Antifungal Effect of Volatile Organic Compounds from Bacillus velezensis CT32 against Verticillium dahliae and Fusarium oxysporum
Abstract
1. Introduction
2. Materials and Methods
2.1. Phytopathogenic Fungi and Culture Conditions
2.2. Isolation of Bacteria from Maize Straw Compost Tea
2.3. Screening for Bacteria Producing Antifungal Volatiles
2.4. Analysis of Bacterial VOCs by HS-SPME-GC-MS
2.4.1. Sample Preparation
2.4.2. HS-SPME Procedure
2.4.3. GC-MS Analysis
2.5. Effect of Synthetic VOCs on Mycelial Growth of V. dahliae and FOF
2.6. Identification of Strain CT32
2.7. Statistical Analysis
3. Results
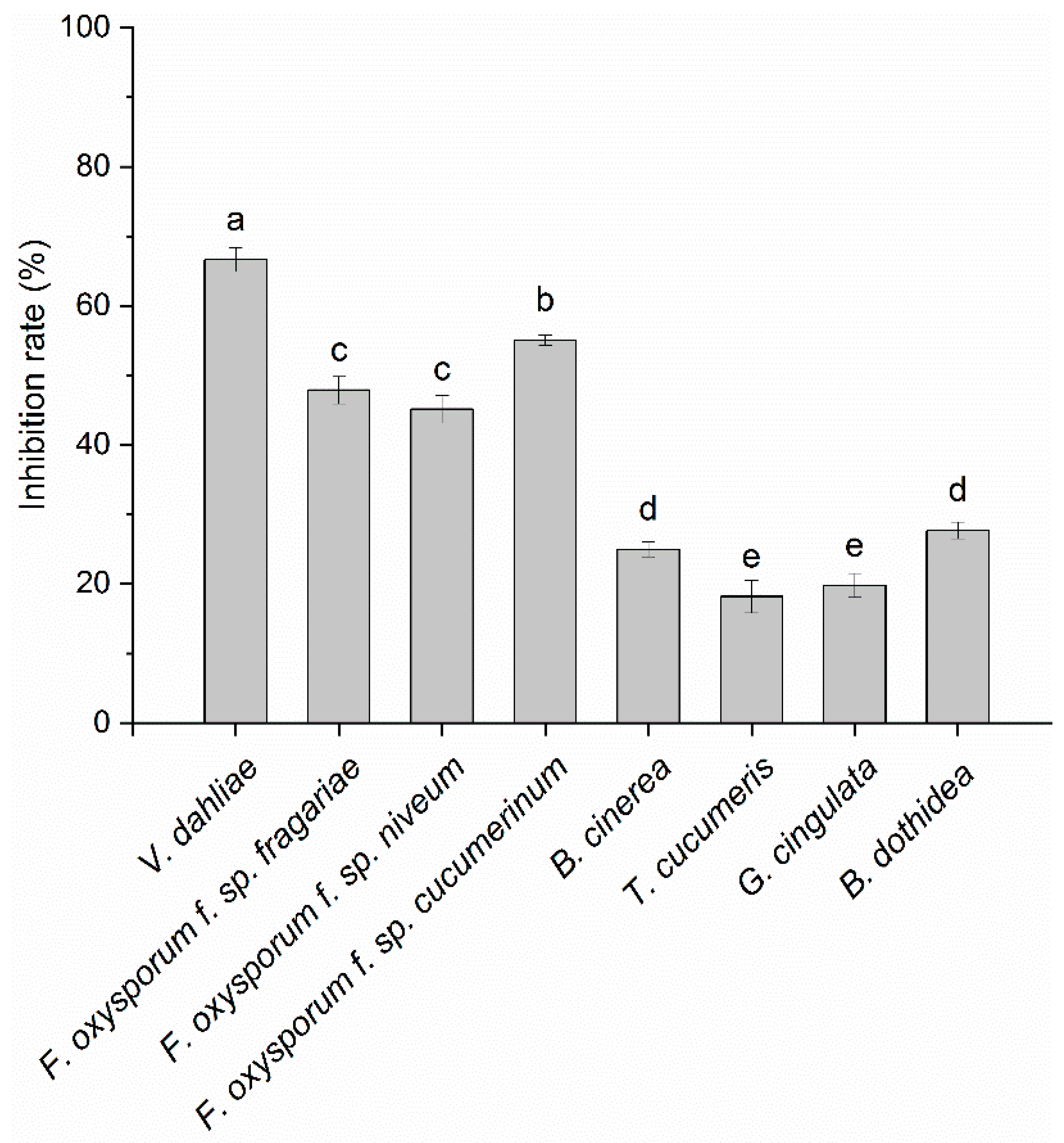
3.1. Screening for Bacteria with Volatile-Mediated Antagonistic Activity
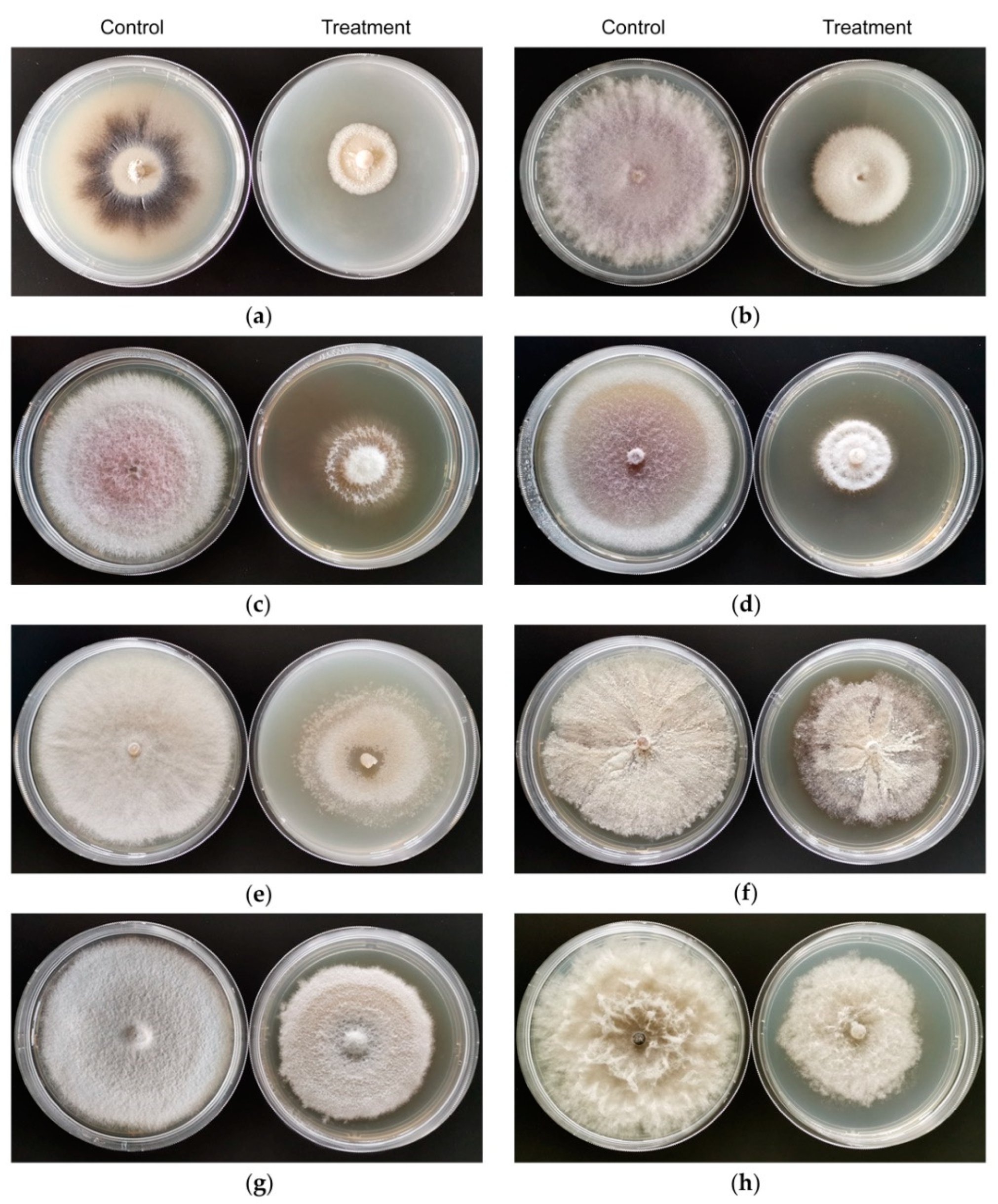
3.2. In Vitro Antifungal Activity of VOCs Produced by Strain CT32
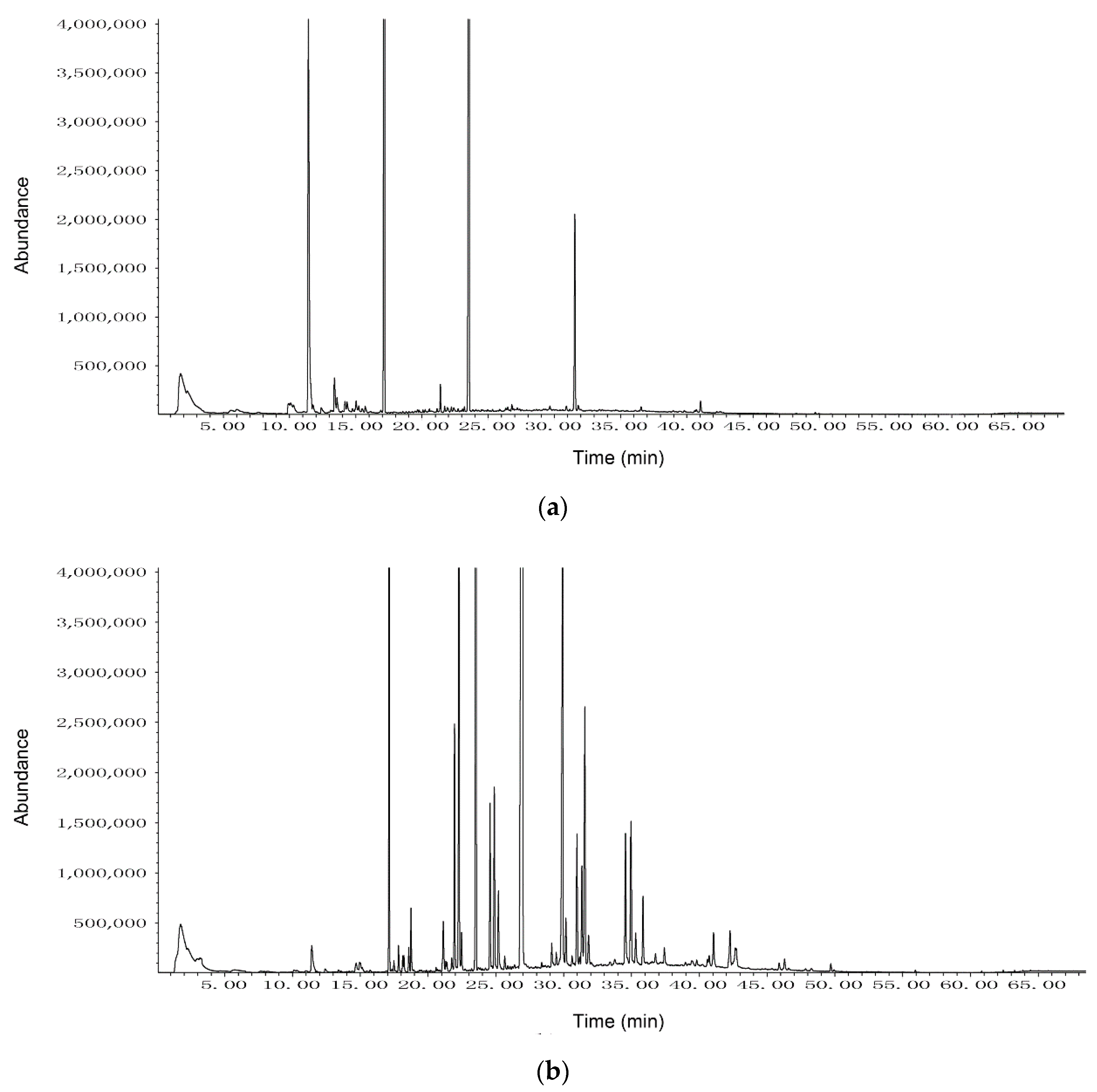
3.3. HS-SPME-GC-MS Analysis of VOCs Produced by Strain CT32
3.4. Antifungal Activity of Synthetic VOCs against V. dahliae and FOF
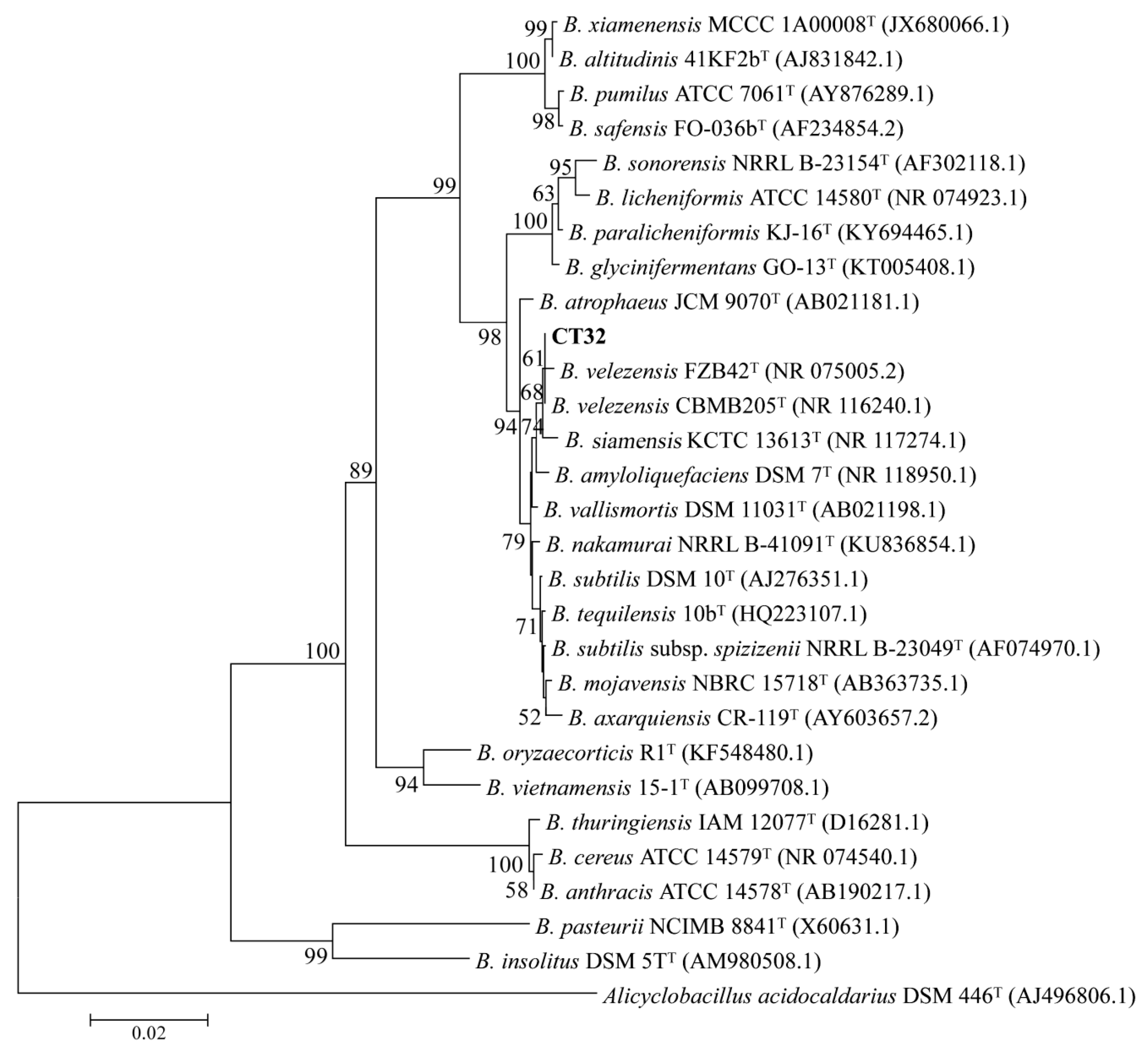
3.5. Identification of Antagonistic Bacterium CT32
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eljounaidi, K.; Lee, S.K.; Bae, H. Bacterial endophytes as potential biocontrol agents of vascular wilt diseases—Review and future prospects. Biol. Control 2016, 103, 62–68. [Google Scholar] [CrossRef]
- Yadeta, K.A.; Thomma, B.P.H.J. The xylem as battleground for plant hosts and vascular wilt pathogens. Front. Plant Sci. 2013, 4, 97. [Google Scholar] [CrossRef]
- Bell, C. Fumigation in the 21st century. Crop. Prot. 2000, 19, 563–569. [Google Scholar] [CrossRef]
- Roux-Michollet, D.; Czarnes, S.; Adam, B.; Berry, D.; Commeaux, C.; Guillaumaud, N.; Le Roux, X.; Clays-Josserand, A. Effects of steam disinfestation on community structure, abundance and activity of heterotrophic, denitrifying and nitrifying bacteria in an organic farming soil. Soil Biol. Biochem. 2008, 40, 1836–1845. [Google Scholar] [CrossRef]
