Pressurized Liquid Extraction of Cannabinoids from Hemp Processing Residues: Evaluation of the Influencing Variables
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
2.2. Study Design
2.3. PLE: Schott Flasks
2.4. PLE: Speed Extractor (40 mL)
2.5. PLE: Autoclave Reactor (1 L)
2.5.1. Influence of Extraction Time
2.5.2. Corroboration of Extraction Conditions: Extraction of Hemp Finola
2.6. Determination of Cannabinoids
2.6.1. Calculation of the Cannabinoid Extraction Yield
2.6.2. Determination of Total Extraction Yield of Cannabinoids in the Feedstocks
3. Results
3.1. Determination of Total Extraction Yield of Cannabinoids in the Feedstocks
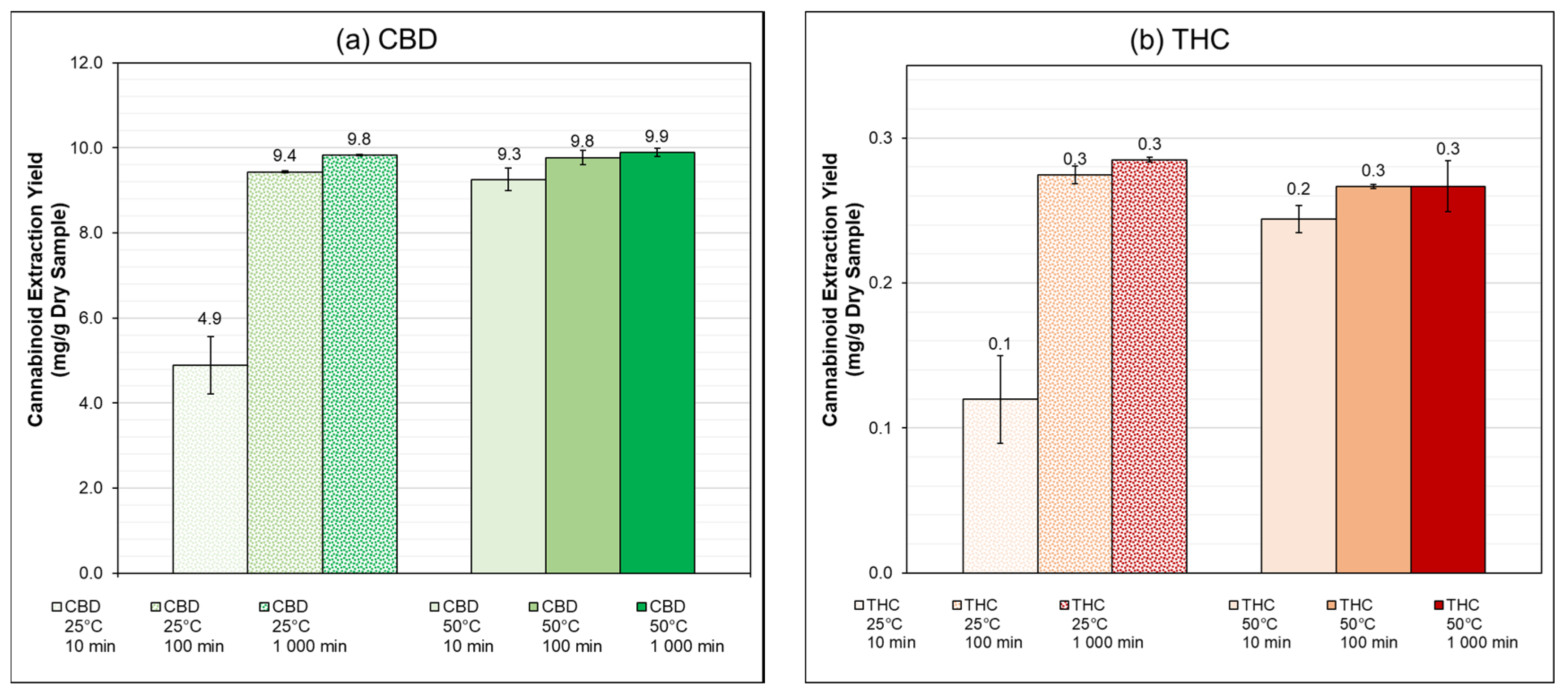
3.2. PLE: Schott Flasks
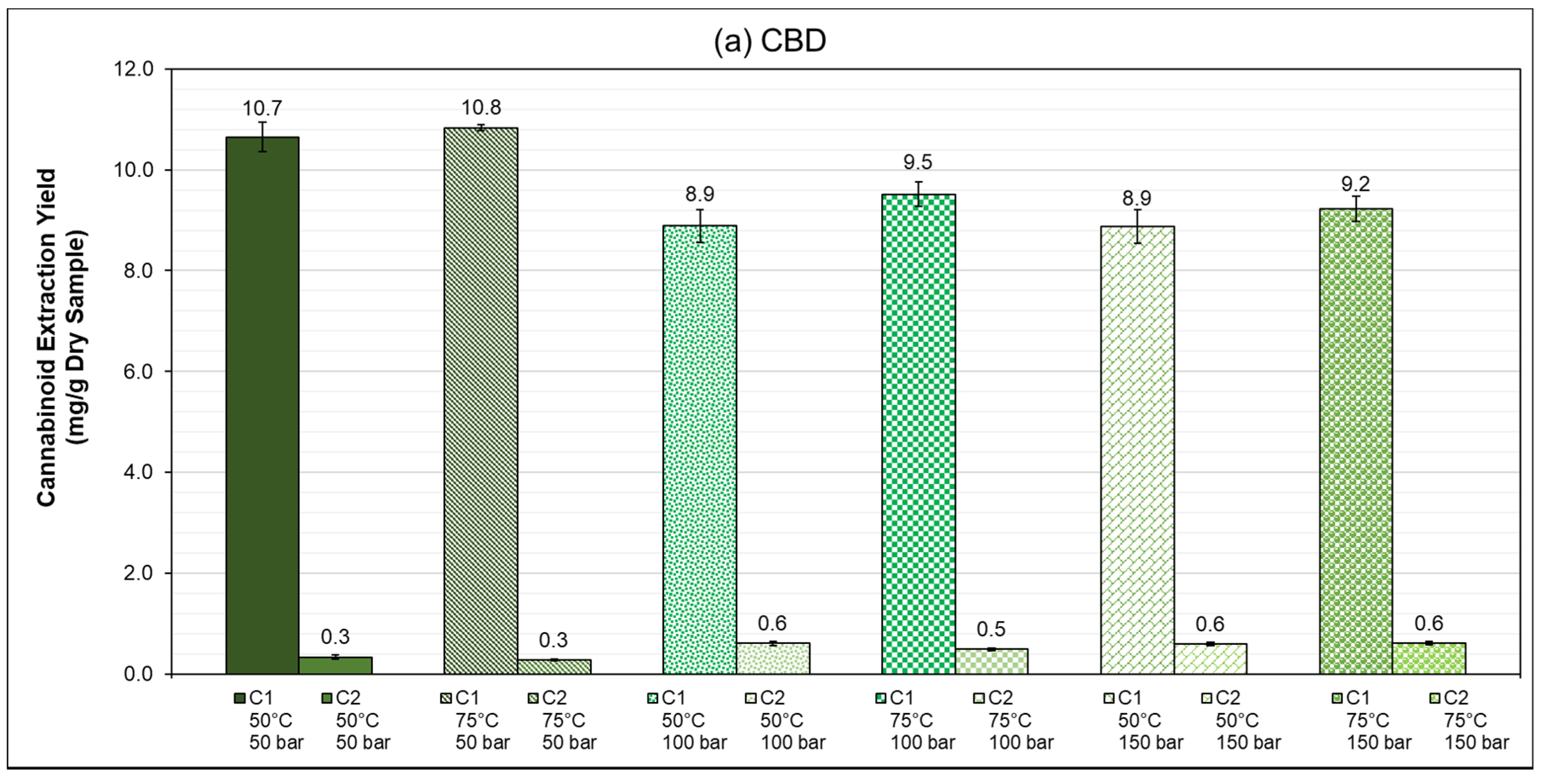
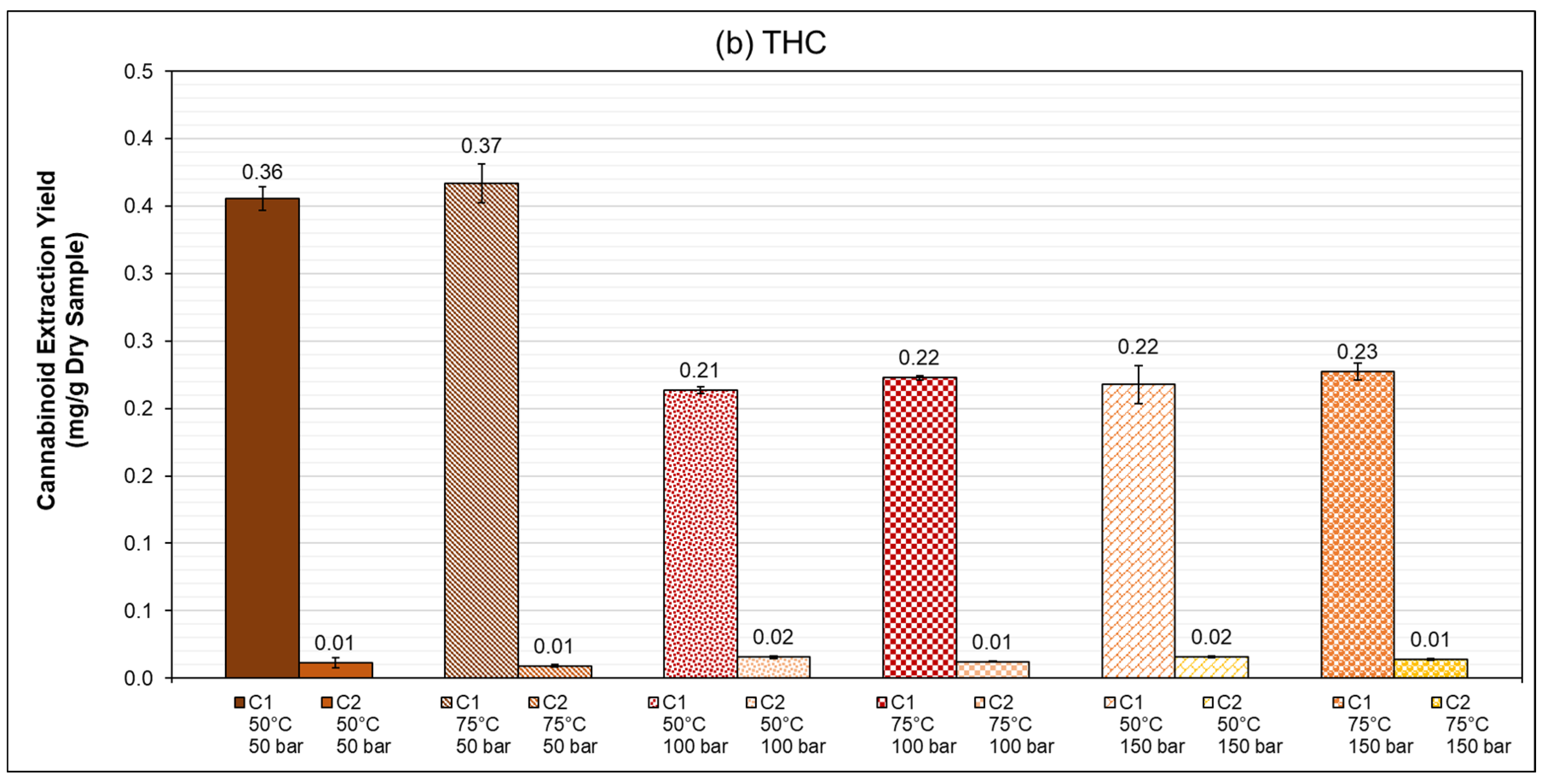
