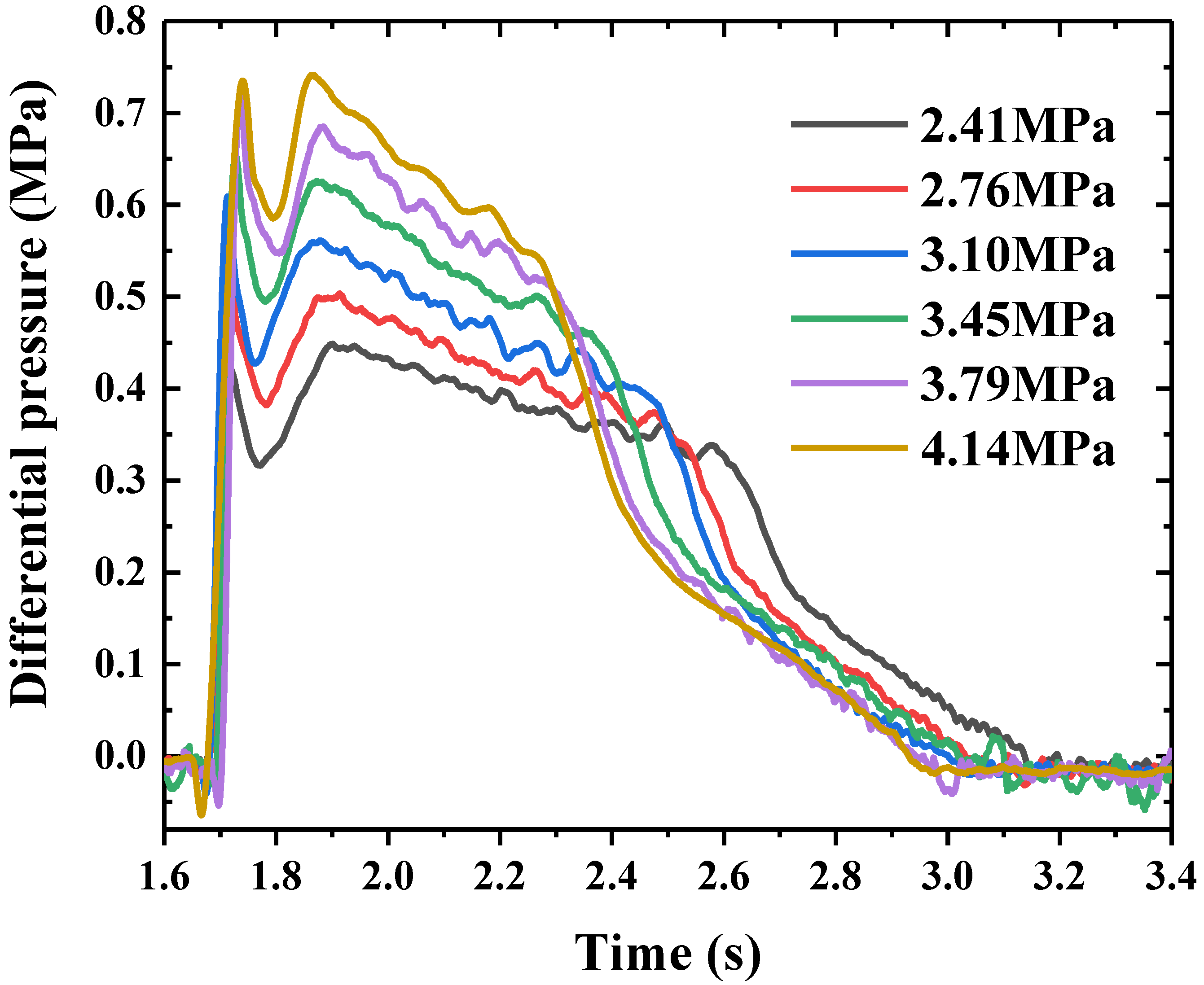
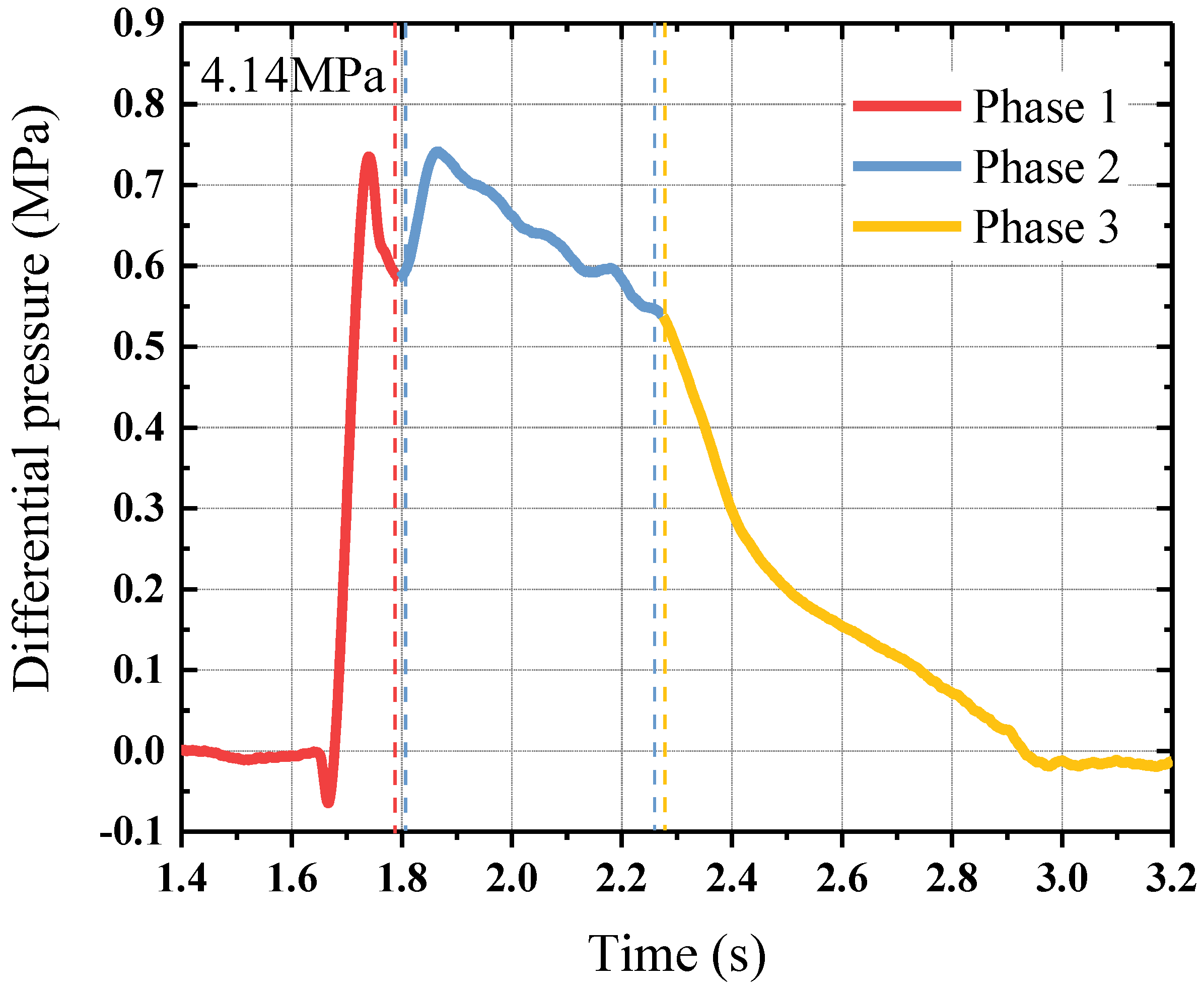
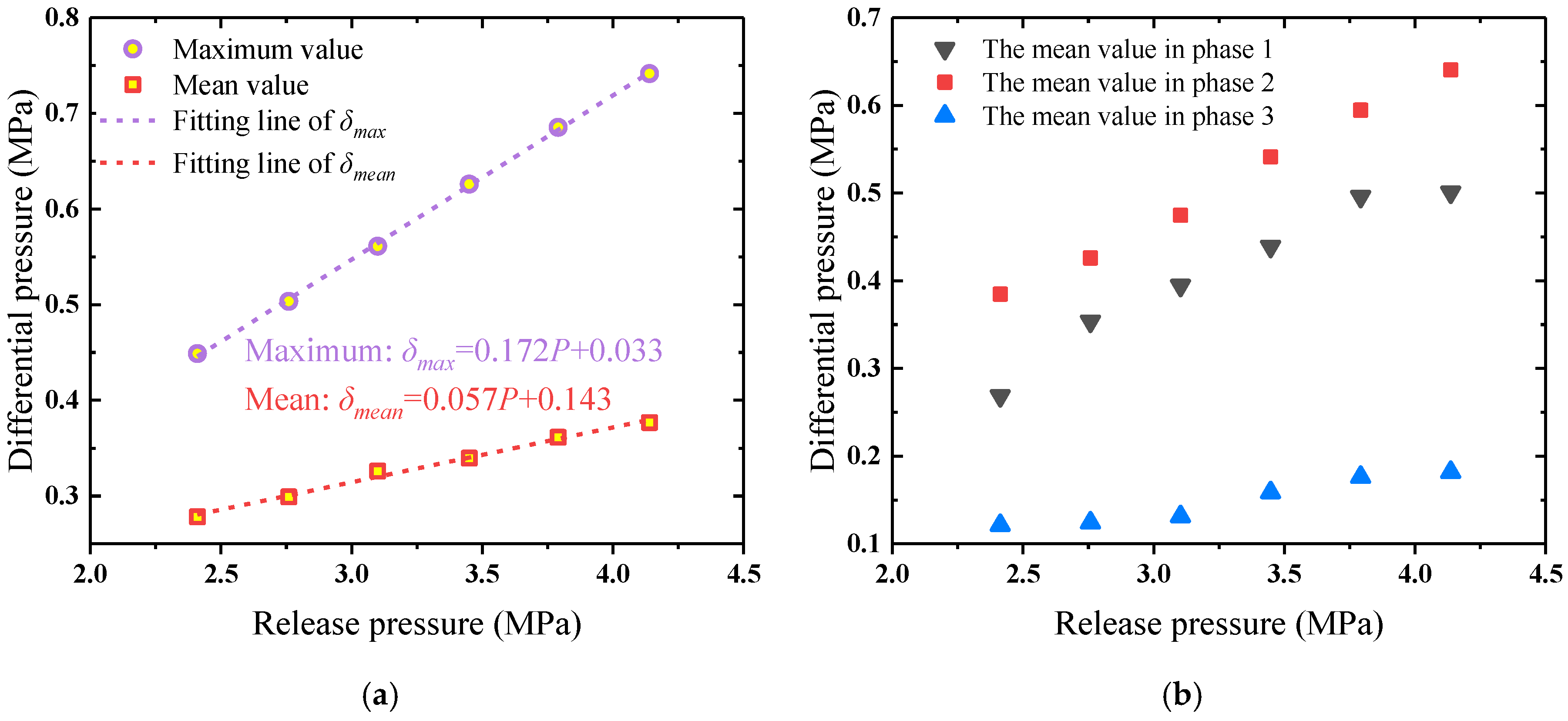
4.3. Pressure Loss in Pipeline under Different Release Pressures
- (1)
- Phase 1: Pipeline filling
- (2)
- Phase 2: Stable Flow
- (3)
- Phase 3: Mixed Gases Release
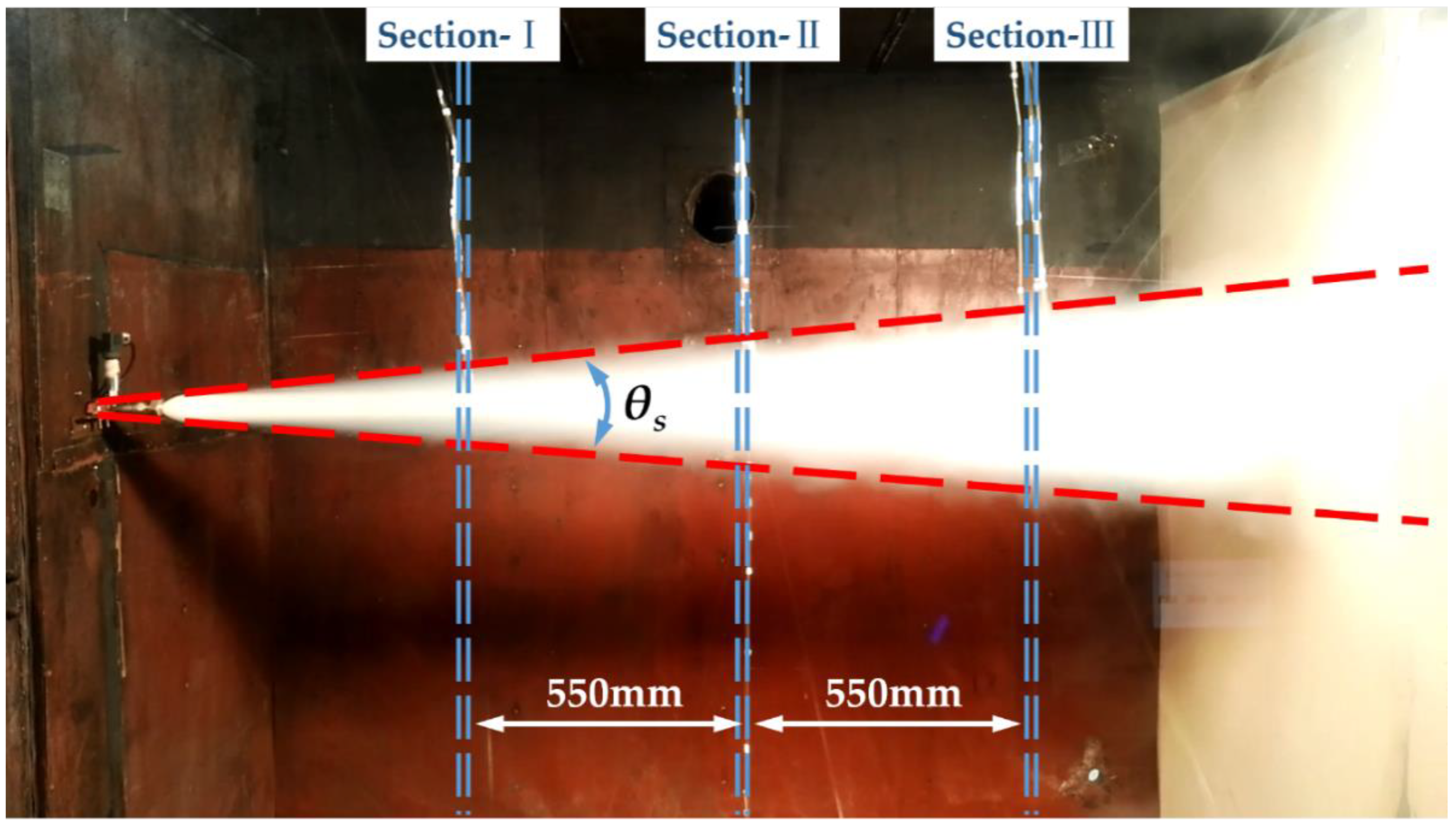
4.4. The Variation Characteristics of the Jet Structure in Near Field
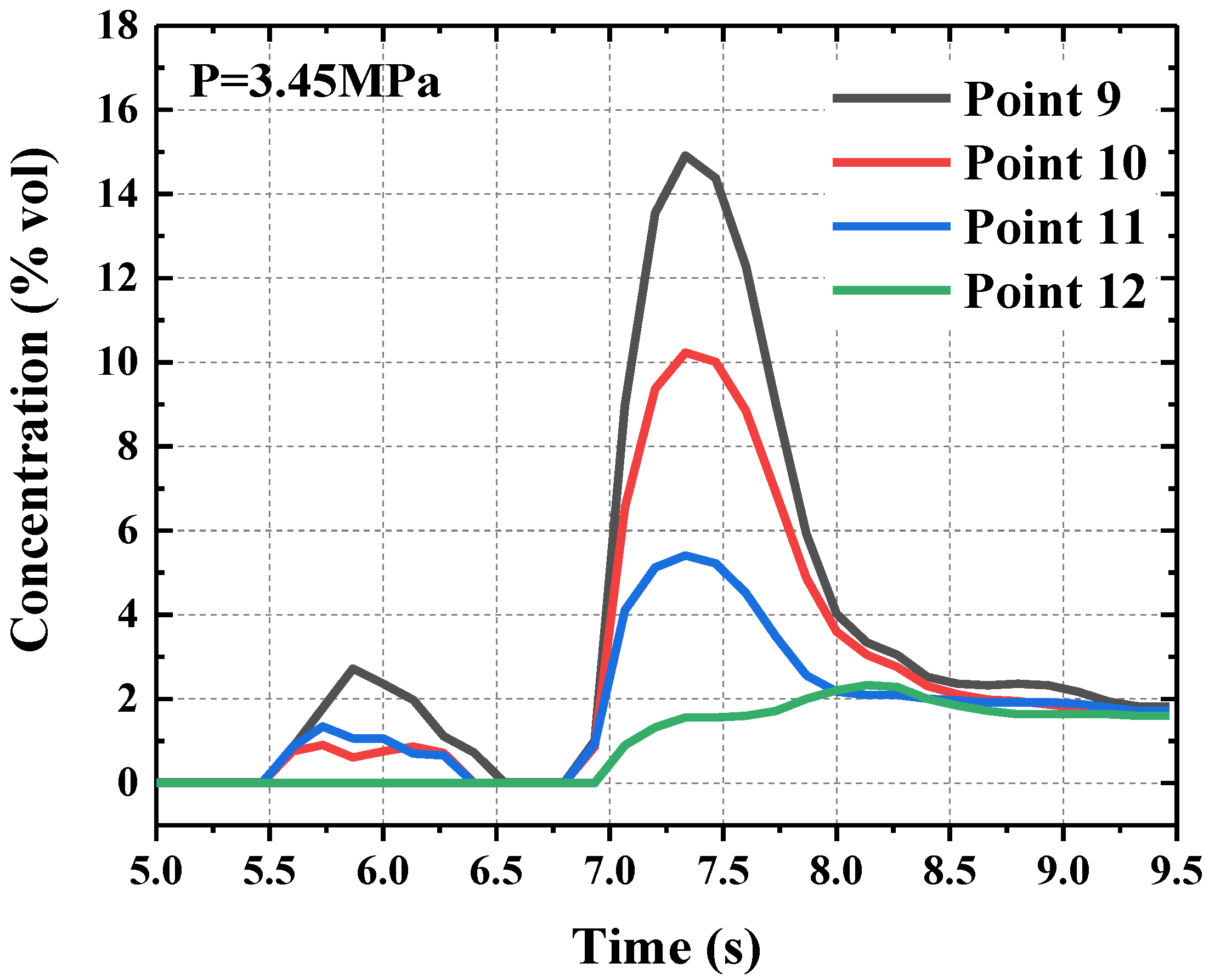
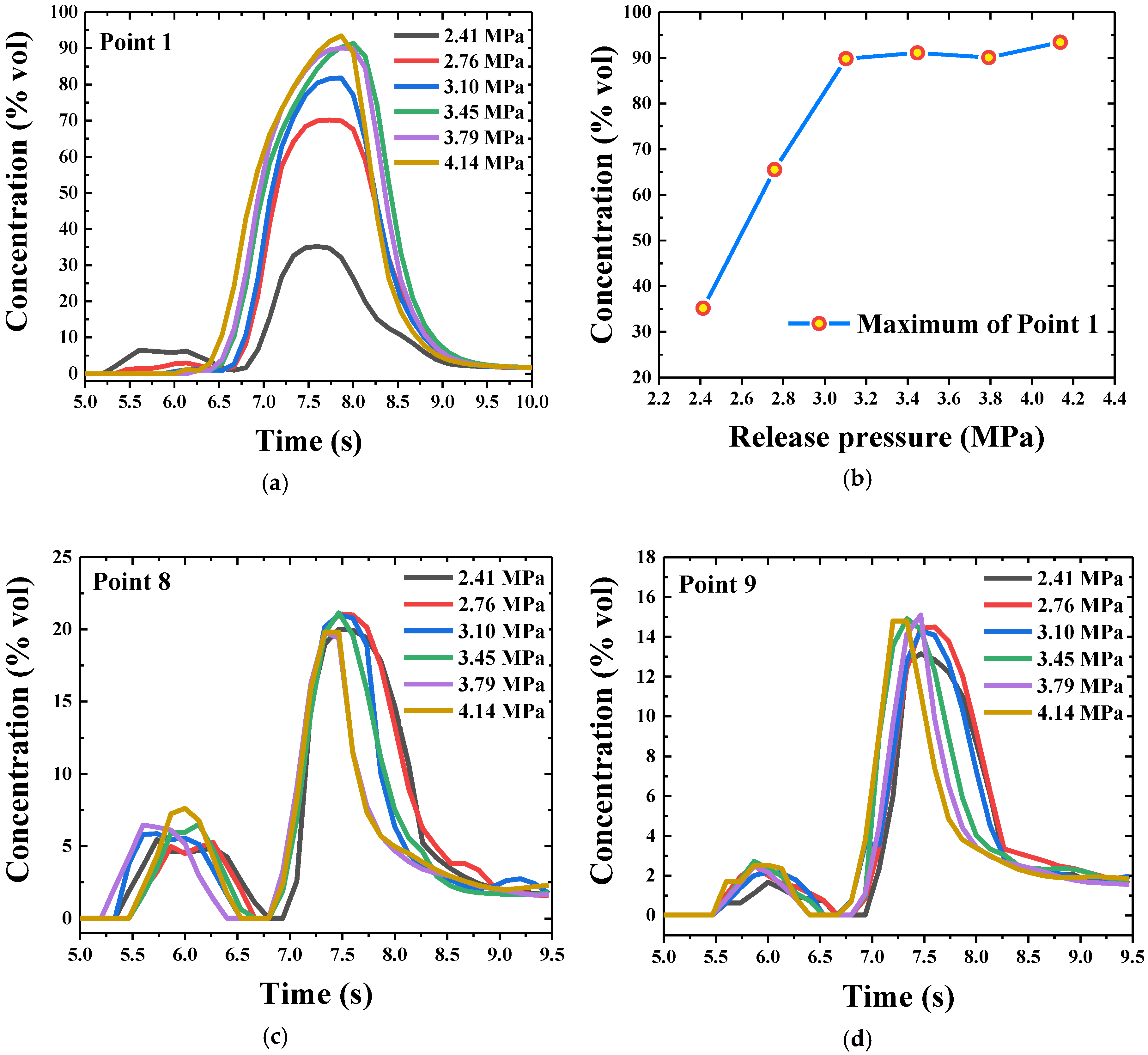
4.5. Concentration Distribution of HFC-125 Agent under Different Release Pressures
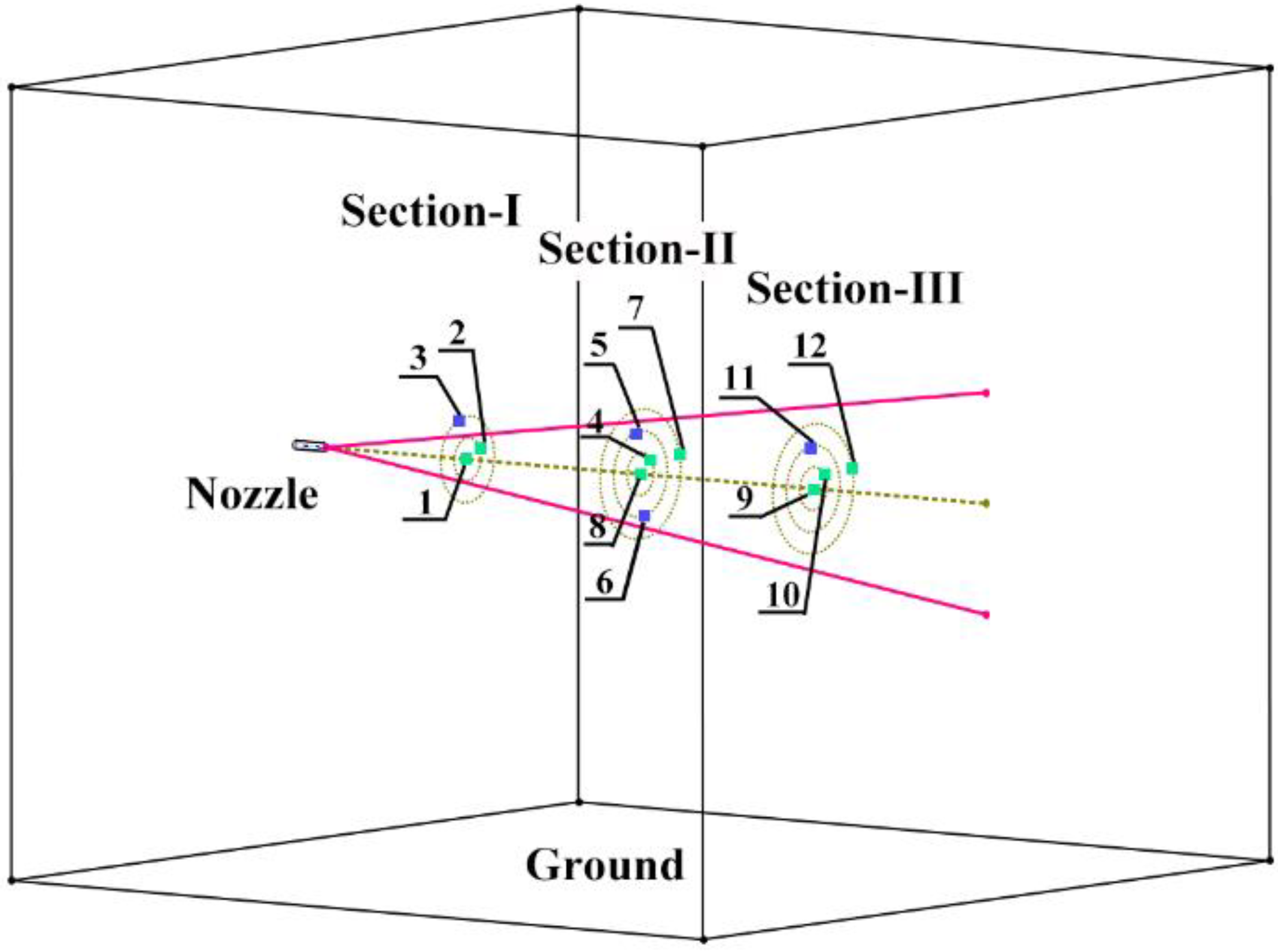
4.5.1. Concentration Distribution in Radial Section
