Novel Study for Energy Recovery from the Cooling–Solidification Stage of Synthetic Slag Manufacturing: Estimation of the Potential Energy Recovery
Abstract
:1. Introduction
1.1. Background
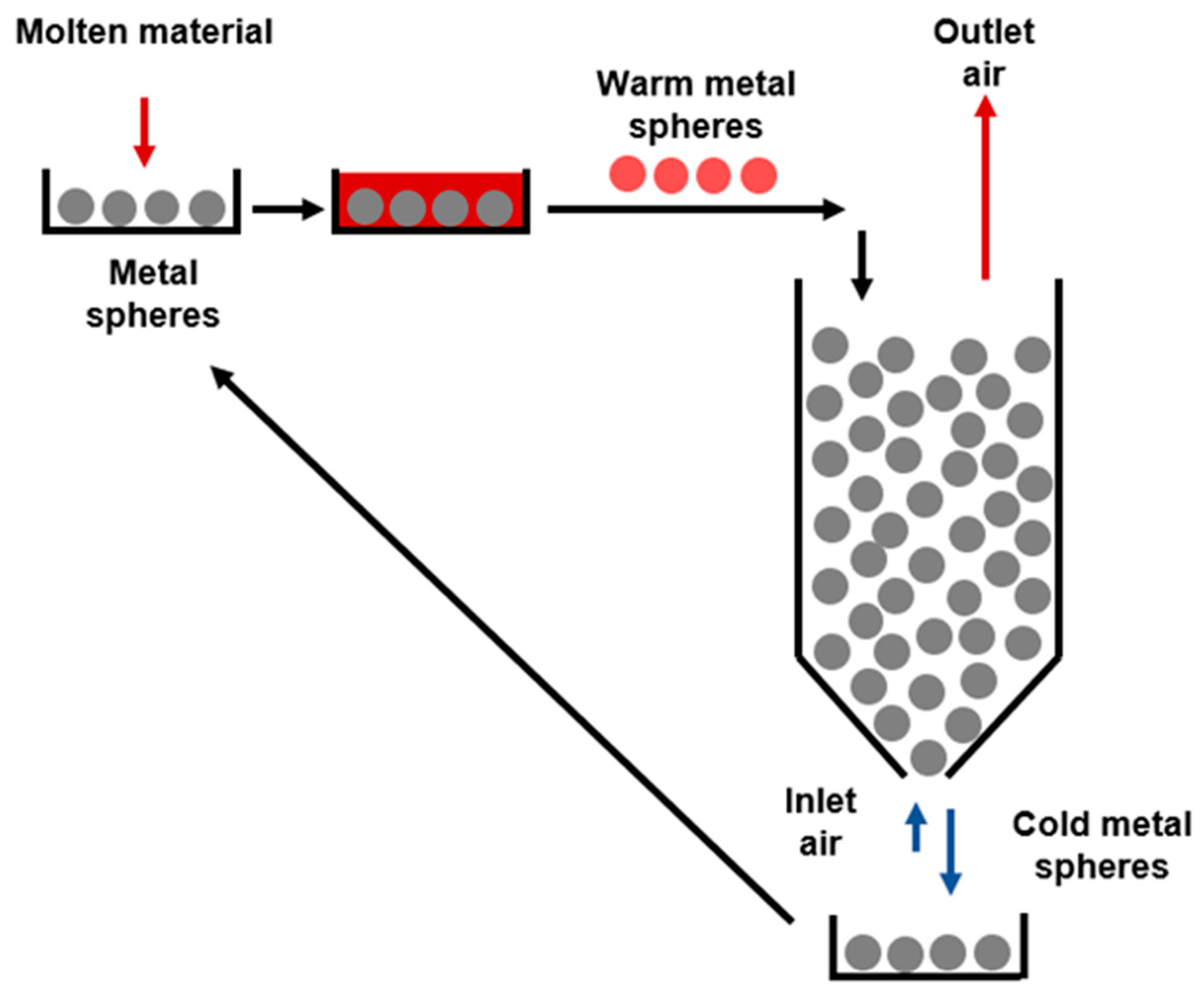
1.2. Goal and Scope
- -
- Justification of the temperature reached by the metal spheres at various molten mass (mm)/metal spheres (ms) mass ratio.
- -
- Corroboration of the industrial feasibility of the proposed system in Figure 1 by means of fixed bed (or regenerator) height calculation. For this purpose, the calculation of the convective heat transfer coefficient by solid–air convection is needed. The definition of this step is crucial for checking that the process proposed is of industrial interest.
- -
- Estimation of the potential energy recovered per kg of molten material.
2. Materials and Methods
- (1)
- Melt the waste mixture in an oven in appropriate proportions to achieve a composition similar to blast furnace slags. This stage was addressed in depth in our previous work [11].
- (2)
- Obtain the temperature reached by the metal spheres when the molten material is poured with different melting/metal mass ratios. Determination of the characteristics of the vitreous phase obtained in order to ensure the vitreous properties of the material solidified.
- (3)
- Evaluate the energy that can be extracted from a fixed bed of metal spheres at the temperature reached in the previous phase by exchanging energy with an air current.
