A Review of Zein as a Potential Biopolymer for Tissue Engineering and Nanotechnological Applications
Abstract
1. Introduction
2. Zein
2.1. Physico-Chemical Properties
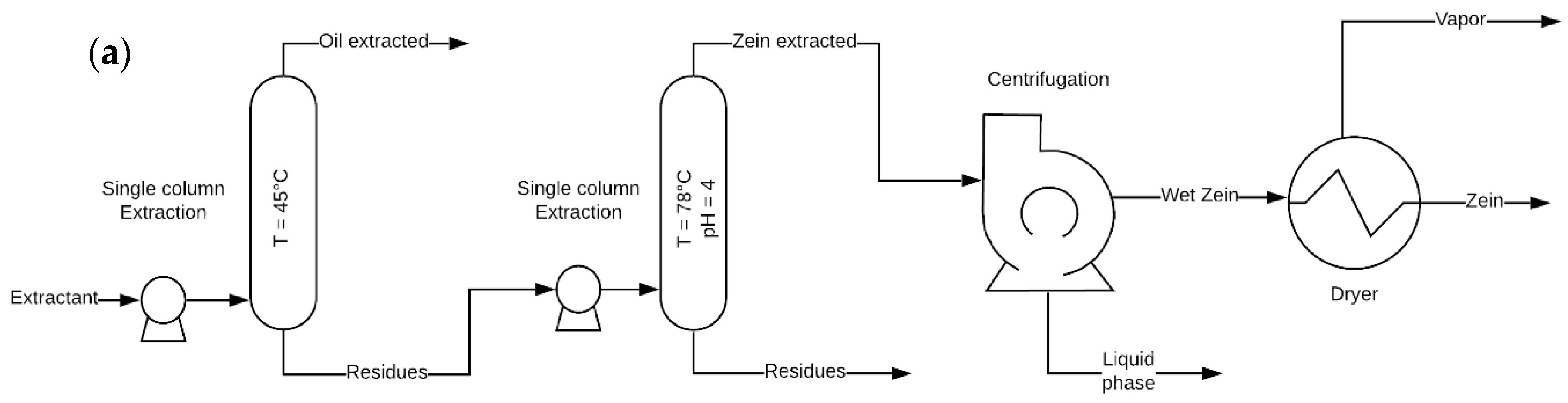
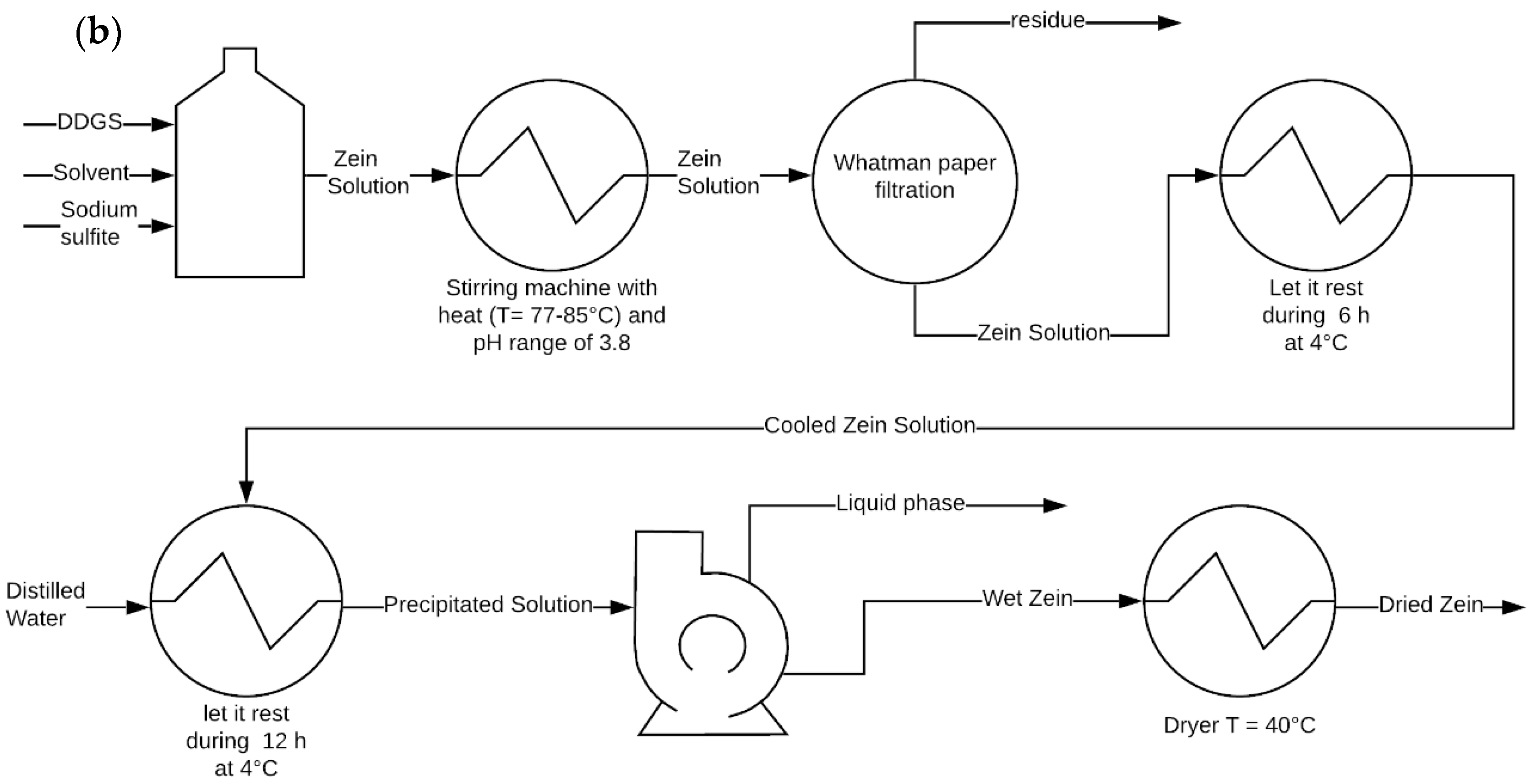
2.2. Zein Extraction
2.3. Zein as a Biopolymer
3. Uses of Zein in Tissue Engineering: Current State of the Art
3.1. An Overview of Tissue Engineering (TE)
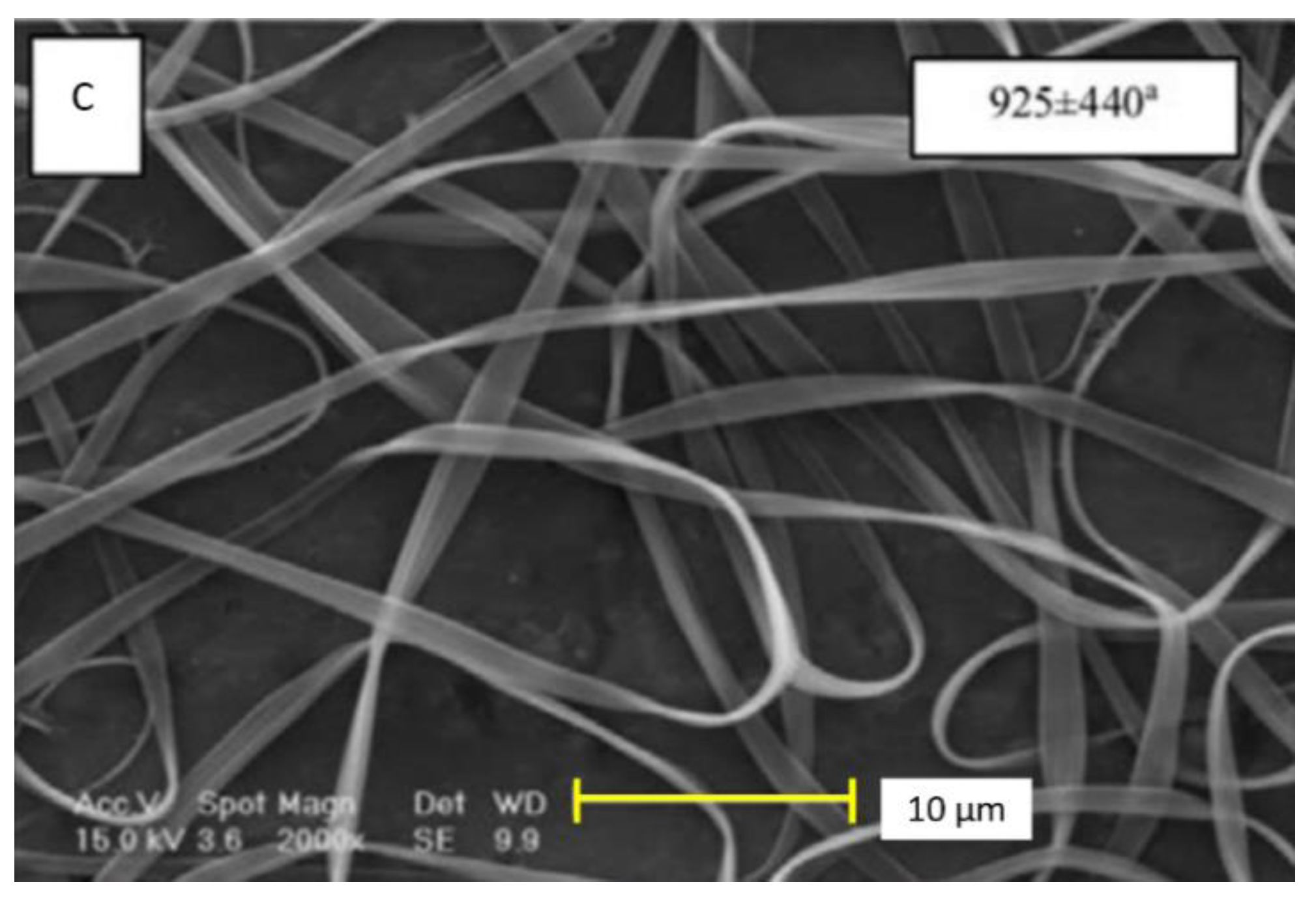
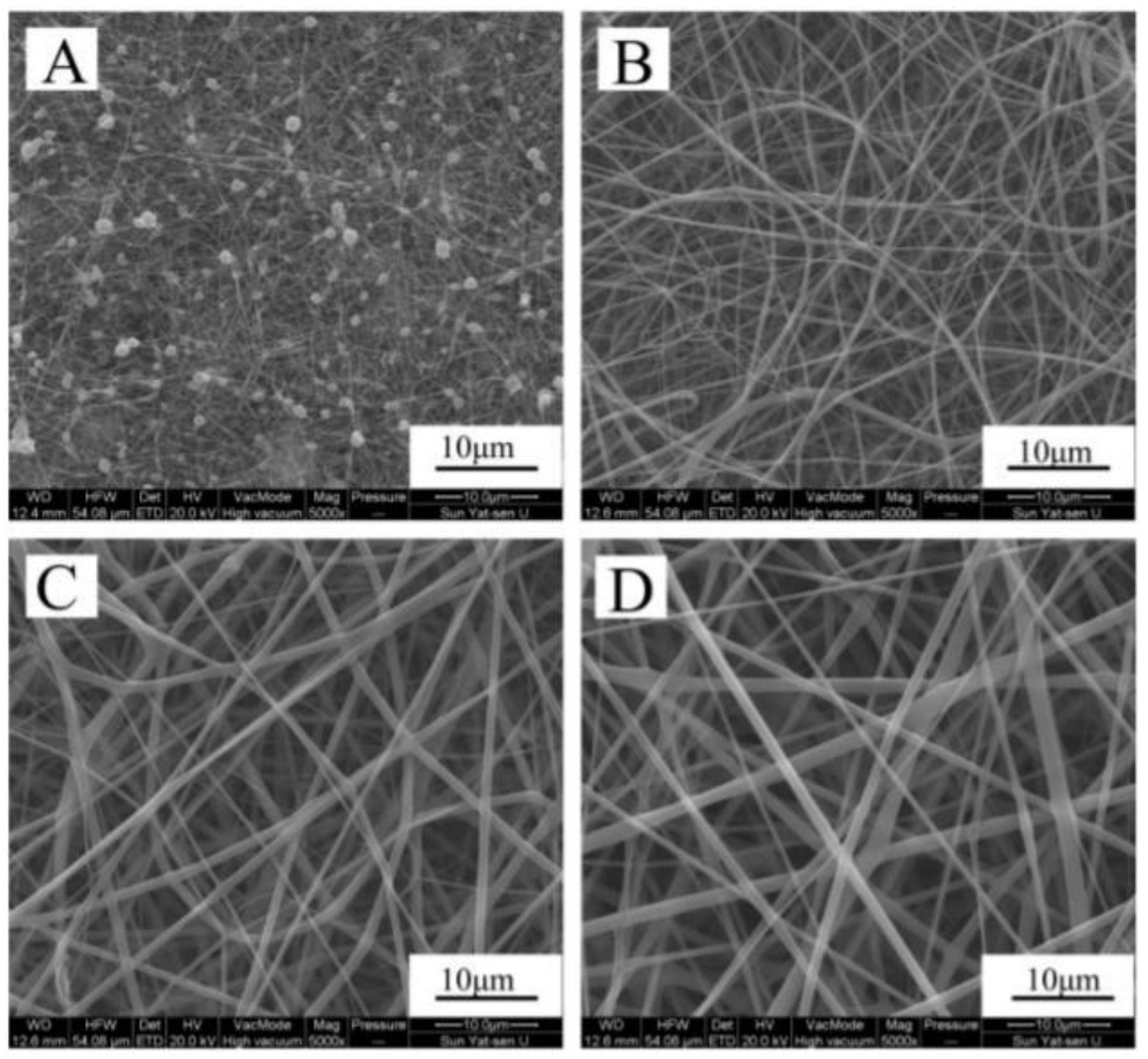
3.2. Zein on Tissue Engineering
4. The Role of Zein in Nanotechnological Applications
4.1. Zein-Based Delivery Systems
4.2. Zein-Based Nanoparticles in the Food Industry
4.3. Zein as a Carrier of Pesticides
4.4. Zein as a Potential Material for Bandages
5. Concluding Remarks and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goranov, V.; Shelyakova, T.; De Santis, R.; Haranava, Y.; Makhaniok, A.; Gloria, A.; Tampieri, A.; Russo, A.; Kon, E.; Marcacci, M.; et al. 3D Patterning of cells in Magnetic Scaffolds for Tissue Engineering. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Obisesan, O.B.; Oluwole, B.; Adebiyi, V.A.; Usman, H.; Khan, A. Mechanical characterization of a polymeric scaffold for bone implant. J. Mater. Sci. 2020, 55, 9057–9069. [Google Scholar] [CrossRef]
- Mehrian, M.; Lambrechts, T.; Papantoniou, I.; Geris, L. Computational Modeling of Human Mesenchymal Stromal Cell Proliferation and Extra-Cellular Matrix Production in 3D Porous Scaffolds in a Perfusion Bioreactor: The Effect of Growth Factors. Front. Bioeng. Biotechnol. 2020, 8, 376. [Google Scholar] [CrossRef]
- Li, H.; Pan, S.; Xia, P.; Chang, Y.; Fu, C.; Kong, W.; Yu, Z.; Wang, K.; Yang, X.; Qi, Z. Advances in the application of gold nanoparticles in bone tissue engineering. J. Biol. Eng. 2020, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Keshav Narayan, A.; Logith Kumar, R.; Viji Chandran, S.; Vairamani, M.; Selvamurugan, N. Proliferation and differentiation of mesenchymal stem cells on scaffolds containing chitosan, calcium polyphosphate and pigeonite for bone tissue engineering. Cell Prolif. 2018, 51, e12408. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Q.; Yang, M.; Zhan, X.; Lan, F.; He, J.; Gu, Z.; Wu, Y. Protein Corona of Magnetic Hydroxyapatite Scaffold Improves Cell Proliferation via Activation of Mitogen-Activated Protein Kinase Signaling Pathway. ACS Nano 2017, 11, 3690–3704. [Google Scholar] [CrossRef] [PubMed]
- Cícero, A.M.; Mardegan, P.J.I.; Feldman, S. Matrices de tercera generación en la ingeniería de tejidos óseos. Actual. Osteol. 2017, 13, 157–176. [Google Scholar]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; González-Valdez, J.; Ahmad, M.Z. High-performance pervaporation chitosan-based membranes: New insights and perspectives. Rev. Chem. Eng. 2020, 20190051. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef]
- Yang, F.; Miao, Y.; Wang, Y.; Zhang, L.M.; Lin, X. Electrospun zein/gelatin scaffold-enhanced cell attachment and growth of human periodontal ligament stem cells. Materials 2017, 10, 1168. [Google Scholar] [CrossRef]
- Moradkhannejhad, L.; Abdouss, M.; Nikfarjam, N.; Mazinani, S.; Heydari, V. Electrospinning of zein/propolis nanofibers; antimicrobial properties and morphology investigation. J. Mater. Sci. Mater. Med. 2018, 29, 165. [Google Scholar] [CrossRef]
- Fuenmayor, C.; Cosio, M. Encapsulation of Antioxidant Phenolic Compounds in Zein Ultra-Thin Fibers Via Electrospinning. Rev. EIA 2016, 3, 13–25. [Google Scholar] [CrossRef]
