3.1. Simulated Gas Utilization Scenarios
In the scope of the methanol synthesis process simulation, four gas utilization scenarios were considered. Prior to its utilization in the methanol process, the considered steel-mill gas streams were enriched with hydrogen corresponding to a stoichiometric number (SN) of 2.05. Two scenarios relate to the unconverted steel-mill gas streams BFG and BOFG. The CO and CO
2 shares remain unchanged. Additionally, a gas composition resulting from a mixture of BFG and BOFG in the ratio of 1:1 was simulated. The latter make-up gases exhibit a significant share of inert nitrogen. Furthermore, it was assumed that the CO share of BFG or BOFG was converted to CO
2 in a CO shift unit. Subsequently, the CO
2 fraction of the resulting gas stream was removed by a CO
2 separation process. The generated pure CO
2 stream, exhibiting no inert nitrogen, is the basis of another scenario. The resulting process feed gas compositions with respect to the four gas utilization scenarios are presented in
Table 2. The COG was not considered as one of the basic scenarios generating methanol because it exhibits a comparatively low amount of COx. However, the COG is currently considered as a source for pure H
2 due to its high H
2 concentration (cf.
Figure 1).
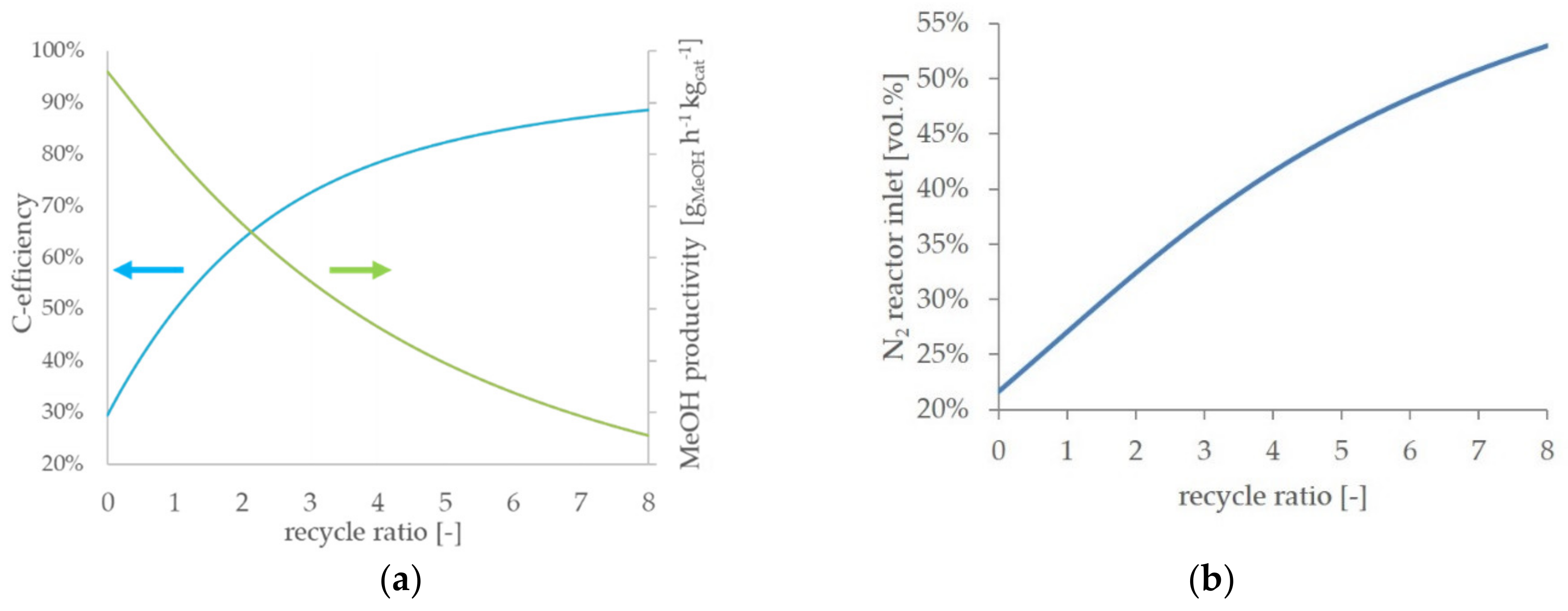
As mentioned before, the RR has a crucial effect on the process efficiency. Hence, the RR was varied and the simulation results are presented as a function of the RR. In
Figure 5a, the simulation results of the
BFG direct scenario are presented to point out the general effects of an increasing recycle ratio on the process efficiency. An RR of 0 corresponds to a one-pass process, and therefore, the highest methanol productivity is achieved. With an increasing RR, the methanol productivity in the reactor decreases. In contrast, the carbon efficiency increases with an increasing RR. As one of the major aims of the described work is a reduction of the CO
2 emissions, an operational point with low carbon efficiency is undesirable.