2.1. Materials
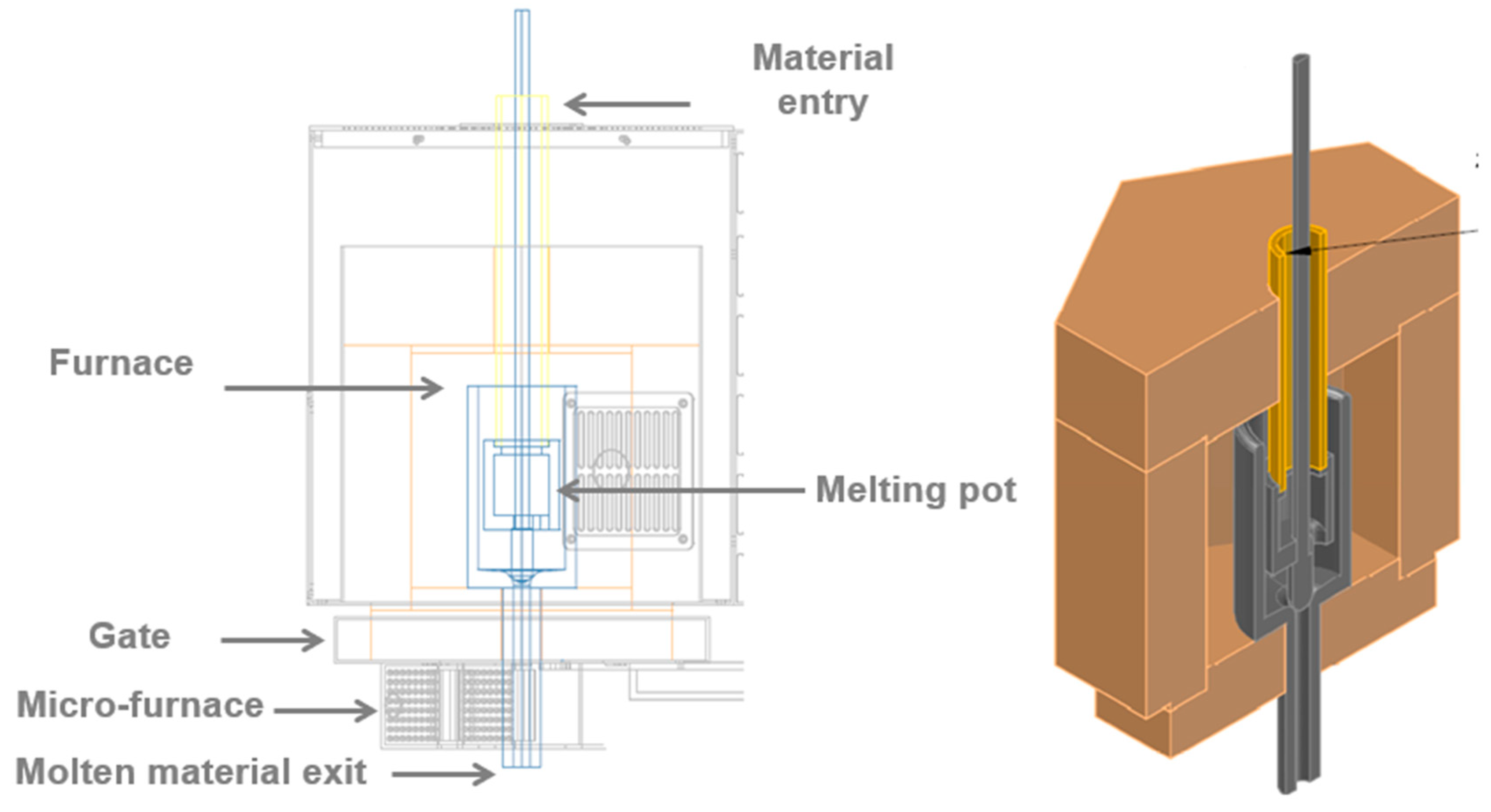
2.2. Experimental Setup
2.2.1. Melting Furnace
2.2.2. Metal Spheres: Cooling of the Molten Material
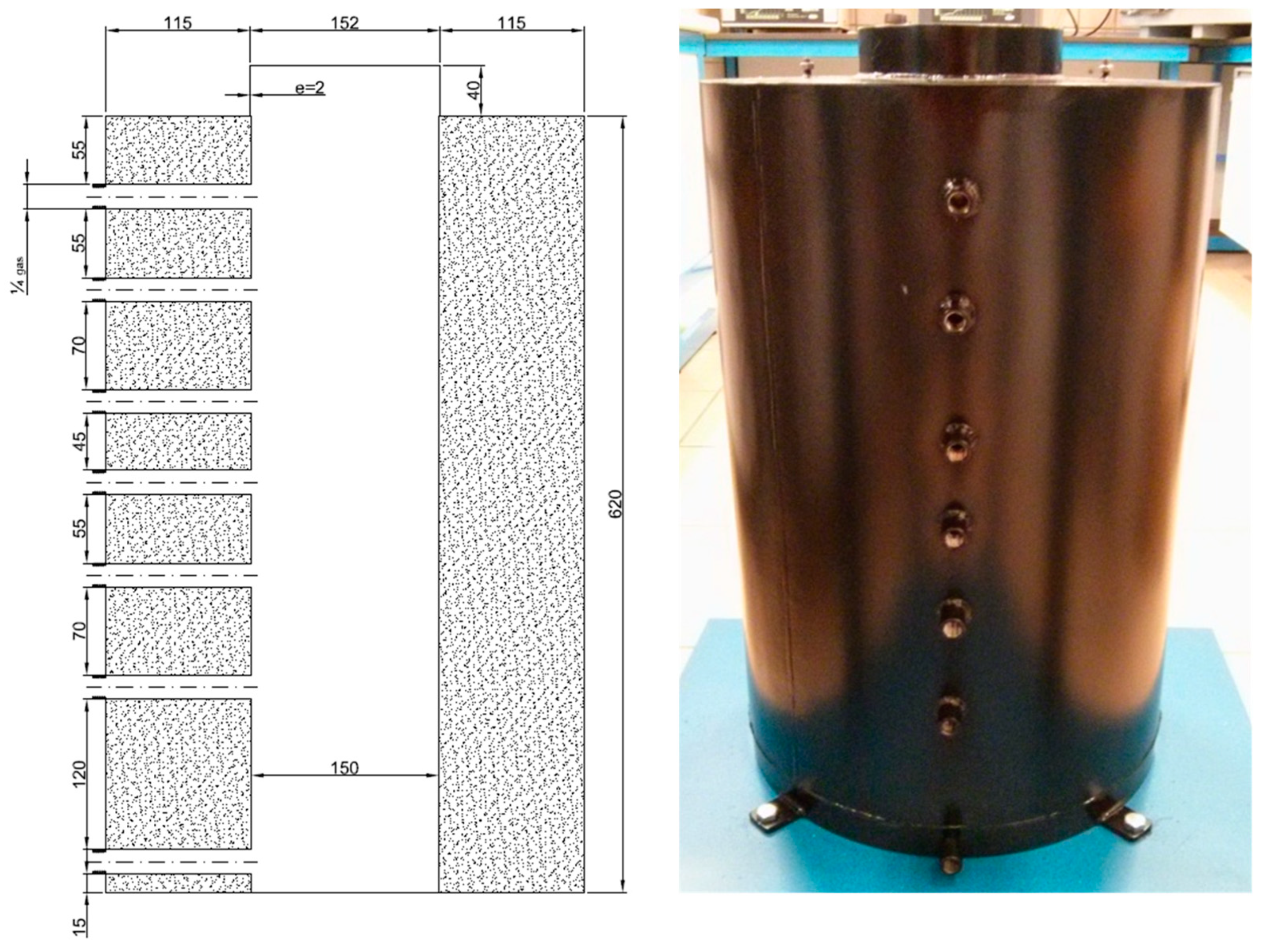
2.2.3. Metal Spheres Packing Device for Energy Recovery
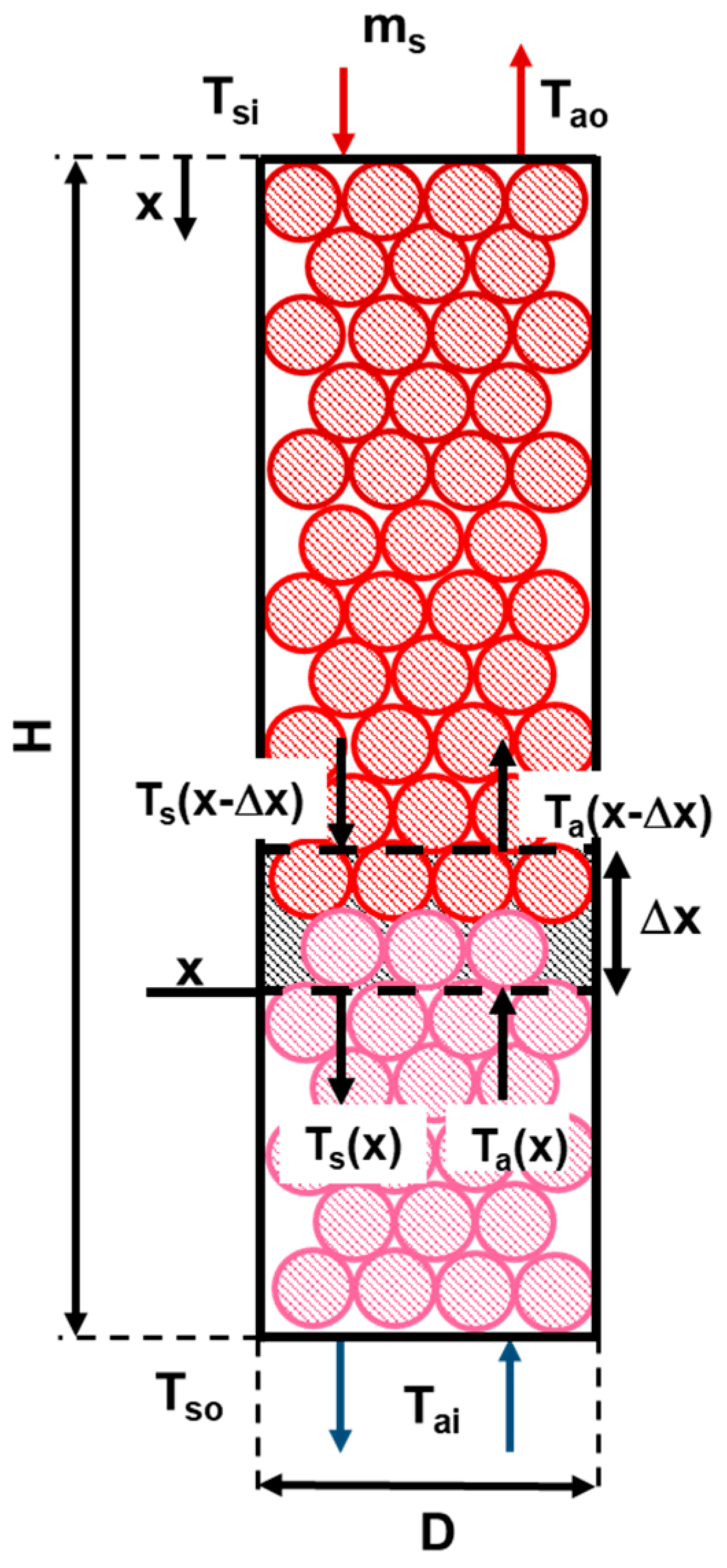
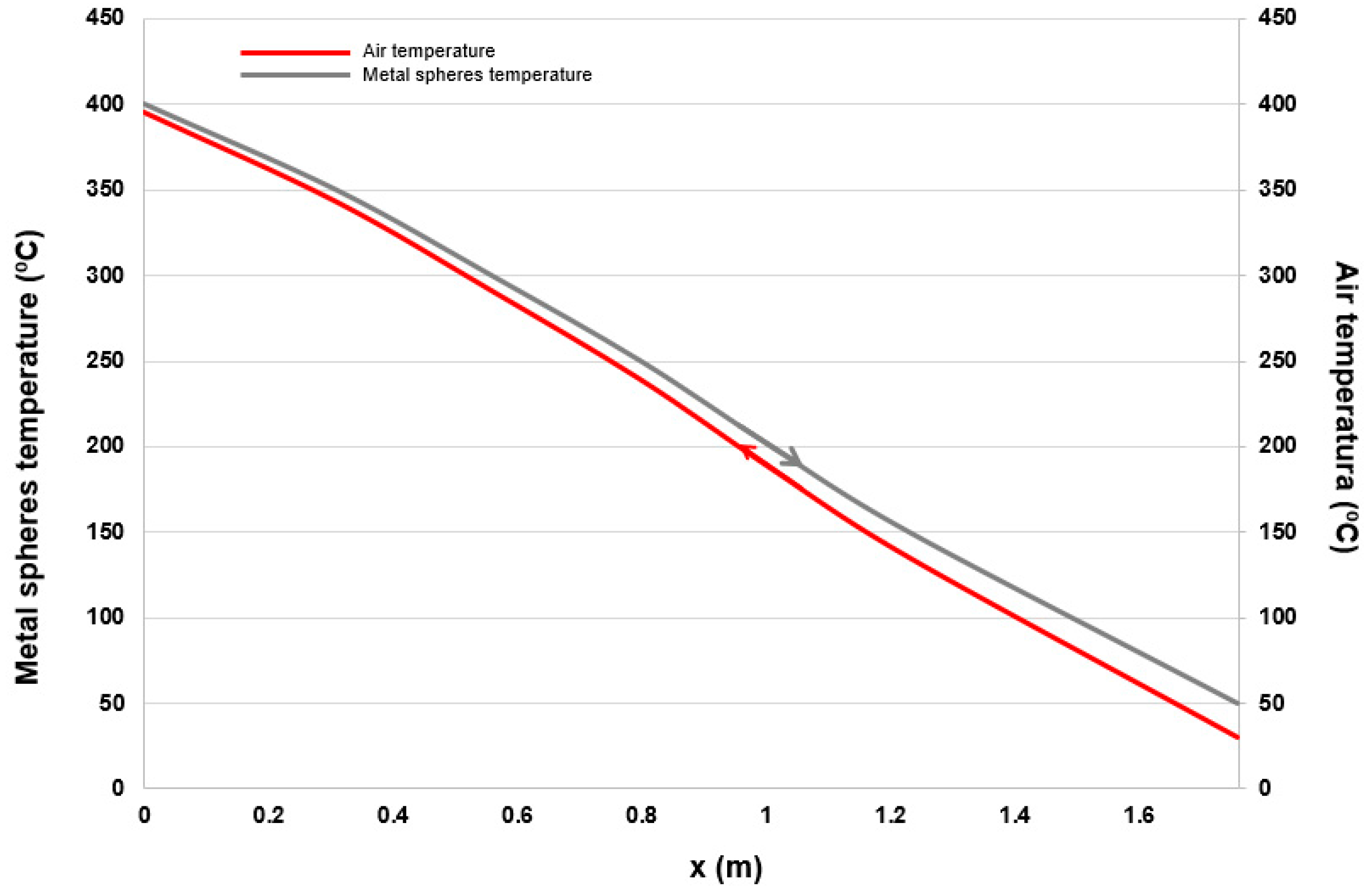
2.2.4. Fixed Bed Height Estimation
2.3. Physicochemical Characterization
2.4. Experimental Plan
3. Results
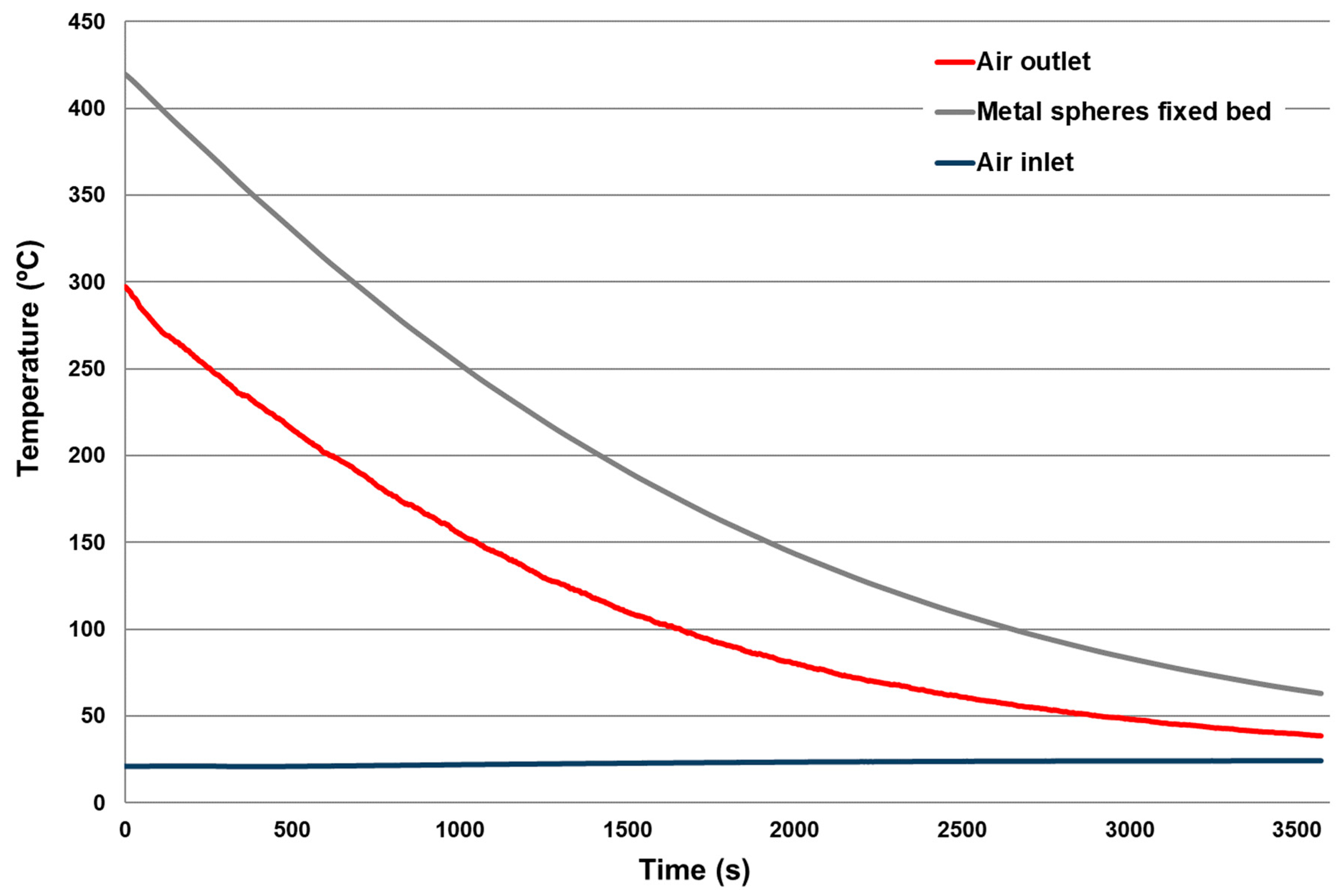
3.1. Energy Transfer from the Molten Material to Metal Spheres
3.2. Properties of the Cooled Material
3.3. Estimation of the Energy Recovery Using a Fixed Bed of Metal Spheres with Air
3.4. Potential Use of the Energy Recovered in the Regenerator in the Manufacture of Synthetic Slags
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| mm | molten mass |
| ms | spheres mass |
| Tsi | spheres temperature inlet |
| Tso | spheres temperature outlet |
| Tai | air temperature inlet |
| Tao | air temperature outlet |
| Ts | spheres temperature |
| Ta | air temperature |
| H | fixed bed regenerator height |
| D | fixed bed regenerator diameter |
| h | convective heat transfer coefficient |
| h′ | convective heat transfer coefficient variation |
| Cs | spheres calorific value |
| Ca | air calorific value |
| A(“∆” x) | transfer area metal spheres–air |
Appendix A

References