- Emmert, E.A.B.; Handelsman, J. Biocontrol of plant disease: A (Gram-) positive perspective. FEMS Microbiol. Lett. 1999, 171, 1–9. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Fira, D.; DimkiC, I.; Beric, T.; Lozo, J.; Stankovic, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef]
- Kai, M.; Haustein, M.; Molina, F.; Petri, A.; Scholz, B.; Piechulla, B. Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 2009, 81, 1001–1012. [Google Scholar] [CrossRef]
- Effmert, U.; Kalderás, J.; Warnke, R.; Piechulla, B. Volatile Mediated Interactions Between Bacteria and Fungi in the Soil. J. Chem. Ecol. 2012, 38, 665–703. [Google Scholar] [CrossRef]
- Farag, M.A.; Zhang, H.; Ryu, C.-M. Dynamic Chemical Communication between Plants and Bacteria through Airborne Signals: Induced Resistance by Bacterial Volatiles. J. Chem. Ecol. 2013, 39, 1007–1018. [Google Scholar] [CrossRef]
- Fincheira, P.; Quiroz, A. Microbial volatiles as plant growth inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Kanchiswamy, C.N.; Emalnoy, M.; Maffei, M.E. Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front. Plant Sci. 2015, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Farag, M.A.; Park, H.B.; Kloepper, J.W.; Lee, S.H.; Ryu, C. Induced Resistance by a Long-Chain Bacterial Volatile: Elicitation of Plant Systemic Defense by a C13 Volatile Produced by Paenibacillus polymyxa. PLoS ONE 2012, 7, e48744. [Google Scholar] [CrossRef]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Kloepper, J.W.; Pare, P.W. Bacterial Volatiles Induce Systemic Resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Wei, H.-X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Mercier, J.; Lego, S.F.; Smilanick, J.L. In-package use of Muscodor albus volatile-generating sachets and modified atmosphere liners for decay control in organic table grapes under commercial conditions. Fruits 2010, 65, 31–38. [Google Scholar] [CrossRef]