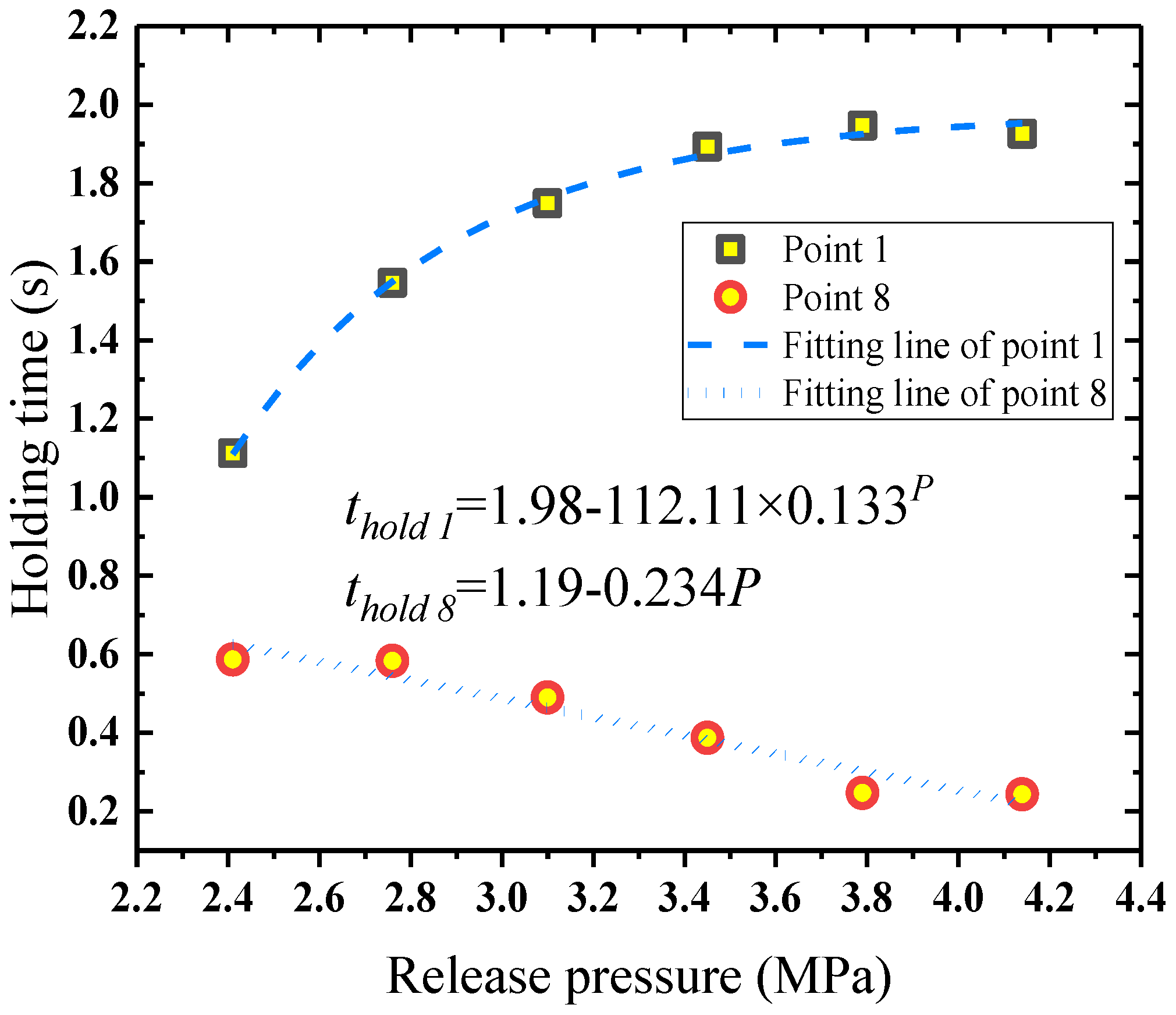
4.5.2. Concentration Distribution along the Jet under Different Release Pressures
5. Conclusions
- The degree of superheat of the HFC-125 in the storage vessel decreased linearly with the release pressure, indicating that high release pressure can assist in improving the fire suppression efficiency.
- The injection duration approximately decreased with the release pressure as a decay exponential function. In addition, the bubble expansion can slow down the pressure drop in the storage vessel, and the bubble expansion occurred earlier with the increase of the release pressure. Moreover, the release process of the HFC-125 agent was a two-phase (liquid and gas) flow.