- Dashdorj, U.; Reyes, M.K.; Unnithan, A.R.; Tiwari, A.P.; Tumurbaatar, B.; Park, C.H.; Kim, C.S. Fabrication and characterization of electrospun zein/Ag nanocomposite mats for wound dressing applications. Int. J. Biol. Macromol. 2015, 80, 1–7. [Google Scholar] [CrossRef]
- Turasan, H.; Cakmak, M.; Kokini, J. Fabrication of zein-based electrospun nanofiber decorated with gold nanoparticles as a SERS platform. J. Mater. Sci. 2019, 54, 8872–8891. [Google Scholar] [CrossRef]
- Ghalei, S.; Asadi, H.; Ghalei, B. Zein nanoparticle-embedded electrospun PVA nanofibers as wound dressing for topical delivery of anti-inflammatory diclofenac. J. Appl. Polym. Sci. 2018, 135, 1–11. [Google Scholar] [CrossRef]
- Li, M.; Zheng, H.; Lin, M.; Zhu, W.; Zhang, J. Characterization of the protein and peptide of excipient zein by the multi-enzyme digestion coupled with nano-LC-MS/MS. Food Chem. 2020, 321, 126712. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; Weng, Y.M. Novel edible composite films fabricated with whey protein isolate and zein: Preparation and physicochemical property evaluation. LWT 2019, 101, 567–574. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, J.; Qin, Y.; Jiang, B.; Zhang, T. Zein/fucoidan-based composite nanoparticles for the encapsulation of pterostilbene: Preparation, characterization, physicochemical stability, and formation mechanism. Int. J. Biol. Macromol. 2020, 158, 461–470. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Sun, Y.; Wang, X.; Li, L. Effect of α-tocopherol antioxidant on rheological and physicochemical properties of chitosan/zein edible films. LWT 2020, 118, 108799. [Google Scholar] [CrossRef]
- Suganya, P.; Vaseeharan, B.; Vijayakumar, S.; Balan, B.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Biopolymer zein-coated gold nanoparticles: Synthesis, antibacterial potential, toxicity and histopathological effects against the Zika virus vector Aedes aegypti. J. Photochem. Photobiol. B Biol. 2017, 173, 404–411. [Google Scholar] [CrossRef]
- Momany, F.A.; Sessa, D.J.; Lawton, J.W.; Selling, G.W.; Hamaker, S.A.H.; Willett, J.L. Structural Characterization of α-Zein. J. Agric. Food Chem. 2006, 54, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Hayat, U.; Bilal, M.; Iqbal, H.M.N.; Wang, J.Y. Zein-based micro- and nano-constructs and biologically therapeutic cues with multi-functionalities for oral drug delivery systems. J. Drug Deliv. Sci. Technol. 2020, 58, 101818. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, R.; Xu, H. Physicochemical properties of zein-based films by electrophoretic deposition using indium tin oxide electrodes: Vertical and horizontal electric fields. Int. J. Food Prop. 2016, 19, 945–957. [Google Scholar] [CrossRef]
- Corradini, E.; Curti, P.S.; Meniqueti, A.B.; Martins, A.F.; Rubira, A.F.; Muniz, E.C. Recent advances in food-packing, pharmaceutical and biomedical applications of zein and zein-based materials. Int. J. Mol. Sci. 2014, 15, 22438–22470. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Xin, Z.; Meng, X.; Sun, S.; Qiao, Q.; Deng, H. Recovering high value-added substances from corn distillers dried grains with solubles: A semi-continuous countercurrent downstream processing method. J. Chem. Technol. Biotechnol. 2016, 91, 1327–1338. [Google Scholar] [CrossRef]
- Demir, M.; Ramos-Rivera, L.; Silva, R.; Nazhat, S.N.; Boccaccini, A.R. Zein-based composites in biomedical applications. J. Biomed. Mater. Res. Part A 2017, 105, 1656–1665. [Google Scholar] [CrossRef]