On the other hand, adequate methanol productivity is required for an economically feasible process. Both a decent carbon efficiency and an acceptable grade of methanol productivity are predicted at medium values of RR in a range between two and five. In
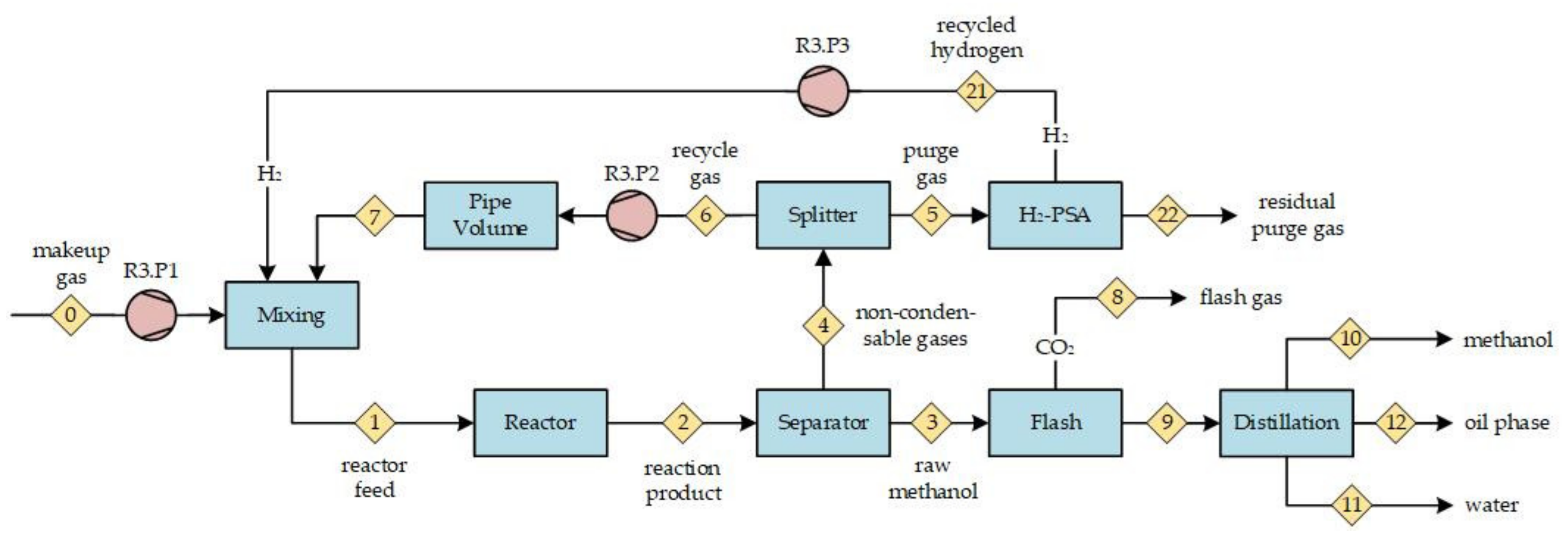
Figure 5b, the nitrogen concentration at the reactor inlet is presented. With an increasing RR, the inert nitrogen accumulates in the gas recycle loop (cf.
Figure 2, point <6>) and results in successive elevated nitrogen concentrations at the reactor inlet. At an RR of five, the nitrogen content nearly doubled compared to the process feed. An increasing nitrogen share dilutes the reactive compounds in the syngas and, therefore, contributes to a decrease in methanol productivity. All scenarios with a process feed significantly containing nitrogen qualitatively show the described trends.
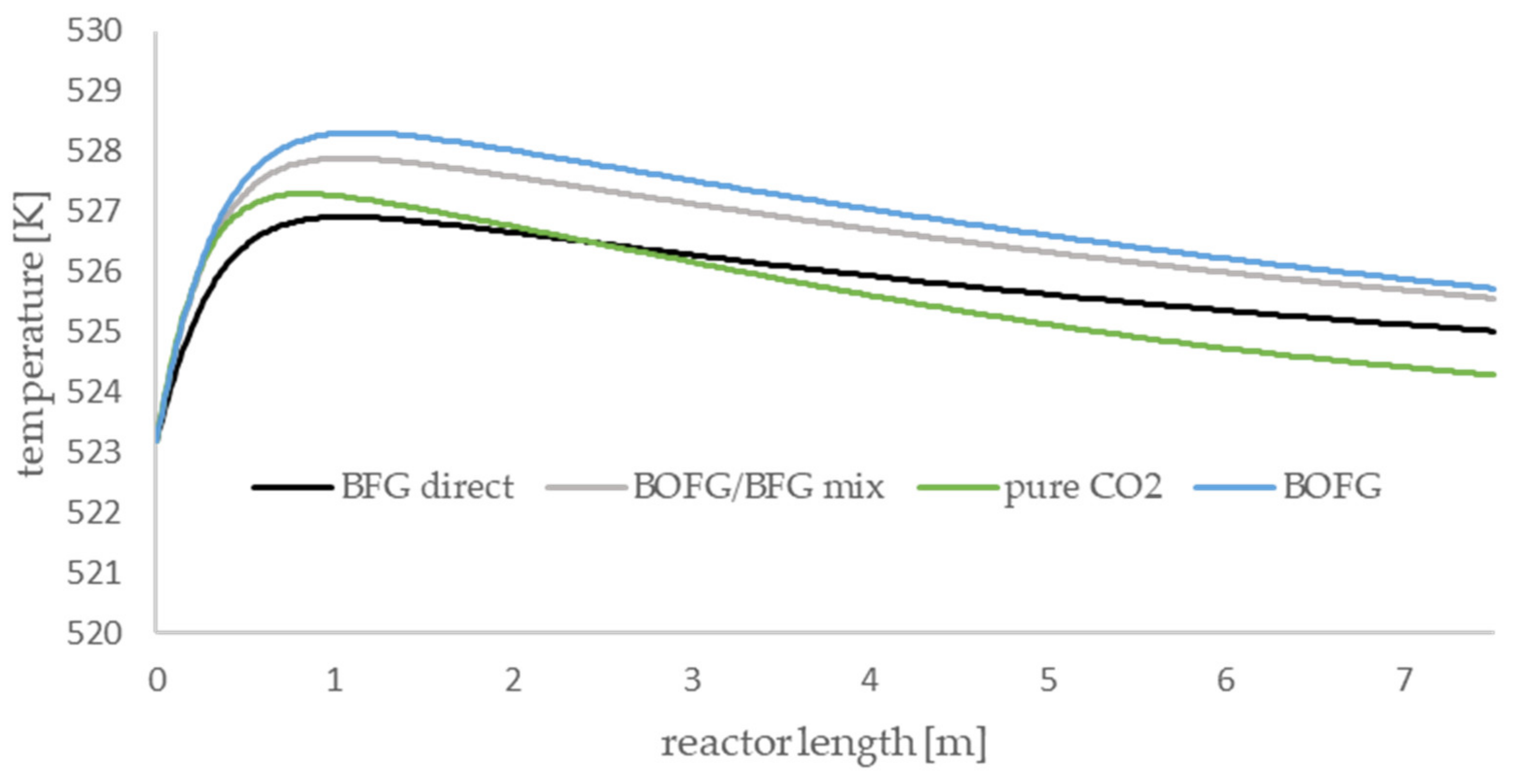
The axial temperature profiles of the methanol-synthesis reactor are presented in
Figure 6. All scenarios show the same qualitative trends, and the occurring temperature gradients are comparatively small. For all scenarios, a temperature maximum is observable in the first section of the reactor. The height of the maximum depends on the gas composition of the syngas. The BOFG case exhibits the highest temperature maximum and the BFG scenario exhibits the lowest maximum. Syngas with a lot of CO produces a higher temperature maximum compared to a syngas with high amounts of CO
2 and inert compounds.