- The flow process of the HFC-125 agent in the pipeline can be divided into three phases: pipeline filling, stable flow, and mixed gases release. The maximum and mean values of the differential pressure in the pipeline increased linearly with the release pressure. The pipeline pressure loss mainly occurred in the first two phases (stable flow and pipeline filling). The mean value of the differential pressure at each phase also increased with the release pressure.
- A typical jet plume structure occurred outside the nozzle. The jet process of the HFC-125 agent in the enclosure space can be divided into three stages: the development stage, the stable release stage, and the rapid decay stage.
- The maximum concentration value decreased with the increase of the distance from the nozzle. Moreover, the jet would deflect upward due to the effects of buoyancy. With the increase of release pressure, the maximum concentration value in the near field from the nozzle increased, and the maximum concentration value in the far field from the nozzle varied little. Furthermore, the backflow resulted by the restriction of the wall could decrease the concentration of the HFC-125 agent.
- The concentration and holding time of the HFC-125 agent could meet the requirement of MPS in some areas. The holding time of HFC-125 could achieve 0.5 s in some areas at low release pressures in the near field of the jet cone. With the increase of the release pressure, the holding time in the near field from the nozzle increased first, and then almost remained constant, which was opposite to that in the far field from the nozzle.
Author Contributions
Funding
Conflicts of Interest
References
- Li, H.; Min, X.; Dai, M.; Dong, X. The biomass potential and GHG (Greenhouse Gas) emissions mitigation of straw-based biomass power plant: A case study in Anhui Province, China. Processes 2019, 7, 608. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.; Ro, D.; Park, Y.K. Estimation, and Framework Proposal of Greenhouse