- Mercier, J.; Jiménez, J.I. Potential of the volatile-producing fungus Muscodor albus for control of building molds. Can. J. Microbiol. 2007, 53, 404–410. [Google Scholar] [CrossRef]
- Riga, E.; Lacey, L.A.; Guerra, N. Muscodor albus, a potential biocontrol agent against plant-parasitic nematodes of economically important vegetable crops in Washington State, USA. Biol. Control 2008, 45, 380–385. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Qiao, X.; Li, Z.; Li, F.; Chen, M.; Wang, Y.; Huang, Y.; Cui, H. Antifungal activity of volatile organic compounds fromStreptomyces alboflavusTD-1. FEMS Microbiol. Lett. 2013, 341, 45–51. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, X.; Dai, C. Antagonistic mechanisms of endophytic Pseudomonas fluorescens against Athelia rolfsii. J. Appl. Microbiol. 2014, 117, 1144–1158. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Z.; Zhang, X.; Bai, W.; Zhang, L.; Pei, H.; Zhang, Y. Control effects of Bacillus siamensis G-3 volatile compounds on raspberry postharvest diseases caused by Botrytis cinerea and Rhizopus stolonifer. Biol. Control 2020, 141, 104135. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Shi, X.; Wang, Q.; Li, X.; Zhang, S. Compost tea-mediated induction of resistance in biocontrol of strawberry Verticillium wilt. J. Plant Dis. Prot. 2019, 127, 257–268. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, B.; Liu, H.; Han, J.; Zhang, Y. Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol. Control 2017, 105, 27–39. [Google Scholar] [CrossRef]
- Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Retention indices in the analysis of food aroma volatile compounds in temperature-programmed gas chromatography: Database creation and evaluation of precision and robustness. J. Sep. Sci. 2007, 30, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Wang, C.; Song, X.; Ding, X.; Wu, L.; Wu, H.; Gao, X.; Borriss, R. Bacillus velezensis FZB42 in 2018: The Gram-Positive Model Strain for Plant Growth Promotion and Biocontrol. Front. Microbiol. 2018, 9, 2491. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Jiang, H.; Hao, J. Effects of Bacillus velezensis strain BAC03 in promoting plant growth. Biol. Control 2016, 98, 18–26. [Google Scholar] [CrossRef]