- Royo, P.; Acevedo, L.; Ferreira, V.J.; García-Armingol, T.; López-Sabirón, A.M.; Ferreira, G. High-temperature PCM-based thermal energy storage for industrial furnaces installed in energy-intensive industries. Energy 2019, 173, 1030–1040. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Reina, T.R.; Vilches, L.F.; Navarrete, B. Regeneration of sodium hydroxide from a biogas upgrading unit through the synthesis of precipitated calcium carbonate: An experimental influence study of reaction parameters. Processes 2018, 6, 205. [Google Scholar] [CrossRef] [Green Version]
- Peceño, B.; Leiva, C.; Alonso-Fariñas, B.; Gallego-Schmid, A. Is recycling always the best option? Environmental assessment of recycling of seashell as aggregates in noise barriers. Processes 2020, 8, 776. [Google Scholar] [CrossRef]
- González-Arias, J.; Sánchez, M.E.; Martínez, E.J.; Covalski, C.; Alonso-Simón, A.; González, R.; Cara-Jiménez, J. Hydrothermal carbonization of olive tree pruning as a sustainableway for improving biomass energy potential: Effect of reaction parameters on fuel properties. Processes 2020, 8, 1201. [Google Scholar] [CrossRef]
- Philip, P.T.; Hannaford, A.L.; Konick, E. Methods of Making Cementitious Compositions from Waste Products. U.S. Patent 4756761A, 16 June 1986. [Google Scholar]
- Iglesias, J.; Tuya, A.; Peña, F. Procedure for Obtaining Calcium Aluminate From Waste Obtained Following Treatment of Saline Dross From the Production of Secondary Aluminium. U.S. Patent 20110293494A1, 18 July 2011. [Google Scholar]
- Akiyama, K. Process for the Manufacture of Aluminous Cement From Aluminum Smelting Residue. U.S. Patent 4071373, 6 July 1976. [Google Scholar]