Gas Emissions of Fluorinated Substitutes for Ozone-Depleting Substances by Application Area in the Republic of Korea. Sustainability 2020, 12, 6355. [Google Scholar] [CrossRef]
- Jou, R.-C.; Chen, T.-Y. Willingness to Pay of Air Passengers for Carbon-Offset. Sustainability 2015, 7, 3071. [Google Scholar] [CrossRef] [Green Version]
- Ingerson, D.A. FAA Engine Compartment Halon Replacement Project Background, Overview, and Current Status. In International Aircraft Fire and Cabin Safety Research Conference; Federal Aviation Administration: Washington, DC, USA, 1998. [Google Scholar]
- Ingerson, D.A. Simulating the Distribution of Halon 1301 in an Aircraft Engine Nacelle With HFC-125; Federal Aviation Administration: Washington, DC, USA, 1999.
- Bennett, M.V.; Bennett, J.M. Aircraft Engine/APU Fire Extinguishing System Design Model (HFC-125); Defense Technical Information Center: Fort Belvoir, VA, USA, 1997.
- Lu, Z.; Zhuang, L.; Dong, L.; Liang, X. Model-Based Safety Analysis for the Fly-by-Wire System by Using Monte Carlo Simulation. Processes 2020, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- FAA. Minimum Performance Standards for Halon 1301 Replacement in the Fire Extinguishing Agents/Systems of Civil Aircraft Engine and Auxiliary Power Unit Compartments(rev04); Federal Aviation Administration: Washington, DC, USA, 2010.