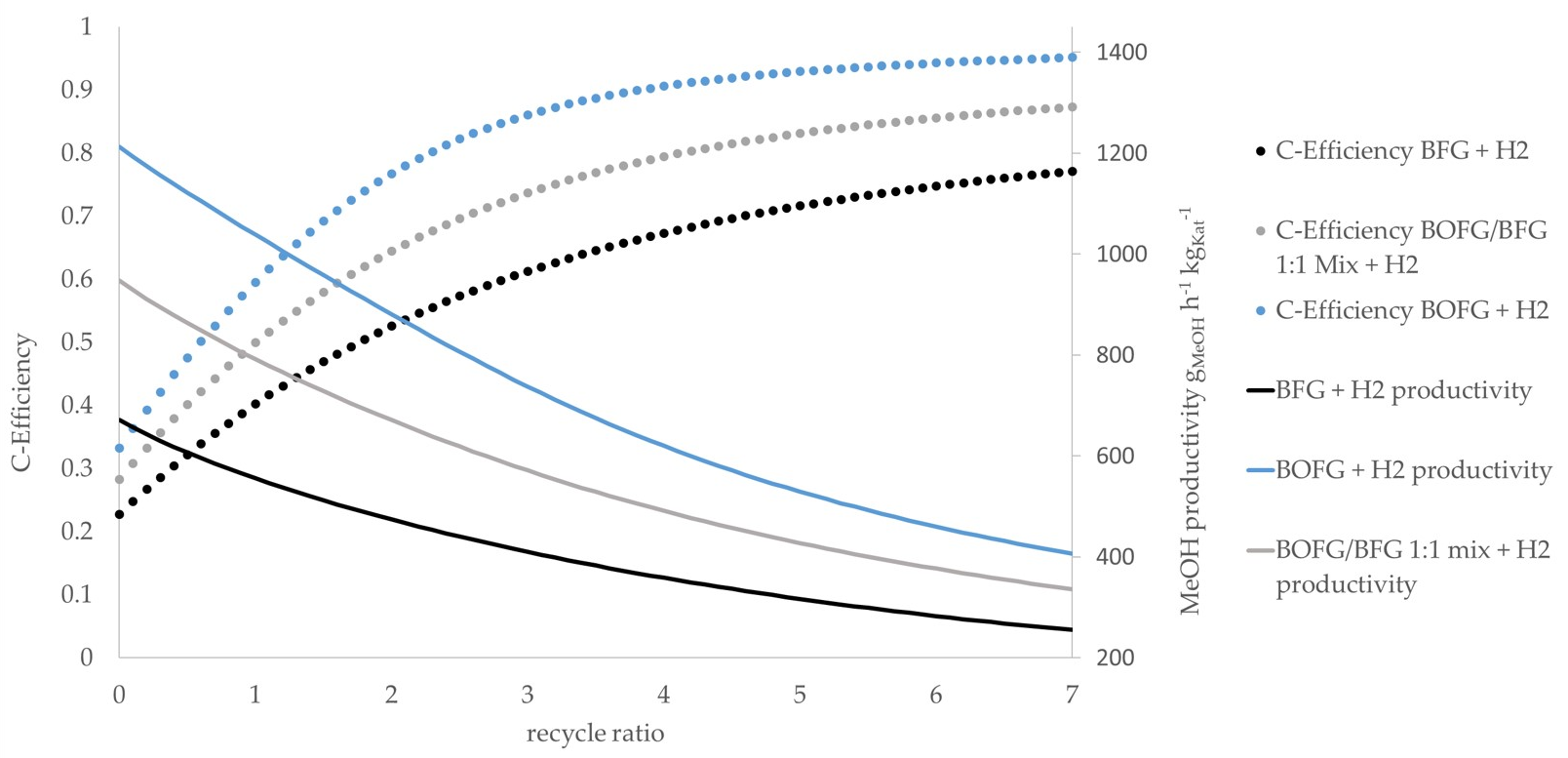
The simulation results of all scenarios are given in
Figure 7. The
BOFG direct scenario exhibits the highest methanol productivity, as it contains the most CO. As expected, the use of syngas with a high CO/CO
2 ratio results in higher methanol productivity compared to syngas with lower CO/CO
2 ratios while keeping the total amount of CO
x constant [
16]. The
BFG direct scenario shows the lowest methanol productivity as it is the syngas stream with the highest nitrogen share. Moreover, it contains higher CO
2 concentrations compared to the
BOFG scenario. The methanol productivity of the
BOFG/BFG mix scenario is between the
BOFG and the
BFG scenarios. Referring to the carbon efficiency, an increasing RR leads to an increasing carbon efficiency, as described before. Above an RR of approximately three, the gain in additional carbon efficiency slows down significantly, while the reactor efficiency keeps decreasing. It is assumed that the medium RR range between three and four represents a promising operational point as it shows a high carbon efficiency as well as sufficient methanol productivity.