- Leiva, C.; Arenas, C.; Alonso-Fariñas, B.; Vilches, L.F.; Peceño, B.; Rodriguez-Galán, M.; Baena, F. Characteristics of fired bricks with co-combustion fly ashes. J. Build. Eng. 2016, 5. [Google Scholar] [CrossRef]
- Karellas, S.; Leontaritis, A.D.; Panousis, G.; Bellos, E.; Kakaras, E. Energetic and exergetic analysis of waste heat recovery systems in the cement industry. Energy 2013, 58, 147–156. [Google Scholar] [CrossRef]
- Hara, T.; Shima, H.; Yoshida, Y.; Matsuhashi, R. Model analysis of an inter-industrial and inter-regional waste recycling system in Japan. Energy 2007, 32, 609–618. [Google Scholar] [CrossRef]
- Rodríguez-Galán, M.; Alonso-Fariñas, B.; Baena-Moreno, F.M.; Leiva, C.; Navarrete, B.; Vilches, L.F. Synthetic slag production method based on a solid waste mix vitrification for the manufacturing of slag-cement. Materials 2019, 12, 208. [Google Scholar] [CrossRef] [Green Version]
- Motz, H.; Geiseler, J. Products of steel slags an opportunity to save natural resources. Waste Manag. 2001. [Google Scholar] [CrossRef]
- Zhu, J.; Zhong, Q.; Chen, G.; Li, D. Effect of particlesize of blast furnace slag on properties of Portland cement. Procedia Eng. 2012, 27, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Puertas, F. Escorias de alto horno: Composición y comportamiento hidráulico. Mater. Construcción 1993, 43, 37–48. [Google Scholar] [CrossRef] [Green Version]
- BSI. Granulated Slag Ground from High Blast Furnace for Use in Concrete, Mortar and Paste; British Standards Institution: London, UK, 2006. [Google Scholar]
