The Effect of Slurry Wet Mixing Time, Thermal Treatment, and Method of Electrode Preparation on Membrane Capacitive Deionisation Performance
Abstract
:1. Introduction
- A large total surface area with appropriate pore size distribution and porosity. Such large surface areas are created using materials with large specific surface area. Several commercially available activated carbons meet the requirements and are currently used as a cost-effective substance in commercial CDI systems [12,13].
- Appropriately developed multi-dimensional electron conductive links between this large specific surface area material and an external power source. Carbon black is typically mixed with the active material and the mixture is deposited onto a current collector. These current collectors are eventually connected to an external power source [14,15].
- Electrode stability. As long as CDI electrode structure provide continuous, low resistive pathways for both ions (from the entire surface area of the activated carbon to the feed water source) and electrons (from the entire surface area of the activated carbon to the external power source), the electrode is of value. The quantity and type of binders play an important role in maintaining the structural integrity of the electrode [16,17].
2. Materials and Methods
2.1. Chemicals
2.2. Electrode Preparation
2.3. Slurry Preparation
2.4. Slurry/”Ink” Deposition
2.4.1. Slurry Infiltration by Calendering
2.4.2. Ink Infiltration Dropwise (Wet Mixing Time 60 min)
2.4.3. Ink Deposition by Spray Coating (Wet Mixing Time 60 min)
2.5. Electrode Characterisation
2.5.1. Scanning Electron Microscopy Energy Dispersive X-ray Spectroscopy (SEM EDS)
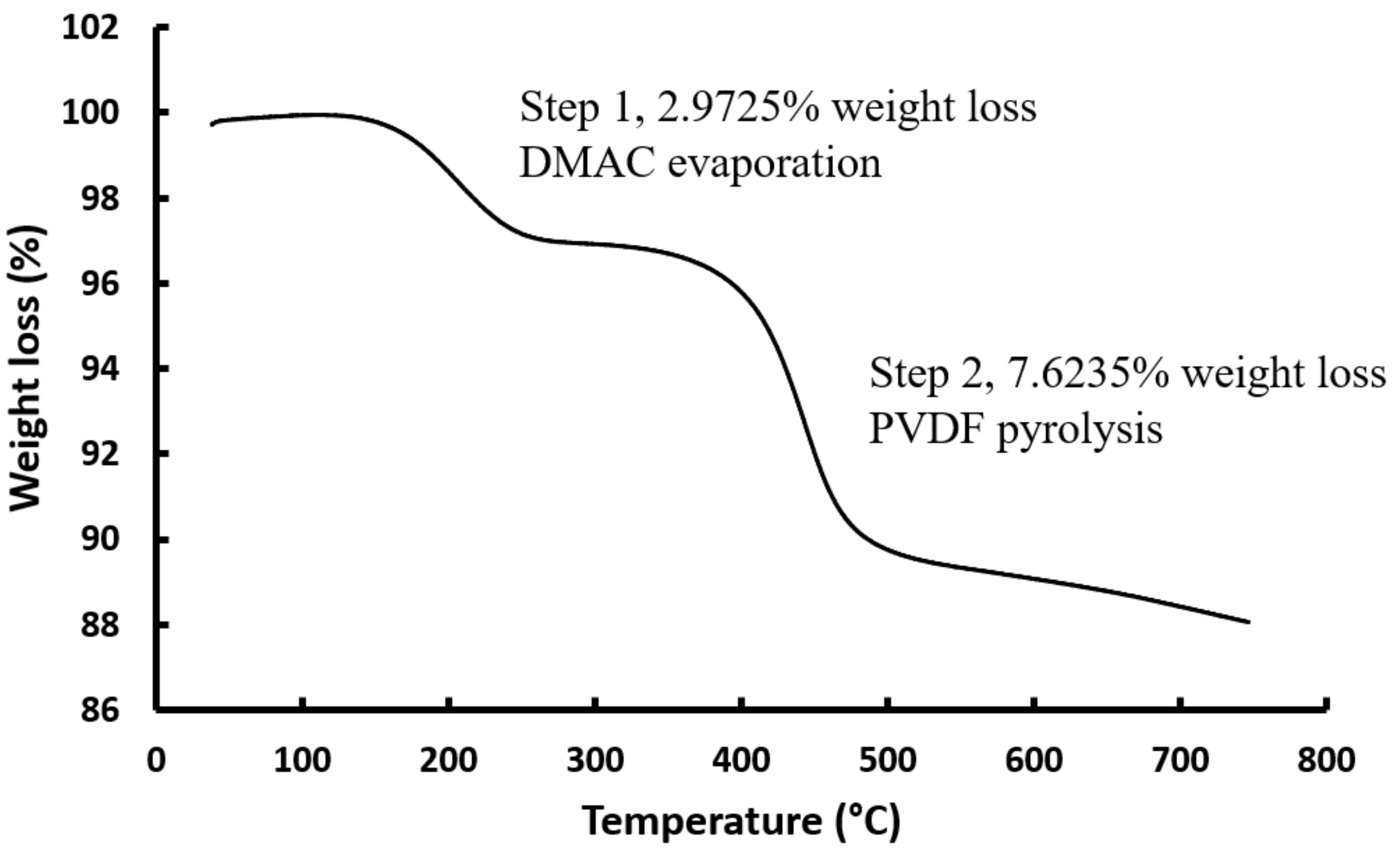
2.5.2. Thermogravimetric Analysis Coupled with Mass Spectrometry (TGA-MS)