- Gupta, J.; Wilson, B.W.; Vadlani, P.V. Evaluation of green solvents for a sustainable zein extraction from ethanol industry DDGS. Biomass Bioenergy 2016, 85, 313–319. [Google Scholar] [CrossRef]
- Yabueng, N.; Napathorn, S.C. Toward non-toxic and simple recovery process of poly(3-hydroxybutyrate) using the green solvent 1,3-dioxolane. Process Biochem. 2018, 69, 197–207. [Google Scholar] [CrossRef]
- Chouchene, K.; Rocha-Santos, T.; Ksibi, M. Types, occurrence, and distribution of microplastics and metals contamination in sediments from south west of Kerkennah archipelago, Tunisia. Environ. Sci. Pollut. Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Mostafa, Y.S.; Alrumman, S.A.; Alamri, S.A.; Ot, K.A. Bioplastic ( poly-3-hydroxybutyrate ) production by the marine bacterium Pseudodonghicola xiamenensis through date syrup valorization and structural assessment of the biopolymer. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.; Fathi, M.; Soleimanian-zad, S. Incorporation of zein nanofibers produced by needle-less electrospinning within the casted gelatin film for improvement of its physical properties. Food Bioprod. Process. 2020, 122, 193–204. [Google Scholar] [CrossRef]
- Wu, J.; Wang, N.; Zhao, Y.; Jiang, L. Electrospinning of multilevel structured functional micro-/nanofibers and their applications. J. Mater. Chem. A 2013, 1, 7290–7305. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, W.; Ma, D.; Ma, Q.; Bridges, D.; Ma, Y.; Hu, A. Electrospinning of Nanofibers and Their Applications for Energy Devices. J. Nanomater. 2015, 2015, 140716. [Google Scholar] [CrossRef]
- Niu, B.; Zhan, L.; Shao, P.; Xiang, N.; Sun, P.; Chen, H.; Gao, H. International Journal of Biological Macromolecules Electrospinning of zein-ethyl cellulose hybrid nanofibers with improved water resistance for food preservation. Int. J. Biol. Macromol. 2020, 142, 592–599. [Google Scholar] [CrossRef]
- Liebschner, M.A.K. Biomechanical considerations of animal models used in tissue engineering of bone. Biomaterials 2004, 25, 1697–1714. [Google Scholar] [CrossRef]
- Choi, J.H.; Gimble, J.M.; Lee, K.; Marra, K.G.; Rubin, J.P.; Yoo, J.J.; Vunjak-Novakovic, G.; Kaplan, D.L. Adipose tissue engineering for soft tissue regeneration. Tissue Eng. Part B Rev. 2010, 16, 413–426. [Google Scholar] [CrossRef]
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003, 21, 157–161. [Google Scholar] [CrossRef]
- Rose, J.B.; Pacelli, S.; El Haj, A.J.; Dua, H.S.; Hopkinson, A.; White, L.J.; Rose, F.R.A.J. Gelatin-based materials in ocular tissue engineering. Materials 2014, 7, 3106–3135. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, K.; Kai, D.; Li, Z.; Loh, X.J. Polyester elastomers for soft tissue engineering. Chem. Soc. Rev. 2018, 47, 4545–4580. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomater. Silver Jubil. Compend. 2000, 21, 175–189. [Google Scholar]
- Zhou, X.; Feng, Y.; Zhang, J.; Shi, Y.; Wang, L. Recent advances in additive manufacturing technology for bone tissue engineering scaffolds. Int. J. Adv. Manuf. Technol. 2020, 108, 3591–3606. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, P.; Sharma, R.; Bhatt, V.D.; Dhot, P.S. Tissue Engineering; Current Status & Futuristic Scope. J. Med. Life 2019, 12, 225–229. [Google Scholar]
- Filardo, G.; Andriolo, L.; Soler, F.; Berruto, M.; Ferrua, P.; Verdonk, P.; Rongieras, F.; Crawford, D.C. Treatment of unstable knee osteochondritis dissecans in the young adult: Results and limitations of surgical strategies—The advantages of allografts to address an osteochondral challenge. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1726–1738. [Google Scholar] [CrossRef]