- Wang, K.S.; Lin, K.L.; Tzeng, B.Y. Latent hydraulic reactivity of blended cement incorporating slag made from municipal solid waste incinerator fly ash. J. Air Waste Manag. Assoc. 2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reino García, H. Supercem®. Experiencia de Holcim (España) con cementos con escorias de alto horno altamente adicionados. Patol. Cim. Estruct. Hormig. 2013. [Google Scholar]
- Li, J.; Mou, Q.; Zeng, Q.; Yu, Y. Experimental study on precipitation behavior of spinels in stainless steel-making slag under heating treatment. Processes 2019, 7, 487. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, H.; Zhu, X.; Qiu, Y.J.; Li, K.; Chen, R.; Liao, Q. A review of waste heat recovery technologies towards molten slag in steel industry. Appl. Energy 2013, 112, 956–966. [Google Scholar] [CrossRef]
- Xiong, B.; Chen, L.; Meng, F.; Sun, F. Modeling and performance analysis of a two-stage thermoelectric energy harvesting system from blast furnace slag water waste heat. Energy 2014, 77, 562–569. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Q.; Tian, Q.; Guo, X. Simulation study and industrial application of enhanced arsenic removal by regulating the proportion of concentrates in the SKS copper smelting process. Processes 2020, 8, 385. [Google Scholar] [CrossRef] [Green Version]
- Ishaq, H.; Dincer, I.; Naterer, G.F. Exergy and cost analyses of waste heat recovery from furnace cement slag for clean hydrogen production. Energy 2019, 172, 1243–1253. [Google Scholar] [CrossRef]