2.5.3. Differential Scanning Calorimetry (DSC)
2.5.4. Specific Surface Area Nitrogen Adsorption
2.5.5. Electrode Conductivity
2.5.6. Contact Angle Measurements
2.6. Salt Adsorption Capacity
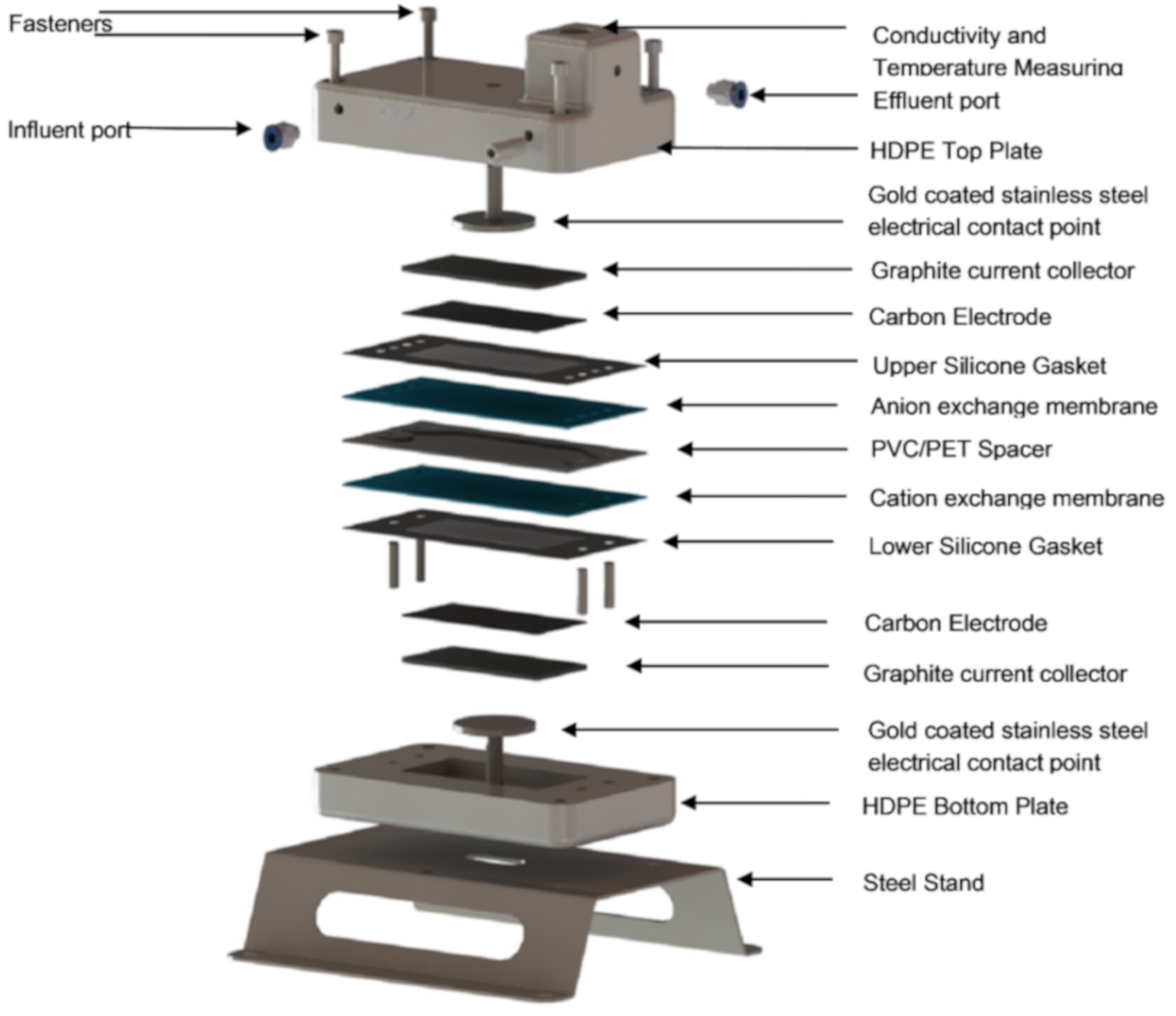
2.6.1. Cell Assembly Procedure
2.6.2. Experimental Desalination Setup
2.6.3. Determination of mSAC
- Mw is the molecular weight of NaCl (58.443 g/mol)
- Ci and Co are the influent and effluent concentrations (mM), respectively
- φ is the flowrate (mL/min)
- t is the time during which charging occurred (min)
- me is the mass of the active material in the electrode pair (g)
2.6.4. Pre-Conditioning and Test Procedure
3. Results and Discussion
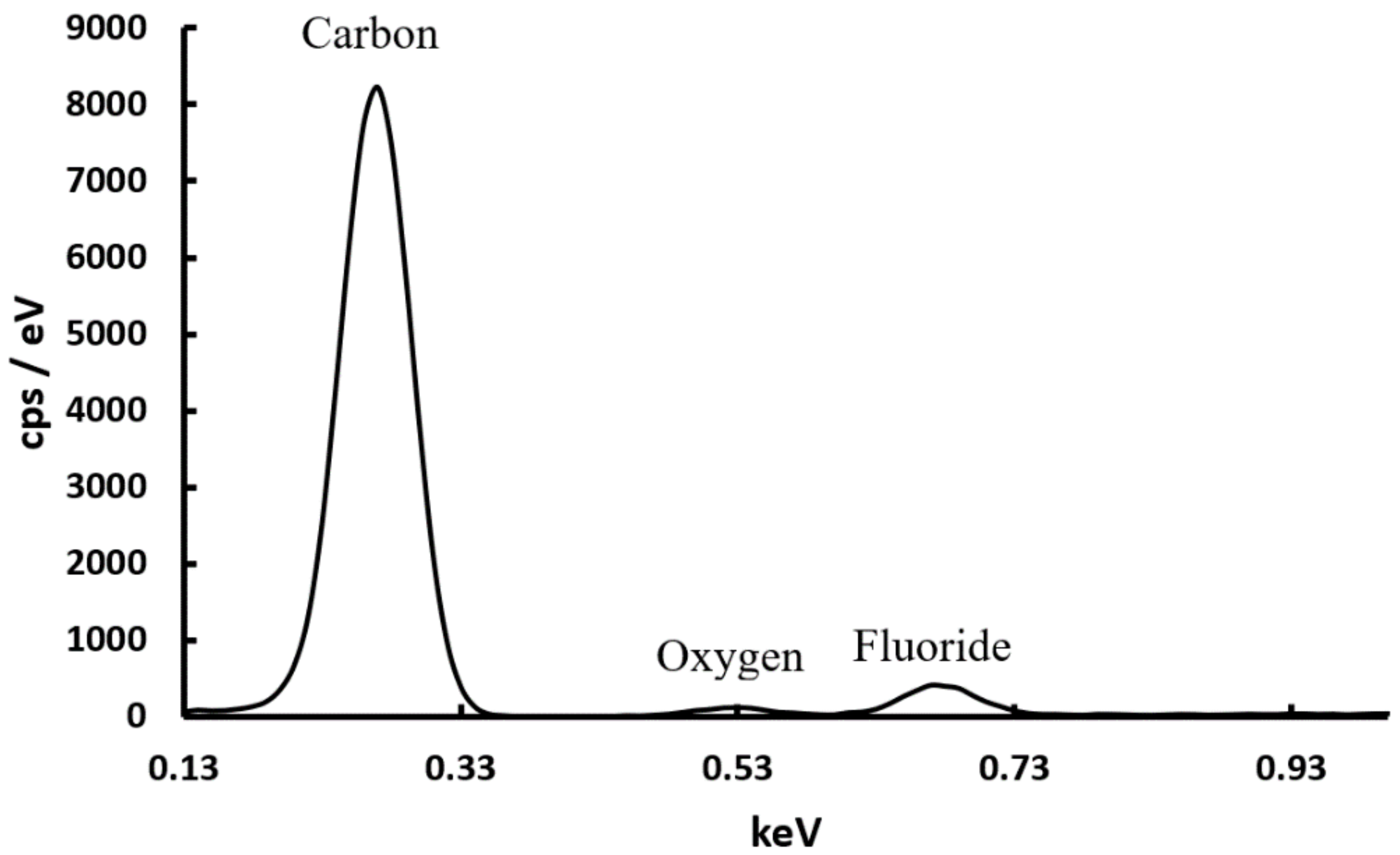
3.1. Scanning Electron Microscopy Energy Dispersive X-ray Spectroscopy (SEM EDS)
3.2. Maximum Salt Adsorption Capacity (mSAC)
3.3. Influence of Wet Mixing Time on Electrode Performance
3.4. Influence of Thermal Treatment on Electrode Performance
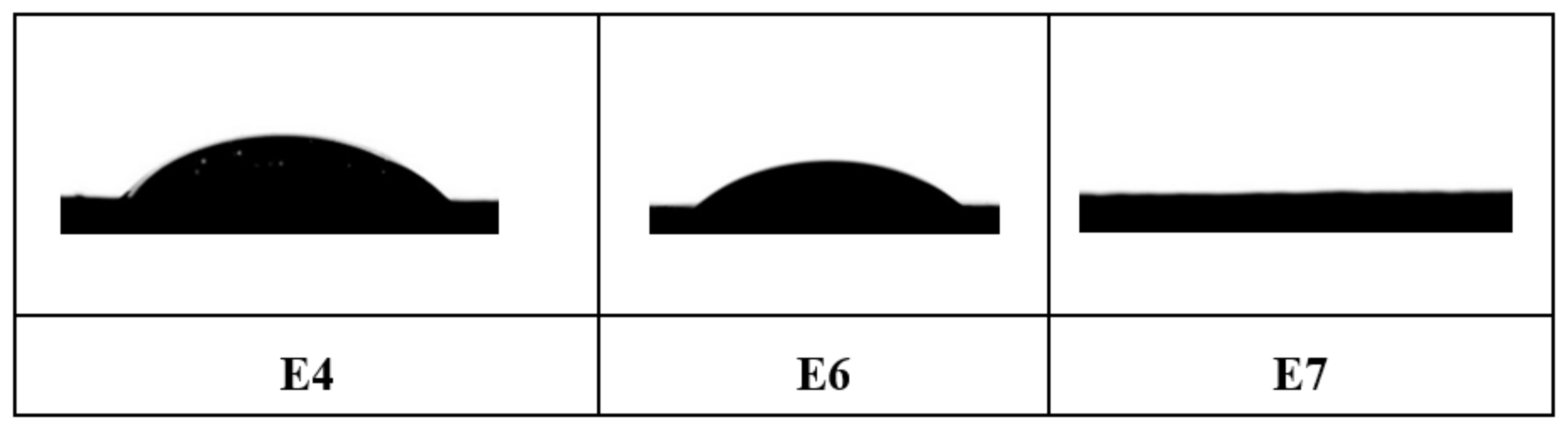
3.5. Contact Angle
3.6. Application and Future Perspective of CDI Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Dou, B.; Zheng, L.; Zhang, G.; Liu, Z.; Hao, Z. Effective desalination by capacitive deionization with functional graphene nanocomposite as novel electrode material. Desalination 2012, 299, 96–102. [Google Scholar] [CrossRef]
- Almarzooqi, F.A.; Al Ghaferi, A.A.; Saadat, I.; Hilal, N. Application of Capacitive Deionisation in water desalination: A review. Desalination 2014, 342, 3–15. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; van der Wal, A.; Presser, V.; Biesheuwel, P.M. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef] [Green Version]
- Bian, Y.H.; Yang, X.F.; Liang, P.; Jiang, Y.; Zhang, C.Y.; Huang, X. Enhanced desalination performance of membrane capacitive deionization cells by packing the flow chamber with granular activated carbon. Water Res. 2015, 85, 371–376. [Google Scholar] [CrossRef]