- Scholz, R.; Molohon, K.J.; Nachtigall, J.; Vater, J.; Markley, A.L.; Süssmuth, R.D.; Mitchell, D.A.; Borriss, R. Plantazolicin, a Novel Microcin B17/Streptolysin S-Like Natural Product from Bacillus amyloliquefaciens FZB42. J. Bacteriol. 2010, 193, 215–224. [Google Scholar] [CrossRef]
- Scholz, R.; Vater, J.; Budiharjo, A.; Wang, Z.; He, Y.; Dietel, K.; Schwecke, T.; Herfort, S.; Lasch, P.; Borriss, R. Amylocyclicin, a Novel Circular Bacteriocin Produced by Bacillus amyloliquefaciens FZB42. J. Bacteriol. 2014, 196, 1842–1852. [Google Scholar] [CrossRef]
- Che, J.; Liu, B.; Liu, G.; Chen, Q.; Lan, J. Volatile organic compounds produced by Lysinibacillus sp. FJAT-4748 possess antifungal activity against Colletotrichum acutatum. Biocontrol Sci. Technol. 2017, 27, 1349–1362. [Google Scholar] [CrossRef]
- Chaves-López, C.; Serio, A.; Gianotti, A.; Sacchetti, G.; Ndagijimana, M.; Ciccarone, C.; Stellarini, A.; Corsetti, A.; Paparella, A. Diversity of food-borne Bacillus volatile compounds and influence on fungal growth. J. Appl. Microbiol. 2015, 119, 487–499. [Google Scholar] [CrossRef]
- Dhouib, H.; Zouari, I.; Ben Abdallah, D.; Belbahri, L.; Taktak, W.; Triki, M.A.; Tounsi, S. Potential of a novel endophytic Bacillus velezensis in tomato growth promotion and protection against Verticillium wilt disease. Biol. Control 2019, 139, 104092. [Google Scholar] [CrossRef]
- Jangir, M.; Pathak, R.; Sharma, S.; Sharma, S. Biocontrol mechanisms of Bacillus sp., isolated from tomato rhizosphere, against Fusarium oxysporum f. sp. lycopersici. Biol. Control 2018, 123, 60–70. [Google Scholar] [CrossRef]