- Barati, M.; Esfahani, S.; Utigard, T.A. Energy recovery from high temperature slags. Energy 2011, 36, 5440–5449. [Google Scholar] [CrossRef]
- Company, N. Metodo Para Enfriar Material Abrasivo Aluminoso Fundido. ES Patent ES399446, 3 February 1972. [Google Scholar]
- Hulek, A.; Ritzberguer, F. Method of Continuously Producing Vitreous Blast Furnace Slag. U.S. Patent 6250109B1, 10 July 1998. [Google Scholar]
- Jirou, K.; Yasuto, T.; Ohkoshi, K. Apparatus for Manufacturing Vitreous Slag. U.S. Patent 4330264A, 18 May 1982. [Google Scholar]
- Nakatani, G.; Kanai, K.; Itoh, H.; Takasaki, Y.; Ohkoshi, K.; Yanagida, Y. Apparatus for Manufacturing Rapidly Cooled Solidified Slag. U.S. Patent 4420304A, 13 December 1983. [Google Scholar]
- Unit, A.G. Low Energy Slag and Cement Production. U.S. Patent 9233485B1, 12 January 2016. [Google Scholar]
- Gresesqui-Lobaina, E.; Rodríguez-González, I.; Fernández-Columbié, T. Characterization of steel 70XL used in the manufacture of balls for the clinker’s milling. Min. Geol. 2017. Available online: https://www.redalyc.org/jatsRepo/2235/223553249009/html/index.html (accessed on 24 November 2020).
- Shakurov, A.G.; Shkol’nik, Y.S.; Parshin, V.M.; Chertov, A.D.; Zhuravlev, V.V. Cooling and solidification of slag melt in spherical packing. Steel Transl. 2012. [Google Scholar] [CrossRef]
- Mills, K.C. The estimation of slag properties. In Proceedings of the Southern African Pyrometallurgy International Conference, Johannesburg, South Africa, 6–9 March 2011. [Google Scholar]