- Liang, P.; Yuan, L.L.; Yang, X.F.; Zhou, S.J.; Huang, X. Coupling ion-exchangers with inexpensive activated carbon fiber electrodes to enhance the performance of capacitive deionization cells for domestic wastewater desalination. Water Res. 2013, 47, 2523–2530. [Google Scholar] [CrossRef]
- Lee, L.Y.; Ng, H.Y.; Ong, S.L.; Tao, G.; Kekre, K.; Viswanath, B.; Lay, W.; Seah, H. Integrated pretreatment with capacitive deionization for reverse osmosis reject recovery from water reclamation plant. Water Res. 2009, 43, 4769–4777. [Google Scholar] [CrossRef]
- Jande, Y.A.C.; Kim, W.-S. Integrating reverse electrodialysis with constant current operating capacitive deionization. J. Environ. Manag. 2014, 146, 463–469. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Qin, M.H.; Deshmukh, A.; Epsztein, R.; Patel, S.K.; Owoseni, O.M.; Walker, W.S.; Elimelech, M. Comparison of energy consumption in desalination by capacitive deionization and reverse osmosis. Desalination 2019, 455, 100–114. [Google Scholar] [CrossRef]
- Wang, C.; Song, H.; Zhang, Q.; Wang, B.; Li, A. Parameter optimization based on capacitive deionization for highly efficient desalination of domestic wastewater biotreated effluent and the fouled electrode regeneration. Desalination 2015, 365, 407–415. [Google Scholar] [CrossRef]
- ACS Publications. Available online: https://pubs.acs.org/doi/10.1021/ba-1960-0027 (accessed on 8 December 2020).
- Arnold, B.B.; Murphy, G.W. Studies on the Electrochemistry of Carbon and Chemically-Modified Carbon Surfaces. J. Phys. Chem. 1961, 65, 135–138. [Google Scholar] [CrossRef]
- Marsh, H.; Rodriguez-Reinoso, F. Activated Carbon, 1st ed.; Elsevier: London, UK, 2006; pp. 13–77. [Google Scholar]