- A Farag, M.; Song, G.C.; Park, Y.; Audrain, B.; Lee, S.; Ghigo, J.-M.; Kloepper, J.W.; Ryu, C.-M. Biological and chemical strategies for exploring inter- and intra-kingdom communication mediated via bacterial volatile signals. Nat. Protoc. 2017, 12, 1359–1377. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.; da Silva, E.; Câmara, J.S.; Khadem, M. Molecular identification and VOMs characterization of Saccharomyces cerevisiae strains isolated from madeira region winery environments. Processes 2020, 8, 1058. [Google Scholar] [CrossRef]
- Ghader, M.; Shokoufi, N.; Es-Haghi, A.; Kargosha, K. Headspace solid-phase microextraction (HS-SPME) combined with GC–MS as a process analytical technology (PAT) tool for monitoring the cultivation of C. tetani. J. Chromatogr. B 2018, 1083, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Romoli, R.; Papaleo, M.C.; De Pascale, D.; Tutino, M.L.; Michaud, L.; Logiudice, A.; Fani, R.; Bartolucci, G. Characterization of the volatile profile of Antarctic bacteria by using solid-phase microextraction-gas chromatography-mass spectrometry. J. Mass Spectrom. 2011, 46, 1051–1059. [Google Scholar] [CrossRef]
- Zhao, L.-J.; Yang, X.-N.; Li, X.-Y.; Mu, W.; Liu, F. Antifungal, Insecticidal and Herbicidal Properties of Volatile Components from Paenibacillus polymyxa Strain BMP-11. Agric. Sci. China 2011, 10, 728–736. [Google Scholar] [CrossRef]
- Zou, C.-S.; Mo, M.-H.; Gu, Y.-Q.; Zhou, J.; Zhang, K.-Q. Possible contributions of volatile-producing bacteria to soil fungistasis. Soil Biol. Biochem. 2007, 39, 2371–2379. [Google Scholar] [CrossRef]
- Agarwal, S.; Gandhi, D.; Kalal, P. Benzothiazole: A Versatile and Multitargeted Pharmacophore in the Field of Medicinal Chemistry. Lett. Org. Chem. 2017, 14, 729–742. [Google Scholar] [CrossRef]
- Mei, X.; Liu, Y.; Huang, H.; Du, F.; Huang, L.; Wu, J.; Li, Y.; Zhu, S.; Yang, M. Benzothiazole inhibits the growth of Phytophthora capsici through inducing apoptosis and suppressing stress responses and metabolic detoxification. Pestic. Biochem. Physiol. 2019, 154, 7–16. [Google Scholar] [CrossRef]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Ingram, L.O.; Buttke, T.M. Effects of Alcohols on Micro-Organisms. Adv. Microb. Physiol. 1985, 25, 253–300. [Google Scholar] [CrossRef]