- Castillo Neyra, P. Manual Práctico de Combustión y Clinkerización. 1990. Available online: https://dl-manual.com/download/manual-practico-de-combustion-y-clinkerizacion-6vj3xerk20oe?hash=168fd0ddbedc13b8ad71e8ec972e7a70 (accessed on 30 November 2020).
- Incropera, F.P.; deWitt, D.P. Fundamentos de Transferencia de Calor; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 1999; ISBN 970-17-0170-4. [Google Scholar]









| Composition Range | SiO2 | CaO | Al2O3 | MgO | Fe2O3 | SO3 | Na2O | K2O |
| 27–40 | 30–50 | 5–15 | 1–15 | 0.2–2.5 | 1–2.5 | 0.1–3 | 0.1–3 |
| First Experimental Stage | |||
| Test | Sphere mass (g) | Melt mass (g) | mm/ms |
| 1 | 260 | 157.5 | 0.61 |
| 2 | 260 | 122.5 | 0.47 |
| 3 | 510 | 157.5 | 0.31 |
| 4 | 510 | 122.5 | 0.24 |
| 5 | 510 | 192.5 | 0.38 |
| Second Experimental Stage | |||
| Test | Metal spheres diameter (mm) | Air inlet temperature (°C) | Experimental time (s) |
| 6 | 50 | 20–25 | 3000 |
| 7 | 50 | 150 | 2500 |
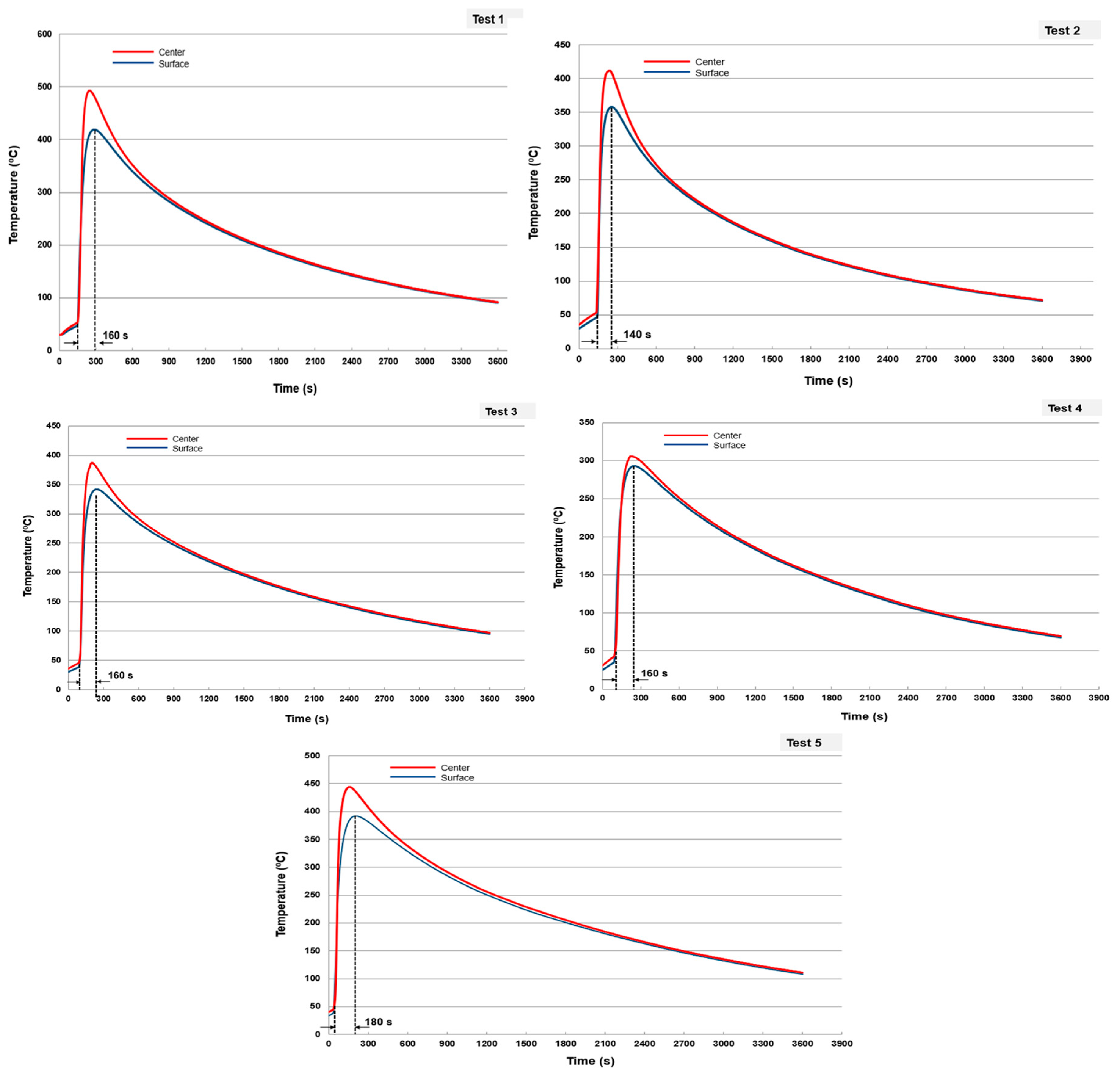
| Test | Sphere Mass (g) | Melt Mass (g) | mm/ms | Temperature in the Center of the Sphere (°C) | Temperature in the Surface of the Sphere (°C) |
|---|---|---|---|---|---|
| 1 | 260 | 157.5 | 0.61 | 410 | 482 |
| 2 | 260 | 122.5 | 0.47 | 350 | 405 |
| 3 | 510 | 157.5 | 0.31 | 340 | 383 |
| 4 | 510 | 122.5 | 0.24 | 295 | 302 |
| 5 | 510 | 192.5 | 0.38 | 390 | 438 |
| SiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | TiO2 | P2O5 | SO3 | C.L. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before melting | 25.2 | 8.4 | 1.39 | 0.04 | 1.12 | 30.93 | 1.03 | 0.66 | 0.2 | 0.07 | 0.28 | 27.81 |