- Stoeckli, F.; Guillot, A.; Slasli, A.M.; Hugi-Cleary, D. Microporosity in carbon blacks. Carbon 2002, 40, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Cohen, I.; Avraham, E.; Bouhadana, Y.; Soffer, A.; Aubach, D. Long term stability of capacitive de-ionization processes for water desalination: The challenge of positive electrodes corrosion. Electrochim. Acta 2013, 106, 91–100. [Google Scholar] [CrossRef]
- Jo, K.; Baek, Y.; Kim, S.; Hong, S.P.; Yoon, J. Evaluation of long-term stability in capacitive deionization using activated carbon electrodes coated with ion exchange polymers. Korean J. Chem. Eng. 2020, 37, 1199–1205. [Google Scholar] [CrossRef]
- Zhao, R.; Biesheuvel, P.M.; Miedema, H.; Bruning, H.; van der Wal, A. Charge Efficiency: A Functional Tool to Probe the Double-Layer Structure Inside of Porous Electrodes and Application in the Modeling of Capacitive Deionization. J. Phys. Chem. Lett. 2010, 1, 205–210. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, J.H. Enhanced desalination efficiency in capacitive deionization with an ion-selective membrane. Sep. Purif. Technol. 2010, 71, 70–75. [Google Scholar] [CrossRef]
- Lu, G.; Wang, G.; Wang, P.H.; Yang, Z.; Yan, H.; Ni, W.; Zhang, L.; Yan, Y.-M. Enhanced capacitive deionization performance with carbon electrodes prepared with a modified evaporation casting method. Desalination 2016, 386, 32–38. [Google Scholar] [CrossRef]
- Bockholt, H.; Haselrieder, W.; Kwade, A. Intensive powder mixing for dry dispersing of carbon black and its relevance for lithium-ion battery cathodes. Powder Technol. 2016, 297, 266–274. [Google Scholar] [CrossRef]
- Bauer, W.; Nötzel, D.; Wenzel, V.; Nirschl, H. Influence of dry mixing and distribution of conductive additives in cathodes for lithium ion batteries. J. Power Sources 2015, 288, 359–367. [Google Scholar] [CrossRef]