- Chaiarwut, S.; Niyompanich, J.; Ekabutr, P.; Chuysinuan, P.; Pavasant, P.; Supaphol, P. Development and characterization of antibacterial hydroxyapatite coated with mangosteen extract for bone tissue engineering. Polym. Bull. 2020, 1–17. [Google Scholar] [CrossRef]
- Lloyd, A. Reinforced polymers for connective tissue. Mater. Today 2004, 7, 19. [Google Scholar] [CrossRef]
- Neo, P.Y.; Teh, T.K.H.; Tay, A.S.R.; Asuncion, M.C.T.; Png, S.N.; Toh, S.L.; Goh, J.C.H. Stem cell-derived cell-sheets for connective tissue engineering. Connect. Tissue Res. 2016, 57, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Bielli, A.; Bernardini, R.; Varvaras, D.; Rossi, P.; Di Blasi, G.; Petrella, G.; Buonomo, O.C.; Mattei, M.; Orlandi, A. Characterization of a new decellularized bovine pericardial biological mesh: Structural and mechanical properties. J. Mech. Behav. Biomed. Mater. 2018, 78, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jia, Y.Y.; Sun, X.; Wang, J. Tissue engineering in female pelvic floor reconstruction. Eng. Life Sci. 2020, 20, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Liao, S.; Ngiam, M.; Chan, C.K.; Ramakrishna, S. Degradation behaviors of electrospun resorbable polyester nanofibers. Tissue Eng. Part B Rev. 2009, 15, 333–351. [Google Scholar] [CrossRef]
- Neeley, W.L.; Redenti, S.; Klassen, H.; Tao, S.; Desai, T.; Young, M.J.; Langer, R. A microfabricated scaffold for retinal progenitor cell grafting. Biomaterials 2008, 29, 418–426. [Google Scholar] [CrossRef]
- Ueda, H.; Hacker, M.C.; Haesslein, A.; Jo, S.; Ammon, D.M.; Borazjani, R.; Kunzler, J.; Mikos, A. Injectable, in situ forming poly(propylene fumarate)-based ocular drug delivery systems. J. Biomed. Mater. Res. Part A 2006, 79, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.A.; Bandello, F.; Belfort, R.; Blumenkranz, M.S.; Gillies, M.; Heier, J.; Loewenstein, A.; Yoon, Y.H.; Jacques, M.L.; Jiao, J.; et al. Randomized, Sham-Controlled Trial of Dexamethasone Intravitreal Implant in Patients with Macular Edema Due to Retinal Vein Occlusion. Ophthalmology 2010, 117, 1134–1146.e3. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for Tissue Engineering Applications. Prog. Mater. Sci. 2020, 100721. [Google Scholar] [CrossRef]
- Armstrong, J.P.K.; Stevens, M.M. Using Remote Fields for Complex Tissue Engineering. Trends Biotechnol. 2020, 38, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, R.; Krishnan, U.M.; Sethuraman, S. Living cardiac patch: The elixir for cardiac regeneration. Expert Opin. Biol. Ther. 2012, 12, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.C.P.; Kaasi, A.; Maciel Filho, R.; Jardini, A.L.; Gabriel, L.P. Cardiac tissue engineering: Current state-of-the-art materials, cells and tissue formation. Einstein (Sao Paulo) 2018, 16, eRB4538. [Google Scholar] [CrossRef]
- Zhang, P.-X.; Han, N.; Kou, Y.-H.; Zhu, Q.-T.; Liu, X.-L.; Quan, D.-P.; Chen, J.-G.; Jiang, B.-G. Tissue engineering for the repair of peripheral nerve injury. Neural Regen. Res. 2019, 14, 51–58. [Google Scholar]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef]
- Limongi, T.; Tirinato, L.; Pagliari, F.; Giugni, A.; Allione, M.; Perozziello, G.; Candeloro, P.; Di Fabrizio, E. Fabrication and Applications of Micro/Nanostructured Devices for Tissue Engineering. NanoMicro Lett. 2016, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Liu, X.; Meng, Z. Enhanced mechanical and osteogenic differentiation performance of hydroxyapatite/zein composite for bone tissue engineering. J. Mater. Sci. 2019, 54, 719–729. [Google Scholar] [CrossRef]