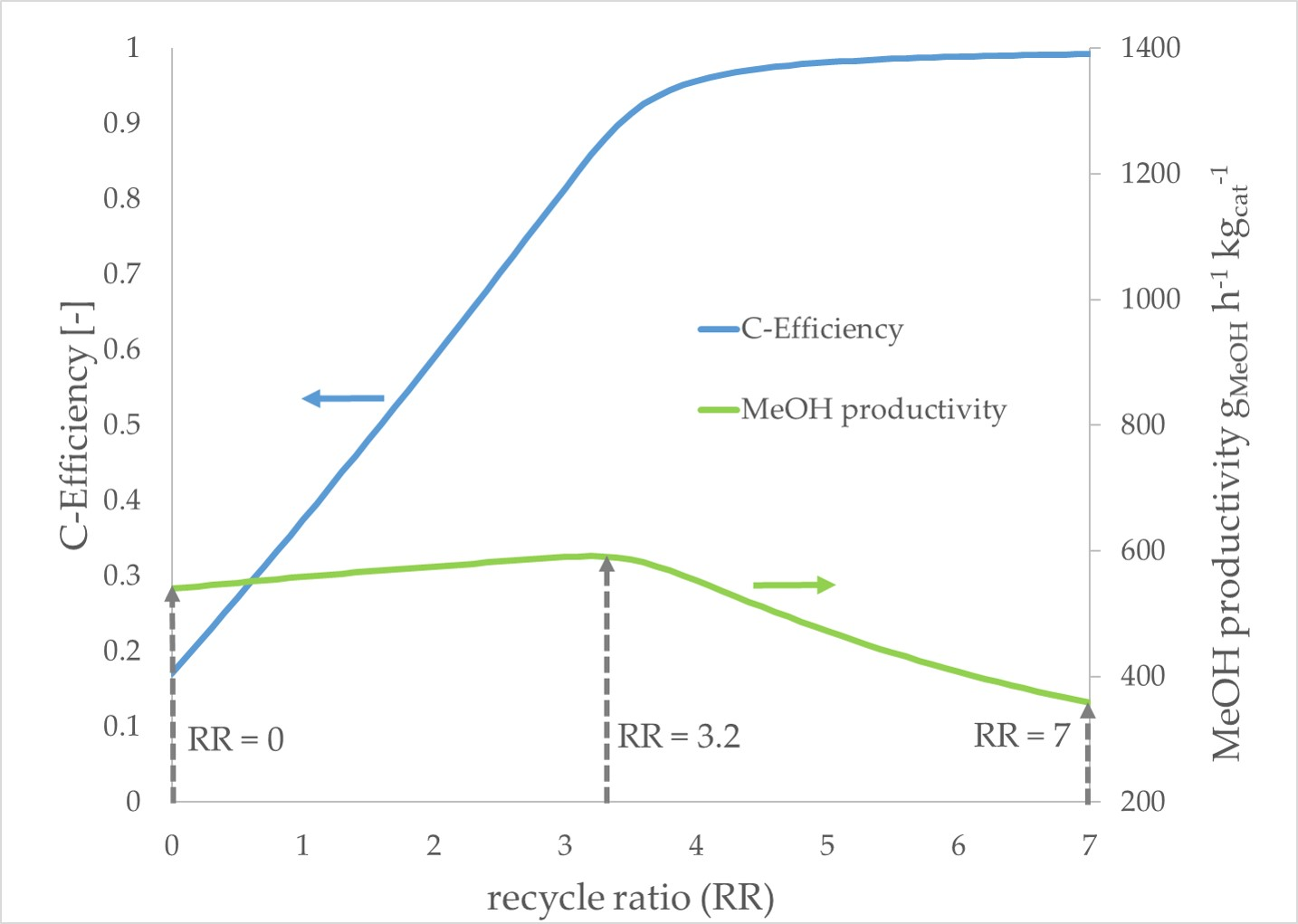
The
pure CO2 scenario exhibits different trends compared to the prior described scenarios. With an increasing RR, the methanol productivity increases slightly until a maximum productivity is reached at an RR of 3.2. A further increase of the RR leads to decreasing productivity. The carbon efficiency increases significantly with a growing RR until an RR of 3.2 is reached. In the following, the gain of carbon efficiency decelerates faster compared to the other scenarios. In
Figure 8, the simulation results of the
pure CO2 scenario are presented. The maximum in methanol productivity at an RR of 3.2. is marked with a dotted line arrow.
In order to reveal the reasons for the deviating productivity trend in the case of the
pure CO2 scenario, the reactor-inlet concentrations for the most productive RR of 3.2 as well as for the extreme cases of a one-pass process with an RR of 0 and a high recycle ratio of 7 are given in
Table 3. At an RR of 0, the reactor-inlet exhibits a nearly stoichiometric gas composition. With an increasing RR, unconverted hydrogen is recycled and induces excess hydrogen. An accumulation of inert compounds does not occur, as there are no inert compounds present. Additionally, CO that was formed by the reverse water–gas shift reaction corresponding to Equation (3) is present at the reactor inlet.