- Yuan, J.; Raza, W.; Shen, Q.; Huang, Q. Antifungal Activity of Bacillus amyloliquefaciens NJN-6 Volatile Compounds against Fusarium oxysporum f. sp. cubense. Appl. Environ. Microbiol. 2012, 78, 5942–5944. [Google Scholar] [CrossRef] [PubMed]
- Fernando, W.G.D.; Ramarathnam, R.; Krishnamoorthy, A.S.; Savchuk, S.C. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 2005, 37, 955–964. [Google Scholar] [CrossRef]
- De Vrieze, M.; Pandey, P.; Bucheli, T.D.; Varadarajan, A.R.; Ahrens, C.H.; Weisskopf, L.; Bailly, A. Volatile Organic Compounds from Native Potato-associated Pseudomonas as Potential Anti-oomycete Agents. Front. Microbiol. 2015, 6, 1295. [Google Scholar] [CrossRef]
- Song, K.C.; Ryu, C.-M. Two Volatile Organic Compounds Trigger Plant Self-Defense against a Bacterial Pathogen and a Sucking Insect in Cucumber under Open Field Conditions. Int. J. Mol. Sci. 2013, 14, 9803–9819. [Google Scholar] [CrossRef] [PubMed]

| Bacterial Isolate | Colony Diameter of Control (mm) | Colony Diameter of Treatment (mm) | Inhibition Rate (%) | |||
|---|---|---|---|---|---|---|
| V. dahliae | FOF | V. dahliae | FOF | V. dahliae | FOF | |
| CT11 | 81.22 ± 0.70 | 87.47 ± 0.64 | 56.12 ± 0.89 | 54.18 ± 2.58 | 32.93 ± 1.17 d 1 | 40.36 ± 3.13 b |
| CT32 | 81.22 ± 0.70 | 87.47 ± 0.64 | 30.20 ± 1.57 | 49.77 ± 1.53 | 66.94 ± 2.06 a | 45.72 ± 1.86 a |
| CT56 | 81.22 ± 0.70 | 87.47 ± 0.64 | 47.85 ± 0.60 | 68.55 ± 1.70 | 43.78 ± 0.79 b | 22.94 ± 2.06 d |
| CT58 | 81.22 ± 0.70 | 87.47 ± 0.64 | 52.55 ± 2.19 | 65.37 ± 1.03 | 37.61 ± 2.87 c | 26.80 ± 1.25 c |
| CT64 | 81.22 ± 0.70 | 87.47 ± 0.64 | 67.82 ± 1.26 | 79.38 ± 0.83 | 17.58 ± 1.65 e | 9.80 ± 1.01 e |
| Compound | Colony Diameter of Control (mm) | Colony Diameter of Treatment (mm) | Inhibition Rate (%) | |||
|---|---|---|---|---|---|---|