- Ghorbani, M.; Nezhad-Mokhtari, P.; Ramazani, S. Aloe vera-loaded nanofibrous scaffold based on Zein/Polycaprolactone/Collagen for wound healing. Int. J. Biol. Macromol. 2020, 153, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, C.; Zhao, Y.; Hu, J.; Zhang, L.-M. Co-electrospun Nanofibrous Membranes of Collagen and Zein for Wound Healing. ACS Appl. Mater. Interfaces 2012, 4, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-W.; Yang, H.; Wu, W.-F.; Zhang, P.; Wang, J.-Y. Design and optimization of a biodegradable porous zein conduit using microtubes as a guide for rat sciatic nerve defect repair. Biomaterials 2017, 131, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Palakurthi, S. Zein in controlled drug delivery and tissue engineering. J. Control. Release 2014, 189, 108–122. [Google Scholar] [CrossRef]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Calendula officinalis extract/PCL/Zein/Gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering. Int. J. Biol. Macromol. 2019, 135, 530–543. [Google Scholar] [CrossRef]
- Gunes, S.; Tamburaci, S.; Tihminlioglu, F. A novel bilayer zein/MMT nanocomposite incorporated with H. perforatum oil for wound healing. J. Mater. Sci. Mater. Med. 2019, 31, 7. [Google Scholar] [CrossRef]
- Dias Antunes, M.; da Silva Dannenberg, G.; Fiorentini, Â.M.; Pinto, V.Z.; Lim, L.-T.; da Rosa Zavareze, E.; Dias, A.R.G. Antimicrobial electrospun ultrafine fibers from zein containing eucalyptus essential oil/cyclodextrin inclusion complex. Int. J. Biol. Macromol. 2017, 104, 874–882. [Google Scholar] [CrossRef]
- Aytac, Z.; Huang, R.; Vaze, N.; Xu, T.; Eitzer, B.D.; Krol, W.; MacQueen, L.A.; Chang, H.; Bousfield, D.W.; Chan-Park, M.B.; et al. Development of Biodegradable and Antimicrobial Electrospun Zein Fibers for Food Packaging. ACS Sustain. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Fabrication and characterization of PCL/zein/gum arabic electrospun nanocomposite scaffold for skin tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Caetano, G.; Ambler, W.S.; Blaker, J.J.; Frade, M.A.; Mandal, P.; Diver, C.; Bártolo, P. Enhancing the hydrophilicity and cell attachment of 3D printed PCL/graphene scaffolds for bone tissue engineering. Materials 2016, 9, 992. [Google Scholar] [CrossRef]
- Meng, Z.X.; Wang, Y.S.; Ma, C.; Zheng, W.; Li, L.; Zheng, Y.F. Electrospinning of PLGA/gelatin randomly-oriented and aligned nanofibers as potential scaffold in tissue engineering. Mater. Sci. Eng. C 2010, 30, 1204–1210. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Hussein, K.H.; Casettari, L.; Khalil, K.A.; Hamdy, A.S. Fabrication of novel high performance ductile poly(lactic acid) nanofiber scaffold coated with poly(vinyl alcohol) for tissue engineering applications. Mater. Sci. Eng. C 2015, 60, 143–150. [Google Scholar] [CrossRef]
- Caballero Aguilar, L.M.; Silva, S.M.; Moulton, S.E. Growth factor delivery: Defining the next generation platforms for tissue engineering. J. Control. Release 2019, 306, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, S.-J.; Zhao, X.-R.; Zhu, Y.-F.; Yu, J.-K. 3D- Printed Poly(ε-caprolactone) Scaffold Integrated with Cell-laden Chitosan Hydrogels for Bone Tissue Engineering. Sci. Rep. 2017, 7, 13412. [Google Scholar] [CrossRef]