It is assumed that a slight excess of hydrogen and an increasing CO concentration at the reactor inlet raise the reaction rate of methanol formation. A further increase of the RR leads to a very high excess of hydrogen, and the total amount of COX decreases drastically. At an RR of 7, the total amount of COx is only 4.8 vol. % compared to a COx amount of 18.4 vol. % at an RR of 3.2.
3.2. Practical Testing of Gas Utilization Scenarios
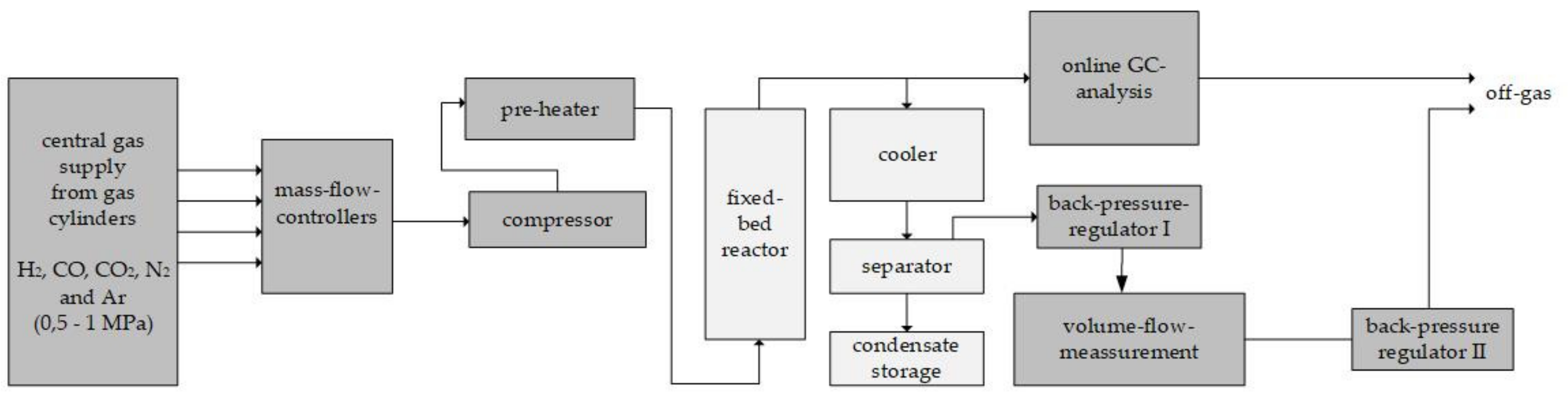
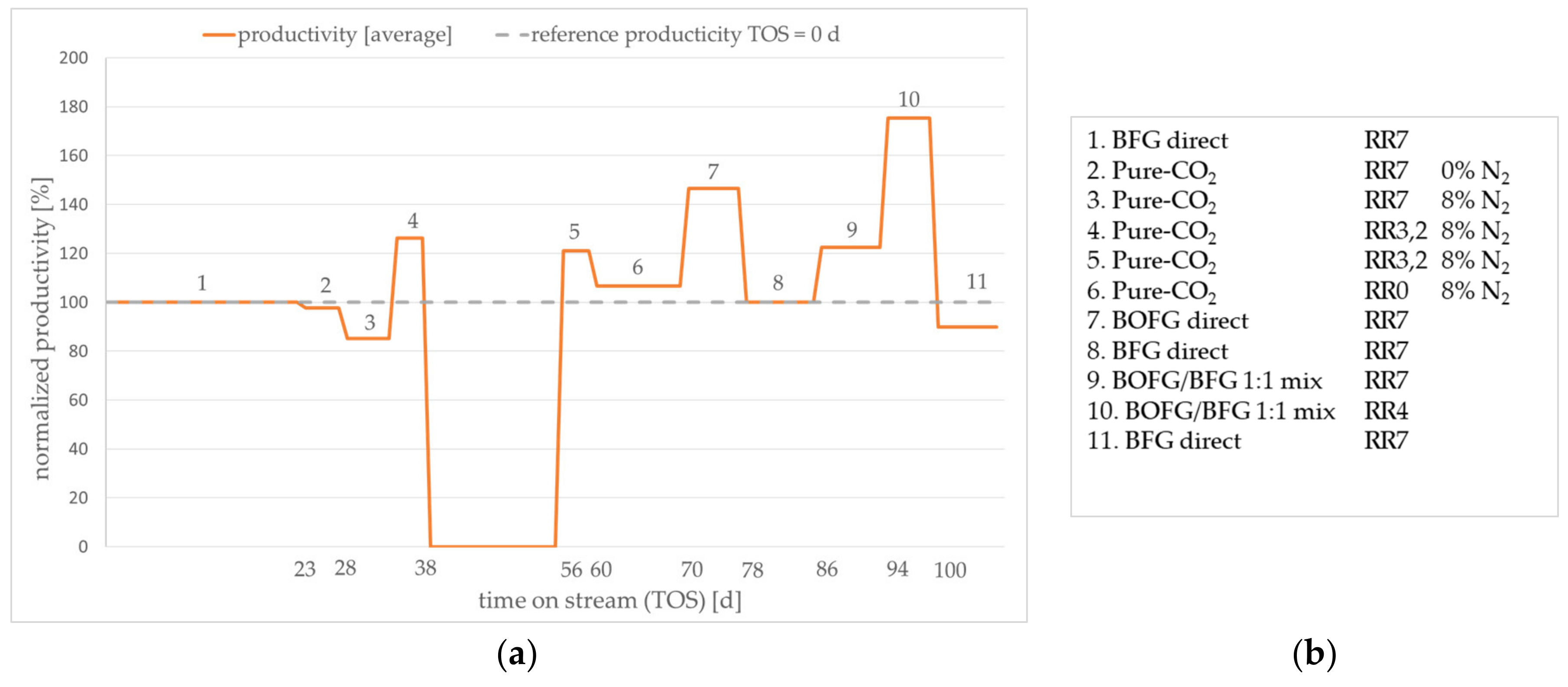
In order to obtain experimental performance data, a practical test series was performed for the considered case scenarios. The average normalized methanol productivity is given in
Figure 9a. Due to nondisclosure agreements among the project partners, it is not possible to present absolute values. The order of tested scenarios and the applied theoretical RR are given in
Figure 9b. The theoretical reactor-inlet gas compositions were taken from the respective simulated scenarios. The theoretical CO share of the pure CO
2 scenario at the reactor inlet was added to the CO
2 share as it was not possible to adjust CO concentrations below 2 vol. % in the test setup. Nitrogen was added to the pure CO
2 scenario only because an internal standard was required to calculate the molar balance of the reactor.