- Bockholt, H.; Haselrieder, W.; Kwade, A. Intensive Dry and Wet Mixing Influencing the Structural and Electrochemical Properties of Secondary Lithium-Ion Battery Cathodes. ECS Trans. 2013, 50, 25–35. [Google Scholar] [CrossRef]
- Westphal, B.G.; Mainusch, N.; Meyer, C.; Haselrieder, W.; Indrikova, M.; Titscher, P.; Bockholt, H.; Viöl, W.; Kwade, A. Influence of high intensive dry mixing and calendering on relative electrode resistivity determined via an advanced two point approach. J. Energy Storage 2017, 11, 76–85. [Google Scholar] [CrossRef]
- Wang, G.; Qian, B.; Dong, Q.; Yang, J.; Zhao, Z.B.; Qiu, J. Highly mesoporous activated carbon electrode for capacitive deionization. Sep. Purif. Technol. 2013, 103, 216–221. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, L.; Xu, X.; Lu, T.; Sun, Z.; Chua, D.H.C. Enhanced desalination efficiency in modified membrane capacitive deionization by introducing ion-exchange polymers in carbon nanotubes electrodes. Electrochimica Acta 2014, 130, 619–624. [Google Scholar] [CrossRef]
- Porada, S.; Weinstein, L.; Dash, R.; van der Wal, A.; Bryjak, M.; Gogotsi, Y.; Biesheuvel, P.M. Water Desalination Using Capacitive Deionization with Microporous Carbon Electrodes. ACS Appl. Mater. Interfaces 2012, 4, 1194–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biesheuvel, P.M.; Zhao, R.; Porada, S.; van der Wal, A. Theory of membrane capacitive deionization including the effect of the electrode pore space. J. Colloid Interface Sci. 2011, 360, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Li, Z. Nanostructured transition metal oxides and their applications in composites. In Physical Properties and Applications of Polymer Nanocomposites; Woodhead Publishing: Cambridge, UK, 2010; pp. 723–742. [Google Scholar]