| V. dahliae | FOF | V. dahliae | FOF | V. dahliae | FOF | |
| Undecane | 83.05 ± 0.83 | 87.75 ± 0.25 | 88.00 ± 0.00 | 88.00 ± 0.00 | –p 1 | –o |
| Decanal | 83.05 ± 0.83 | 87.75 ± 0.25 | 21.13 ± 1.21 | 35.03 ± 0.96 | 79.33 ± 1.54 d 2 | 63.71 ± 1.16 c |
| Benzothiazole | 83.05 ± 0.83 | 87.75 ± 0.25 | 5.00 ± 0.00 | 5.00 ± 0.00 | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| 3-Undecanone | 83.05 ± 0.83 | 87.75 ± 0.25 | 32.75 ± 1.75 | 42.85 ± 0.41 | 64.45 ± 2.24 f | 54.26 ± 0.49 e |
| 2-Undecanone | 83.05 ± 0.83 | 87.75 ± 0.25 | 25.47 ± 2.87 | 40.62 ± 1.62 | 73.78 ± 3.67 e | 56.96 ± 1.95 d |
| 2-Undecanol | 83.05 ± 0.83 | 87.75 ± 0.25 | 24.42 ± 1.23 | 41.83 ± 1.15 | 75.12 ± 1.58 e | 55.49 ± 1.40 de |
| Undecanal | 83.05 ± 0.83 | 87.75 ± 0.25 | 12.05 ± 0.91 | 49.25 ± 1.09 | 90.97 ± 1.17 c | 46.53 ± 1.32 f |
| 2-Dodecanone | 83.05 ± 0.83 | 87.75 ± 0.25 | 54.47 ± 1.50 | 55.35 ± 2.93 | 36.62 ± 1.92 h | 39.15 ± 3.54 g |
| 2-Dodecanol | 83.05 ± 0.83 | 87.75 ± 0.25 | 43.75 ± 2.41 | 64.22 ± 1.55 | 50.35 ± 3.09 g | 28.44 ± 1.87 h |
| 2,4-Dimethyl-6-tert-butylphenol | 83.05 ± 0.83 | 87.75 ± 0.25 | 8.45 ± 0.43 | 12.33 ± 0.38 | 95.58 ± 0.55 b | 91.14 ± 0.46 b |
| Tetradecane | 83.05 ± 0.83 | 87.75 ± 0.25 | 86.75 ± 0.87 | 88.00 ± 0.00 | –nop | –o |
| Dodecanal | 83.05 ± 0.83 | 87.75 ± 0.25 | 61.75 ± 1.24 | 80.58 ± 1.28 | 27.29 ± 1.59 i | 8.66 ± 1.55 j |
| 1-Dodecanol 4 | 83.05 ± 0.83 | 87.75 ± 0.25 | 59.55 ± 1.50 | 71.25 ± 1.91 | 30.11 ± 1.93 i | 19.94 ± 2.31 i |
| Dodecanenitrile | 83.05 ± 0.83 | 87.75 ± 0.25 | 43.17 ± 2.36 | 71.17 ± 1.61 | 51.10 ± 3.03 g | 20.04 ± 1.94 i |
| 2-Tridecanone 3 | 83.05 ± 0.83 | 87.75 ± 0.25 | 82.52 ± 0.23 | 84.13 ± 1.04 | 0.68 ± 0.29 m | 4.37 ± 1.26 kl |
| Pentadecane | 83.05 ± 0.83 | 87.75 ± 0.25 | 86.92 ± 1.01 | 88.00 ± 0.00 | –op | –o |
| 2-Tridecanol 4 | 83.05 ± 0.83 | 87.75 ± 0.25 | 67.47 ± 1.73 | 84.33 ± 0.38 | 19.97 ± 2.22 j | 4.13 ± 0.46 klm |
| Butylated Hydroxytoluene | 83.05 ± 0.83 | 87.75 ± 0.25 | 76.13 ± 0.81 | 85.40 ± 0.26 | 8.86 ± 1.04 l | 2.84 ± 0.31 lmn |