After activating the catalyst, the start-up of test series was carried out with the BFG direct scenario for three weeks (step 1 in
Figure 9b). The average productivity of this period was used as a reference for the following tests and was set to 100% productivity. The following observed changes in methanol productivity were presented relative to this value. Subsequently, the test continued with the gas composition from the pure CO
2 scenario (steps 2–6). The gas compositions of three theoretical recycle ratios were applied corresponding to
Table 3. The test conditions of steps 4 and 5 equal the theoretical productivity maximum presented in
Figure 8 at an RR of 3.2. The two other operational points corresponding to RRs of 0 and 7 showed lower productivity. This trend is in accordance with the observed productivity trends predicted by the simulation. Thus, the assumption of an enhanced reaction rate by a slight excess of hydrogen found in the simulation was verified by the experiment. As there is no CO present in the experimental gas composition, the effect of minor CO concentrations in the reactor feed is less dominating. Subsequently, the BOFG direct scenario was experimentally examined. As expected, the productivity is higher compared to the BFG and the pure CO
2 scenario. The test series proceeded with the reference scenario
BFG direct (step 8) in order to determine whether the catalyst performance was permanently affected by the prior test conditions (steps 1–7). Obviously, irreversible deactivation of the catalyst did not occur. Then, the BOFG/BFG scenario was tested. The resulting productivity was between the productivity of the BOFG and the BFG scenarios, as already predicted by the simulation (cf.