- Alencherry, T.; Naveen, A.R.; Ghosh, A.; Daniel, J.; Venkataraghavan, R. Effect of increasing electrical conductivity and hydrophilicity on the electrosorption capacity of activated carbon electrodes for capacitive deionization. Desalination 2017, 415, 14–19. [Google Scholar] [CrossRef]
- Jia, B.; Zou, L. Wettability and its influence on graphene nansoheets as electrode material for capacitive deionization. Chem. Phys. Lett. 2012, 548, 23–28. [Google Scholar] [CrossRef]
- Nguyen, T. Degradation of poly (vinyl Fluoride) and poly (vinylidene Fluoride). J. Macromol. Sci. Part C 1985, 25, 227–275. [Google Scholar] [CrossRef]
- Byles, B.W.; Cullen, D.A.; More, K.L.; Pomerantseva, E. Tunnel structured manganese oxide nanowires as redox active electrodes for hybrid capacitive deionization. Nano Energy 2018, 44, 476–488. [Google Scholar] [CrossRef]




| Electrode Pair | Wet Mixing Time | Deposition Method | Electrical Conductivity | Electrode Thickness | BET Specific Surface Area | mSAC |
|---|---|---|---|---|---|---|
| (min) | (°C) | S/cm | mm | m2/gAC | (mgNaCl/gAC) | |
| JNT45 | N/A | N/A | 56 | 0.34 | 7 | 0.42 |
| YP80 | N/A | N/A | N/A | N/A | 2316 | N/A |
| E1 | 60 | SIC (25) | 41 | 0.37 | 1589 | 17.0 |
| E2a | 60 | IID (25) | 41 | 0.48 | 1706 | 15.5 |
| E2b | 60 | IID (130) | 33 | 0.44 | 1795 | 14.1 |
| E3 | 60 | IDSC (150) | 32 | 0.68 | 1310 | 12.2 |
| Electrode Pair | Wet Mixing Time | Deposition Method | AC Loading | Electrical Conductivity | Electrode Thickness | BET Specific Surface Area | mSAC |
|---|---|---|---|---|---|---|---|
| (min) | (°C) | mg/cm2 | S/cm | mm | m2/gAC | (mgNaCl/gAC) | |
| E4 | 30 | SIC (25) | 6.9 | 44 | 0.38 | 1993 | 24.8 |
| E1 | 60 | SIC (25) | 6.9 | 41 | 0.37 | 1589 | 17.0 |
| E5 | 120 | SIC (25) | 6.9 | 44 | 0.35 | 1508 | 14.0 |