- Vogt, L.; Liverani, L.; Boccaccini, A.R.; Roether, J.A. Electrospun zein fibers incorporating poly(glycerol sebacate) for soft tissue engineering. Nanomaterials 2018, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Ghaee, A.; Nourmohammadi, J. Poly (sodium 4-styrene sulfonate)-modified hydroxyapatite nanoparticles in zein-based scaffold as a drug carrier for vancomycin. Mater. Sci. Eng. C 2019, 100, 874–885. [Google Scholar] [CrossRef]
- Shahbazarab, Z.; Teimouri, A.; Chermahini, A.N.; Azadi, M. Fabrication and characterization of nanobiocomposite scaffold of zein/chitosan/nanohydroxyapatite prepared by freeze-drying method for bone tissue engineering. Int. J. Biol. Macromol. 2018, 108, 1017–1027. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, Y.; Sun, D.; Ma, W.; Yao, J.; Zhang, M. Preparation and characterization of coaxial electrospinning rhBMP2-loaded nanofiber membranes. J. Nanomater. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Mamidi, N.; Romo, I.L.; Leija Gutiérrez, H.M.; Barrera, E.V.; Elías-Zúñiga, A. Development of forcespun fiber-aligned scaffolds from gelatin-zein composites for potential use in tissue engineering and drug release. MRS Commun. 2018, 8, 885–892. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Buera-González, J.; de la Iglesia, Ó.; Galiano, F.; Fíla, V.; Malankowska, M.; Rubio, C.; Figoli, A.; Téllez, C.; Coronas, J. Towards the dehydration of ethanol using pervaporation cross-linked poly(vinyl alcohol)/graphene oxide membranes. J. Membr. Sci. 2019, 582, 423–434. [Google Scholar] [CrossRef]
- Zhijiang, C.; Qin, Z.; Xianyou, S.; Yuanpei, L. Zein/Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) electrospun blend fiber scaffolds: Preparation, characterization and cytocompatibility. Mater. Sci. Eng. C 2017, 71, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, C.E.; Ramos-Rivera, L.; Conti, B.; Boccaccini, A.R. Zein-Based Electrospun Fibers Containing Bioactive Glass with Antibacterial Capabilities. Macromol. Biosci. 2020. [Google Scholar] [CrossRef]
- Kasaai, M.R. Zein and zein -based nano-materials for food and nutrition applications: A review. Trends Food Sci. Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
- Singh, B.G.; Das, R.P.; Kunwar, A. Protein: A versatile biopolymer for the fabrication of smart materials for drug delivery. J. Chem. Sci. 2019, 131, 1–14. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Borne, M.; Chalier, P.; Gontard, N.; Morel, M.H.; Peyron, S.; Gavara, R.; Hernandez-Munoz, P. Retention and release of cinnamaldehyde from wheat protein matrices. Biomacromolecules 2013, 14, 1493–1502. [Google Scholar] [CrossRef]
- Tavares, W.S.; Tavares-júnior, A.G.; Otero-espinar, F.J.; Martín-pastor, M.; Sousa, F.F.O. Design of ellagic acid-loaded chitosan/zein films for wound bandaging. J. Drug Deliv. Sci. Technol. 2020, 59, 101903. [Google Scholar] [CrossRef]
- Hao, L.; Lin, G.; Lian, J.; Chen, L.; Zhou, H.; Chen, H.; Xu, H.; Zhou, X. Carboxymethyl cellulose capsulated zein as pesticide nano-delivery system for improving adhesion and anti-UV properties. Carbohydr. Polym. 2020, 231, 115725. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Elgohary, M.M.; Kamel, N.M. Implications of Protein- and Peptide-Based Nanoparticles as Potential Vehicles for Anticancer Drugs, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 98. [Google Scholar]
- Zhang, Q.; Wang, J.; Liu, D.; Zhu, W.; Guan, S.; Fan, L.; Cai, D. Targeted delivery of honokiol by zein/hyaluronic acid core-shell nanoparticles to suppress breast cancer growth and metastasis. Carbohydr. Polym. 2020, 240, 116325. [Google Scholar] [CrossRef]