| After melting | 38.05 | 11.42 | 1.77 | 0.07 | 1.44 | 42.63 | 1.36 | 0.79 | 0.32 | 0.1 | 0.68 | 0.21 |
| Deviations | ±0.68 | ±1.13 | ±0.34 | ±0.00 | ±0.06 | ±0.49 | ±0.07 | ±0.04 | ±0.01 | ±0.01 | ±0.21 | ±0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baena-Moreno, F.M.; Rodríguez-Galán, M.; Navarrete, B.; Vilches, L.F. Novel Study for Energy Recovery from the Cooling–Solidification Stage of Synthetic Slag Manufacturing: Estimation of the Potential Energy Recovery. Processes 2020, 8, 1590. https://doi.org/10.3390/pr8121590
Baena-Moreno FM, Rodríguez-Galán M, Navarrete B, Vilches LF. Novel Study for Energy Recovery from the Cooling–Solidification Stage of Synthetic Slag Manufacturing: Estimation of the Potential Energy Recovery. Processes. 2020; 8(12):1590. https://doi.org/10.3390/pr8121590
Chicago/Turabian StyleBaena-Moreno, Francisco M., Mónica Rodríguez-Galán, Benito Navarrete, and Luis F. Vilches. 2020. "Novel Study for Energy Recovery from the Cooling–Solidification Stage of Synthetic Slag Manufacturing: Estimation of the Potential Energy Recovery" Processes 8, no. 12: 1590. https://doi.org/10.3390/pr8121590
APA StyleBaena-Moreno, F. M., Rodríguez-Galán, M., Navarrete, B., & Vilches, L. F. (2020). Novel Study for Energy Recovery from the Cooling–Solidification Stage of Synthetic Slag Manufacturing: Estimation of the Potential Energy Recovery. Processes, 8(12), 1590. https://doi.org/10.3390/pr8121590