- Williamson, H. V Halon 1301 flow in pipelines. Fire Technol. 1976, 12, 18–32. [Google Scholar] [CrossRef]
- Elliott, D.G.; Garrison, P.W.; Klein, G.A.; Moran, K.M.; Zydowicz, M.P. Flow of nitrogen-pressurized Halon 1301 in fire extinguishing system. JPL Publ. 1984, 84–62, 1–124. [Google Scholar]
- Yang, J.C.; Huber, M.L.; Boyer, C.I. A model for calculating alternative agent/nitrogen thermodynamic properties. In Proceedings of the Halon Options Technical Working Conference, Albuquerque, NM, USA, 9–11 May 1995; Volume 11. [Google Scholar]
- Tuzla, K.; Palmer, T.; Chen, J.C.; Sundaram, R.K.; Yeung, W.S. Development of Computer Program for Fire Suppressant Fluid Flow; Lehigh University: Bethlehem, PA, USA, 1998. [Google Scholar]
- Kim, J.; Baek, B.; Lee, J. Numerical analysis of flow characteristics of fire extinguishing agents in aircraft fire extinguishing systems. J. Mech. Sci. Technol. 2009, 23, 1877–1884. [Google Scholar] [CrossRef]
- Sarkos, C.P. Characteristics of Halon 1301 Dispensing Systems for Aircraft Cabin Fire Protection; National Aviation Facilities Experimental Center: Atlantic City, NJ, USA, 1975.
- Niu, X.; Xie, Y.; Hasemi, Y. Analysis of Fire Spread and Fire Extinguishing Agent Distribution in Nacelle of Helicopter under No-ventilation Condition. Procedia Eng. 2013, 62, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Kurokawa, F.Y.; De Andrade, C.R.; Zaparoli, E.L. Numerical simulation of the concentration of fire extinguishing agent in aircraft cargo compartment. In Proceedings of the 10th Brazilian Congress of Thermal Sciences and Engineering, Rio de Janeiro, Brazil, 29 November–4 December 2004. [Google Scholar]
- Kurokawa, F.Y.; De Andrade, C.R.; Zaparoli, E.L. Modeling of aircraft fire suppression system by the lumped parameter approach. Aircr. Eng. Aerosp. Technol. 2016, 88, 535–539. [Google Scholar] [CrossRef]
- Zaparoli, E.L. Numerical simulation of an aircraft cargo compartment fire suppression system using cfd tool. In Proceedings of the 19th International Congress of Mechanical Engineering, Gramado, Brazil, 15–20 November 2009. [Google Scholar]
- Lee, J. Simulation Method for the Fire Suppression Process Inside the Engine Core and APU Compartments. In Proceedings of the Fourth Triennial International Aircraft Fire and Cabin Safety Research Conference, Lisabon, Portugal, 15–18 November 2004. [Google Scholar]
- Xue, R.; Ruan, Y.; Liu, X.; Chen, L.; Liu, L.; Hou, Y. Numerical Study of the Effects of Injection Fluctuations on Liquid Nitrogen Spray Cooling. Processes 2019, 7, 564. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W. Characteristics and Mechanisms of Direct-Injection Liquid Jet and Flash-Boiling Spray; Shanghai Jiao Tong University: Shanghai, China, 2012. [Google Scholar]
- Geanette, P.; Arne, E.H.; George, M. General review of flashing jet studies. J. Hazard. Mater. 2010, 173, 2–18. [Google Scholar] [CrossRef]
- Lamanna, G.; Kamoun, H.; Weigand, B.; Steelant, J. Towards a unified treatment of fully flashing sprays. Int. J. Multiph. Flow 2014, 58, 168–184. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Pan, R.; Chen, R.; Xu, X.; Li, Q. Flow and Diffusion Characteristics of Typical Halon Extinguishing Agent Substitute under Different Release Pressures. Processes 2020, 8, 1684. https://doi.org/10.3390/pr8121684
Jin J, Pan R, Chen R, Xu X, Li Q. Flow and Diffusion Characteristics of Typical Halon Extinguishing Agent Substitute under Different Release Pressures. Processes. 2020; 8(12):1684. https://doi.org/10.3390/pr8121684
Chicago/Turabian StyleJin, Jiaming, Renming Pan, Ruiyu Chen, Xiaokang Xu, and Quanwei Li. 2020. "Flow and Diffusion Characteristics of Typical Halon Extinguishing Agent Substitute under Different Release Pressures" Processes 8, no. 12: 1684. https://doi.org/10.3390/pr8121684
APA StyleJin, J., Pan, R., Chen, R., Xu, X., & Li, Q. (2020). Flow and Diffusion Characteristics of Typical Halon Extinguishing Agent Substitute under Different Release Pressures. Processes, 8(12), 1684. https://doi.org/10.3390/pr8121684