| Electrode Pair | Wet Mixing Time | Deposition Method | Contact Angle (°) | Thermal Treatment | BET Specific Surface Area | mSAC | |
|---|---|---|---|---|---|---|---|
| (min) | (°C) | Front | Back | °C | m2/gAC | (mgNaCl/gAC) | |
| JNT45 | N/A | Untreated | 116 | 116 | 25 | 7 | 0.42 |
| E4 | 30 | SIC (25) | 47 | 68 | 130 | 1993 | 24.8 |
| E6 | 30 | SIC (25) | 23 | 48 | 250 | 1804 | 20.9 |
| E7 | 30 | SIC (25) | 0 | 0 | 350 | 1797 | 20.1 |
| E8 | 30 | SIC (25) | 50 | 50 | 25 | * | 16.0 |
| Liberated Product | m/z | TGA Step |
|---|---|---|
| DMAC | 87 | 1 |
| HF | 20 | 2 |
| Electrode/Reference | mSAC (mg/g) |
|---|---|
| E4 | 24.8 |
| E6 | 20.9 |
| E7 | 20.1 |
| 28 | 14.2 |
| 12.8 | |
| 20 | 14.4 |
| 8.5 | |
| 18 | 12.8 |
| 10.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botha, E.; Smith, N.; Hlabano-Moyo, B.; Bladergroen, B. The Effect of Slurry Wet Mixing Time, Thermal Treatment, and Method of Electrode Preparation on Membrane Capacitive Deionisation Performance. Processes 2021, 9, 1. https://doi.org/10.3390/pr9010001
Botha E, Smith N, Hlabano-Moyo B, Bladergroen B. The Effect of Slurry Wet Mixing Time, Thermal Treatment, and Method of Electrode Preparation on Membrane Capacitive Deionisation Performance. Processes. 2021; 9(1):1. https://doi.org/10.3390/pr9010001
Chicago/Turabian StyleBotha, Ebrahiem, Nafeesah Smith, Bongibethu Hlabano-Moyo, and Bernard Bladergroen. 2021. "The Effect of Slurry Wet Mixing Time, Thermal Treatment, and Method of Electrode Preparation on Membrane Capacitive Deionisation Performance" Processes 9, no. 1: 1. https://doi.org/10.3390/pr9010001
APA StyleBotha, E., Smith, N., Hlabano-Moyo, B., & Bladergroen, B. (2021). The Effect of Slurry Wet Mixing Time, Thermal Treatment, and Method of Electrode Preparation on Membrane Capacitive Deionisation Performance. Processes, 9(1), 1. https://doi.org/10.3390/pr9010001