| 2-Tetradecanone 3 | 83.05 ± 0.83 | 87.75 ± 0.25 | 84.42 ± 0.88 | 88.00 ± 0.00 | –mno | –o |
| 2-Tetradecanol 4 | 83.05 ± 0.83 | 87.75 ± 0.25 | 71.77 ± 1.67 | 85.95 ± 0.51 | 14.46 ± 2.13 k | 2.18 ± 0.61 lmn |
| Hexadecane | 83.05 ± 0.83 | 87.75 ± 0.25 | 86.37 ± 0.64 | 88.00 ± 0.00 | –nop | –o |
| Tetradecanal | 83.05 ± 0.83 | 87.75 ± 0.25 | 75.28 ± 1.34 | 82.50 ± 1.32 | 9.95 ± 1.71 l | 6.34 ± 1.60 k |
| 2,2’,5,5’-Tetramethyl-1,1’-biphenyl | 83.05 ± 0.83 | 87.75 ± 0.25 | 83.30 ± 0.72 | 88.00 ± 0.00 | –m | –o |
| Heptadecane | 83.05 ± 0.83 | 87.75 ± 0.25 | 84.72 ± 0.80 | 86.68 ± 0.70 | –mno | 1.29 ± 0.85 no |
| 2-Hexadecanone 3 | 83.05 ± 0.83 | 87.75 ± 0.25 | 84.58 ± 1.01 | 86.18 ± 0.28 | –mno | 1.89 ± 0.34 mno |
| Octadecane | 83.05 ± 0.83 | 87.75 ± 0.25 | 84.33 ± 1.26 | 85.88 ± 0.81 | –mn | 2.26 ± 0.98 lmn |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, X.; Shi, X.; Wang, B.; Li, M.; Wang, Q.; Zhang, S. Antifungal Effect of Volatile Organic Compounds from Bacillus velezensis CT32 against Verticillium dahliae and Fusarium oxysporum. Processes 2020, 8, 1674. https://doi.org/10.3390/pr8121674
Li X, Wang X, Shi X, Wang B, Li M, Wang Q, Zhang S. Antifungal Effect of Volatile Organic Compounds from Bacillus velezensis CT32 against Verticillium dahliae and Fusarium oxysporum. Processes. 2020; 8(12):1674. https://doi.org/10.3390/pr8121674
Chicago/Turabian StyleLi, Xinxin, Xiuhong Wang, Xiangyuan Shi, Baoping Wang, Meiping Li, Qi Wang, and Shengwan Zhang. 2020. "Antifungal Effect of Volatile Organic Compounds from Bacillus velezensis CT32 against Verticillium dahliae and Fusarium oxysporum" Processes 8, no. 12: 1674. https://doi.org/10.3390/pr8121674
APA StyleLi, X., Wang, X., Shi, X., Wang, B., Li, M., Wang, Q., & Zhang, S. (2020). Antifungal Effect of Volatile Organic Compounds from Bacillus velezensis CT32 against Verticillium dahliae and Fusarium oxysporum. Processes, 8(12), 1674. https://doi.org/10.3390/pr8121674