- Labib, G. Overview on zein protein: A promising pharmaceutical excipient in drug delivery systems and tissue engineering. Expert Opin. Drug Deliv. 2018, 15, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Q.; Li, H.; Yang, L.; Yi, J.Z.; Xie, M.; Zhang, L.M. Long-circulating zein-polysulfobetaine conjugate-based nanocarriers for enhancing the stability and pharmacokinetics of curcumin. Mater. Sci. Eng. C 2020, 109, 110636. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Han, C.; Tian, Y.; Liu, T. Fabrication of curcumin-loaded zein nanoparticles stabilized by sodium caseinate/sodium alginate: Curcumin solubility, thermal properties, rheology, and stability. Process Biochem. 2020, 94, 30–38. [Google Scholar] [CrossRef]
- Maria Leena, M.; Yoha, K.S.; Moses, J.A.; Anandharamakrishnan, C. Edible coating with resveratrol loaded electrospun zein nanofibers with enhanced bioaccessibility. Food Biosci. 2020, 36, 100669. [Google Scholar] [CrossRef]
- Rostami, M.; Ghorbani, M.; Aman mohammadi, M.; Delavar, M.; Tabibiazar, M.; Ramezani, S. Development of resveratrol loaded chitosan-gellan nanofiber as a novel gastrointestinal delivery system. Int. J. Biol. Macromol. 2019, 135, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.; Zou, Y.; Liang, X.; Peng, Y.; McClements, D.J.; Hu, K. Encapsulation of resveratrol in zein/pectin core-shell nanoparticles: Stability, bioaccessibility, and antioxidant capacity after simulated gastrointestinal digestion. Food Hydrocoll. 2019, 93, 261–269. [Google Scholar] [CrossRef]
- Karim, M.; Fathi, M.; Soleimanian-Zad, S. Nanoencapsulation of cinnamic aldehyde using zein nanofibers by novel needle-less electrospinning: Production, characterization and their application to reduce nitrite in sausages. J. Food Eng. 2020, 288, 110140. [Google Scholar] [CrossRef]
- Chen, X.; Cui, F.; Zi, H.; Zhou, Y.; Liu, H.; Xiao, J. Development and characterization of a hydroxypropyl starch/zein bilayer edible film. Int. J. Biol. Macromol. 2019, 141, 1175–1182. [Google Scholar] [CrossRef]
- Kacsó, T.; Neaga, I.O.; Erincz, A.; Astete, C.E.; Sabliov, C.M.; Oprean, R.; Bodoki, E. Perspectives in the design of zein-based polymeric delivery systems with programmed wear down for sustainable agricultural applications. Polym. Degrad. Stab. 2018, 155, 130–135. [Google Scholar] [CrossRef]
- Cui, B.; Gao, F.; Zeng, Z.; Wang, C.; Wang, Y.; Sun, C.; Zhao, X.; Guo, L.; Shen, Y.; Liu, G.; et al. Construction and characterization of avermectin B2 solid nanodispersion. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Lin, M.; Fang, S.; Zhao, X.; Liang, X.; Wu, D. Natamycin-loaded zein nanoparticles stabilized by carboxymethyl chitosan: Evaluation of colloidal/chemical performance and application in postharvest treatments. Food Hydrocoll. 2020, 106, 105871. [Google Scholar] [CrossRef]
- Falahieh, K.H.; Falahieh, K.H.; Sarkaki, A.; Sarkaki, A.; Edalatmanesh, M.; Naseri, M.K.G.; Farbood, Y. Ellagic acid attenuates post-cerebral ischemia and reperfusion behavioral deficits by decreasing brain tissue inflammation in rats. Iran. J. Basic Med. Sci. 2020, 23, 645–653. [Google Scholar]
- Zhang, S.; Vijayavenkataraman, S.; Lu, W.F.; Fuh, J.Y.H. A review on the use of computational methods to characterize, design, and optimize tissue engineering scaffolds, with a potential in 3D printing fabrication. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1329–1351. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, Z.; Venugopal, J.R.; Urbanska, A.M.; Mills, D.K.; Ramakrishna, S.; Mozafari, M. A bird’s eye view on the use of electrospun nanofibrous scaffolds for bone tissue engineering: Current state-of-the-art, emerging directions and future trends. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2181–2200. [Google Scholar] [CrossRef] [PubMed]