Figure 7). The BOFG/BFG mix scenario at an RR of 4 exhibited the highest productivity of the test series. Due to the lower RR compared to the BOFG scenario at an RR 7, the accumulation of nitrogen was lower, resulting in a slightly higher productivity. Finally, the reference conditions were applied (step 11). Here, a slight decrease of the productivity was observed. It is not clear whether deactivation occurred due to the reaction conditions of the prior test conditions. However, the drop of activity was comparatively small. Reproduction of the test series with a fresh catalyst sample might reveal permanent deactivation of the catalyst. Depending on the results, the author suggests further investigations corresponding to the applied gas compositions.
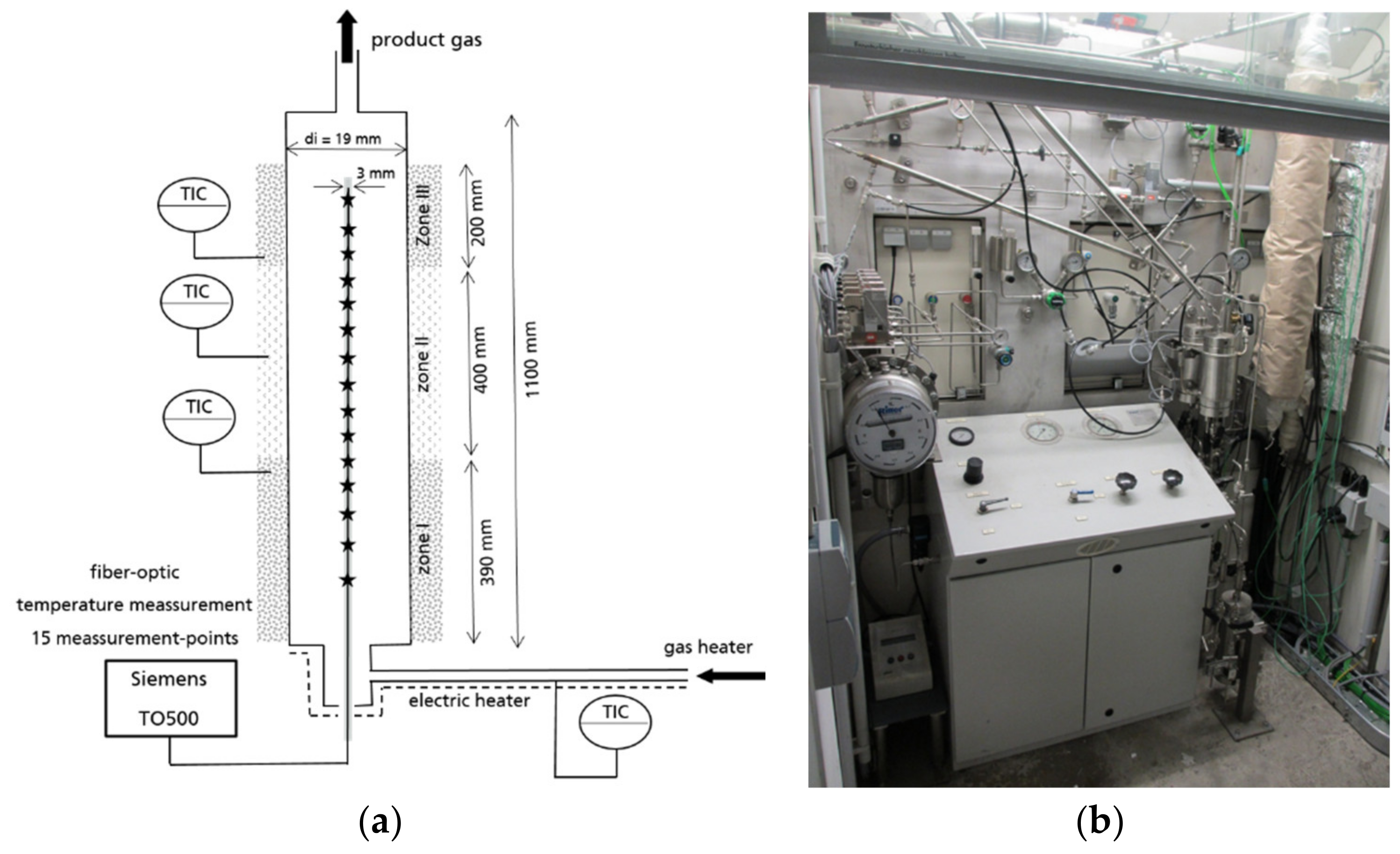
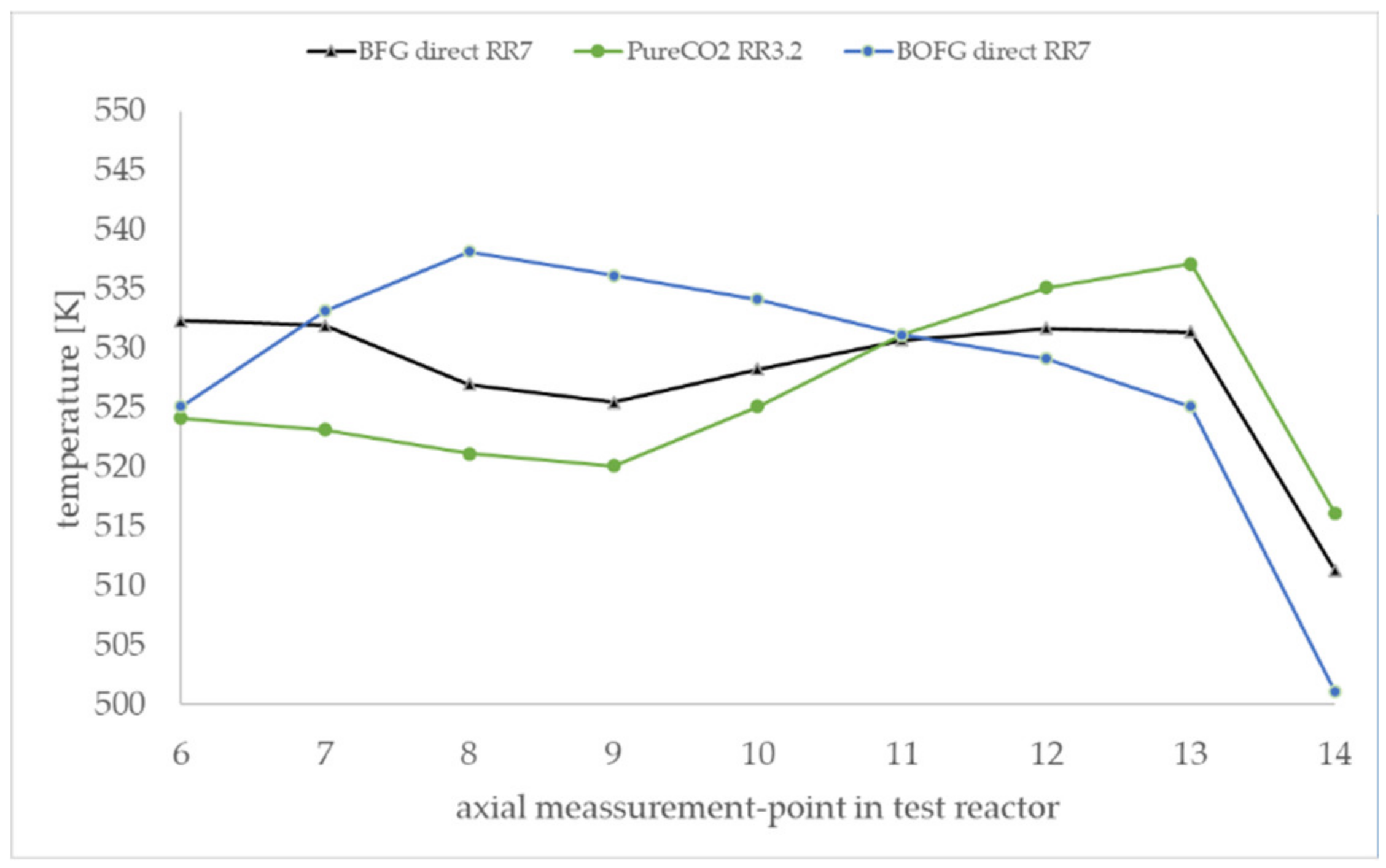
Figure 10 shows the axial temperature inside the active 60-cm catalytic fixed bed during the experiment in steady state. The measurement points 6–14 were positioned inside the active catalytic bed. The measurement points 1–6 and 14 were positioned in the inert supporting bed and are not shown in the diagram. The experimental temperature profiles are clearly influenced by the gas composition. The BOFG case exhibits a temperature maximum in the first section of the reactor. The pure CO
2 case shows a temperature minimum in the first section and a maximum in the rear section of the reactor. In contrast, all simulated temperature profiles in
Figure 6 show a temperature maximum in the first section. A possible reason for the differences of the temperature profiles shown in
Figure 10 is discussed in
Section 4.