- Elkasabgy, N.A.; Mahmoud, A.A. Fabrication Strategies of Scaffolds for Delivering Active Ingredients for Tissue Engineering. AAPS PharmSciTech 2019, 20, 256. [Google Scholar] [CrossRef] [PubMed]




| Property | Value | References |
|---|---|---|
| Denaturation temperature. | 80.32–87.1 °C | [25] |
| Glass transition temperature of α-zein | 165 °C | [26] |
| Thermal degradation point of α-zein | 280 °C | [26] |
| Molecular weight of α-zein | 22–24 kDa | [24] |
| Molecular weight of β-zein | 17 kDa | [24] |
| Molecular weight of δ-zein | 10 kDa | [24] |
| Molecular weight of γ-zein | 18–27 kDa | [24] |
| Degree of polymerization of α-zein | 210–245 | [26] |
| Isoelectric point of α-zein | pH 6.2 | [26,27] |
| Partial specific volume of α-zein | 0.771 | [26] |
| Zein flavor | Flavorless | [25] |
| Zein color | Yellowish | [25] |
| Zein odor | Odorless | [25] |
| Zein composition | Glutamine 21–26% Leucine 20% Proline 10% Alanine 10% | [28] |
| Name of the Method | Solvent | pH | Temperature | Time | Hazard Count | Zein Yield Extraction |
|---|---|---|---|---|---|---|
| One-time single column extraction | Ethanol | 4 | 78 °C | 40 min | 19 | 17.7% |
| Four times single column extraction | Ethanol | 4 | 78 °C | 40 min | 19 | 30.7% |
| Semi-continuous column extraction | Ethanol | 4 | 78 °C | 10 min | 19 | 24.5% |
| Stirred flask extraction | Isopropanol | 3.8–4.4 | 77–85 °C | 20 h | 24 | 70.0% |
| Stirred flask extraction | n-butanol | 3.8–4.4 | 77–85 °C | 20 h | 21 | 10.8% |
| Stirred flask extraction | Isobutanol | 3.8–4.4 | 77–85 °C | 20 h | 18 | 16.7% |
| Stirred flask extraction | 2,3-BDO | 3.8–4.4 | 77–85 °C | 20 h | - | 35.7% |
| Stirred flask extraction | 1,4-BDO | 3.8–4.4 | 77–85 °C | 20 h | - | 9.6% |
| Stirred flask extraction | Isoamyl alcohol | 3.8–4.4 | 77–85 °C | 20 h | 19 | 1.7% |
| Stirred flask extraction | 1,3 dioxolane | 3.8–4.4 | 77–85 °C | 20 h | Green | 23.3% |
| Stirred flask extraction | 1,4 dioxane | 3.8–4.4 | 77–85 °C | 20 h | 29 | 40.8% |
| Stirred flask extraction | Tetrahydrofuran | 3.8–4.4 | 77–85 °C | 20 h | 25 | 3.3% |
| Stirred flask extraction | Acetic acid | 3.8–4.4 | 77–85 °C | 20 h | 24 | 31.3% |
| Stirred flask extraction | Latic acid | 3.8–4.4 | 77–85 °C | 20 h | - | 4.2% |
| Stirred flask extraction | [emim]Ac | 3.8–4.4 | 77–85 °C | 20 h | - | 0.0% |
| Stirred flask extraction | [emim]Br | 3.8–4.4 | 77–85 °C | 20 h | - | 0.0% |
| Stirred flask extraction | Ethanol | 3.8–4.4 | 77–85 °C | 20 h | 19 | 80.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Guzmán, C.J.; Castro-Muñoz, R. A Review of Zein as a Potential Biopolymer for Tissue Engineering and Nanotechnological Applications. Processes 2020, 8, 1376. https://doi.org/10.3390/pr8111376
Pérez-Guzmán CJ, Castro-Muñoz R. A Review of Zein as a Potential Biopolymer for Tissue Engineering and Nanotechnological Applications. Processes. 2020; 8(11):1376. https://doi.org/10.3390/pr8111376
Chicago/Turabian StylePérez-Guzmán, Carlos Joaquín, and Roberto Castro-Muñoz. 2020. "A Review of Zein as a Potential Biopolymer for Tissue Engineering and Nanotechnological Applications" Processes 8, no. 11: 1376. https://doi.org/10.3390/pr8111376
APA StylePérez-Guzmán, C. J., & Castro-Muñoz, R. (2020). A Review of Zein as a Potential Biopolymer for Tissue Engineering and Nanotechnological Applications. Processes, 8(11), 1376. https://doi.org/10.3390/pr8111376