3.3. Comparison of Simulation and Practical Results
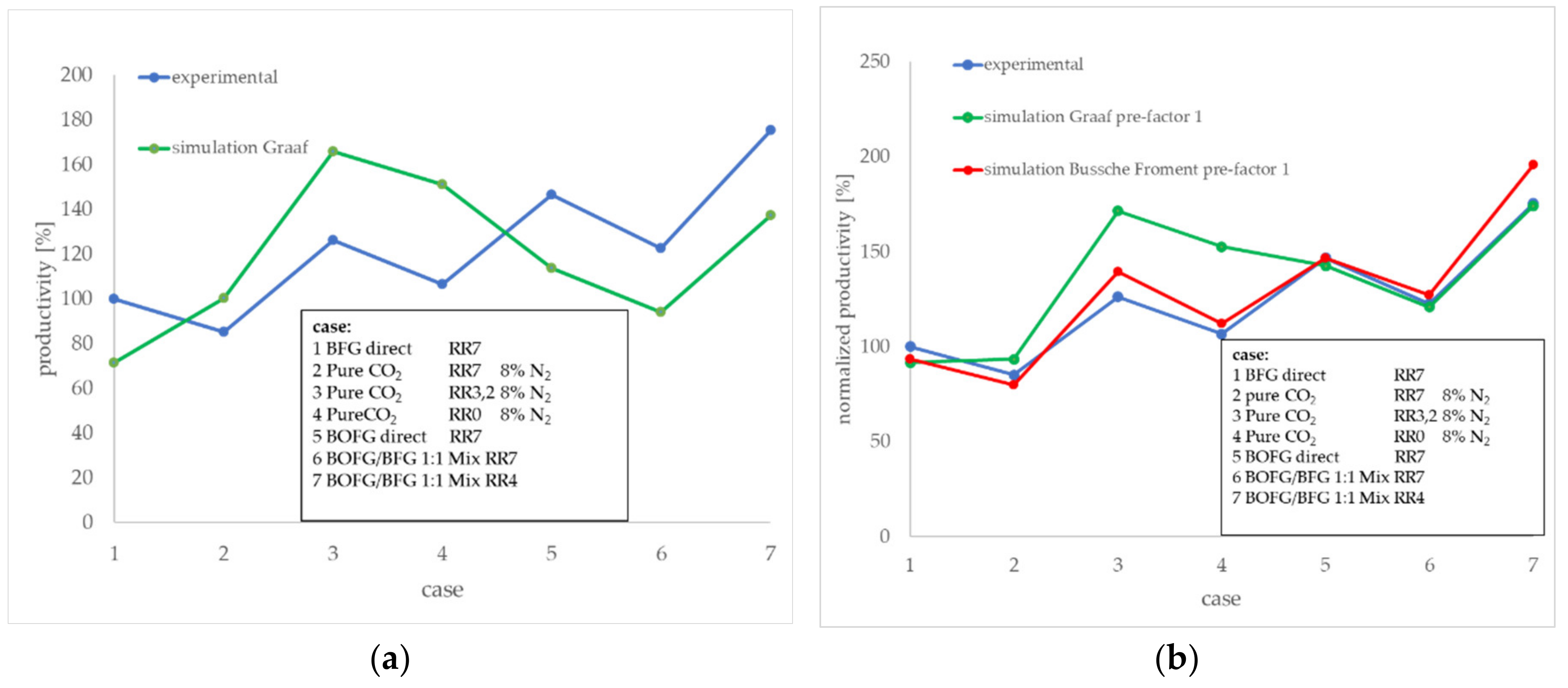
The differences between the experimental test results according to
Figure 9 and the process simulation results at the respective RR and gas mixture are presented in
Figure 11a. Presented here as normalized values, the experimental result of the
BFG direct case at an RR of 7 (case (1)) was used as a reference point and was set to 100% productivity). Apparently, the deviation between the simulation and test results depends on the gas composition. The simulated results based on CO containing process feeds (
BFG direct,
BOFG direct, and
BOFG/BFG 1:1 mix) showed a lower productivity compared to the experimental results. In contrast to these scenarios, the simulation results for the
pure CO2 scenario exhibited higher results compared to the experimental results. Both should be discussed in the following.
The kinetic approach of the reactor model was taken from the literature according to the Graaf kinetic approach. The required kinetic parameters of the model were taken from literature [
9] and diffusion limiting pre-factors were adapted to performance data from Graaf and Beenackers for an industrial methanol process [
8]. The available parameters were determined for conventional syngases, based on mostly used fossil feed stocks like reformed natural gas. The gas compositions of the considered scenarios deviate from these gas compositions in terms of an inreased CO
2/CO-ratio. An adapted set of kinetic parameters, obtained from kinetic studies with the applied gas composition and type of catalyst might lead to better fitting simulation results. Currently, no adapted set of parameters is available, but the pre-factors of the kinetic rate expressions according to diffusion limitation might be changed for this laboratory situation. In addition to the Graaf kinetic approach, the Bussche and Froment kinetic approach was considered in the simulation in order to compare with experimental results at different gas compositions [
17]. In our experiments a broken catalyst with particle sizes much smaller than the original pellet size is used, so catalyst diffusion limitation should not arise significantly. This in mind the kinetic pre-factors of the rate expressions was set to 1.0 for all cases. In a second simulation run
with equal reactor inlet conditions, and after all this approach showed good conformity to the experimental results.
Figure 11b shows a comparison of the two simulated kinetic approaches and the experimental results. The Bussche-Froment approach exhibits better conformity with regard to the
pure CO2 cases (3) and (4). The gas compositions with significant shares of CO seem to be represented good by both kinetic approaches, but slightly better by Graaf. Best compliance to the experimental results was found for the Graaf kinetic approach at the high CO-containing cases (6) and (7).