3.3. PLE: Speed Extractor (40 mL)
Summary of Results
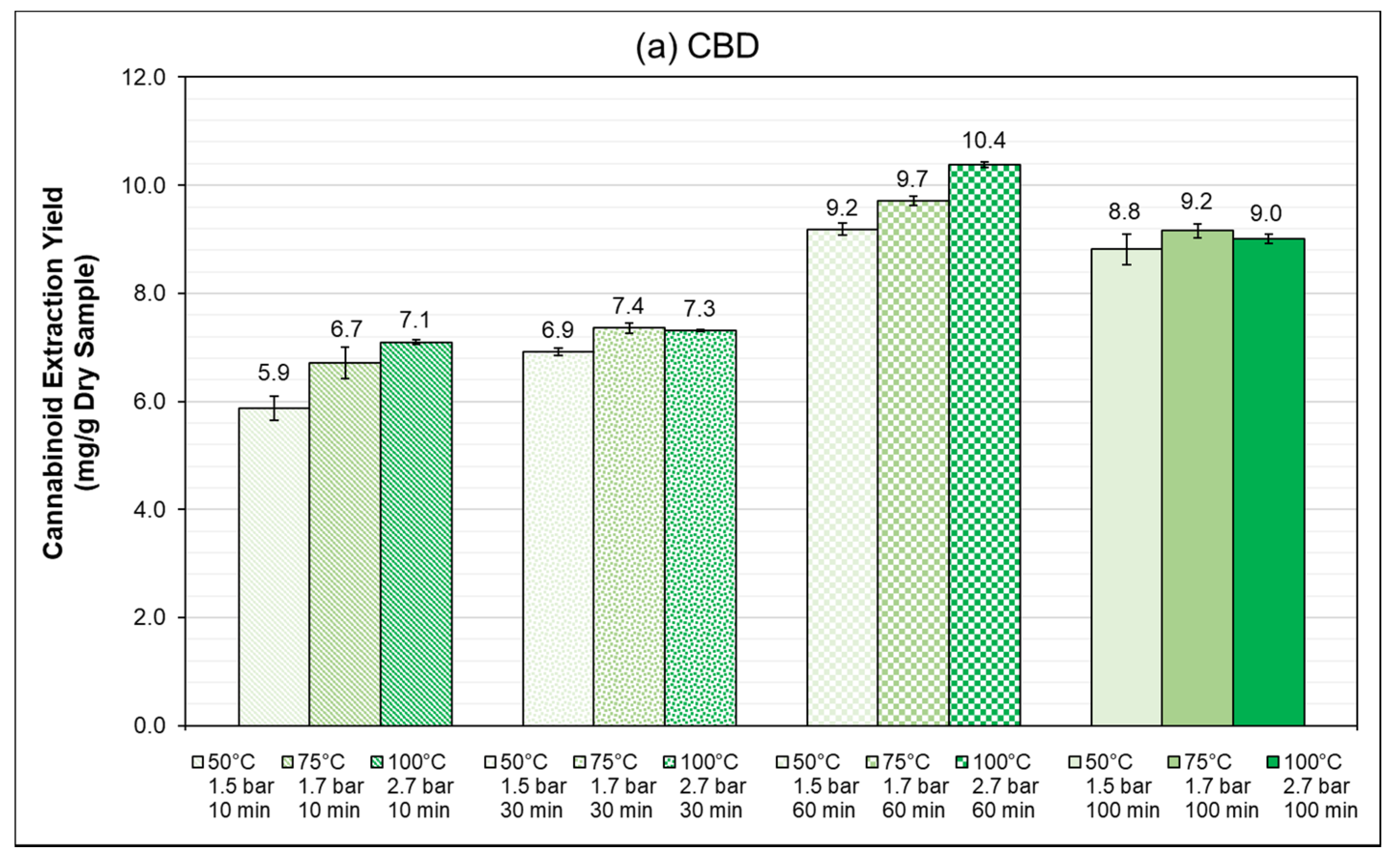
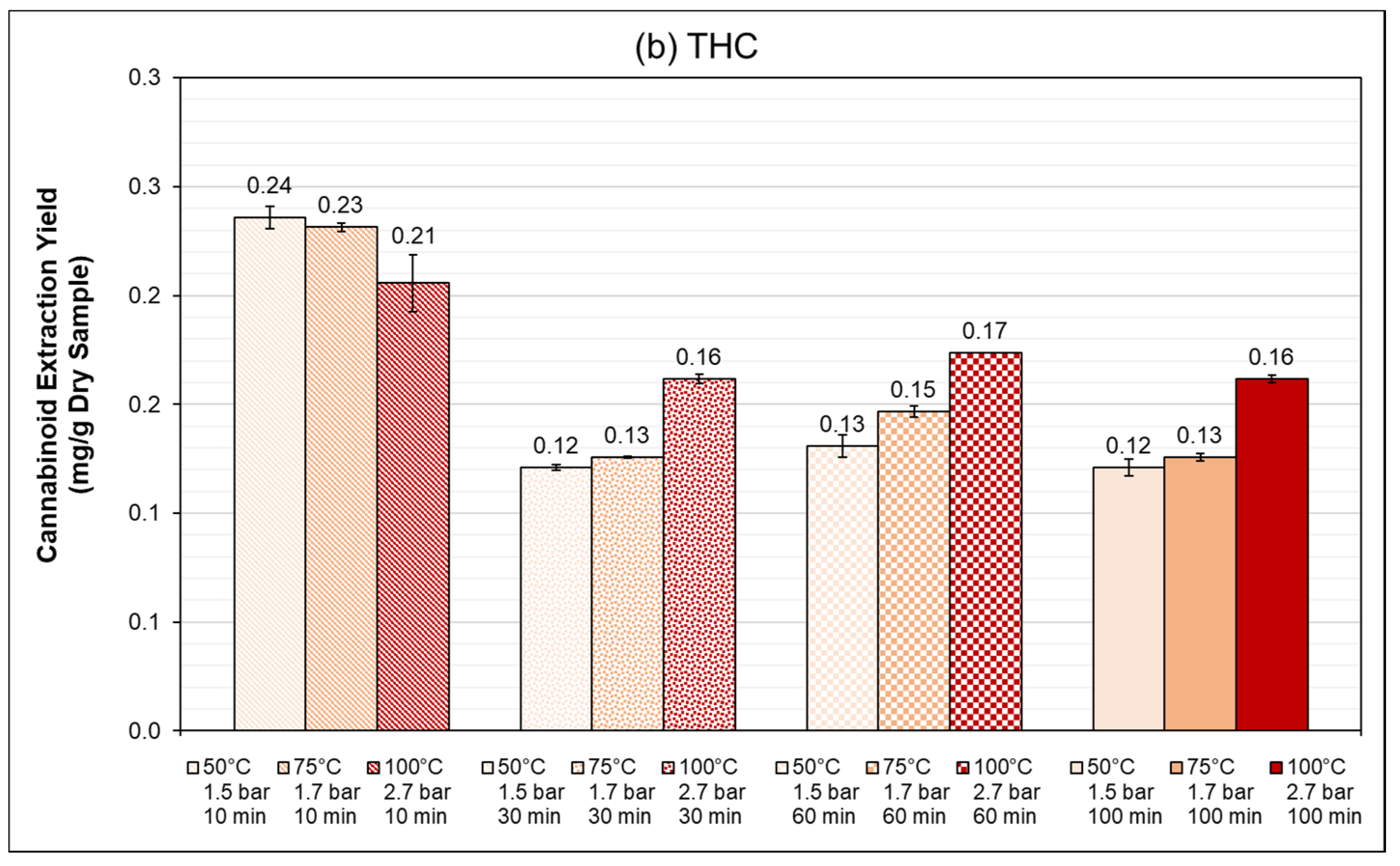
3.4. PLE: Autoclave Reactor (1 L)
3.4.1. Influence of Extraction Time
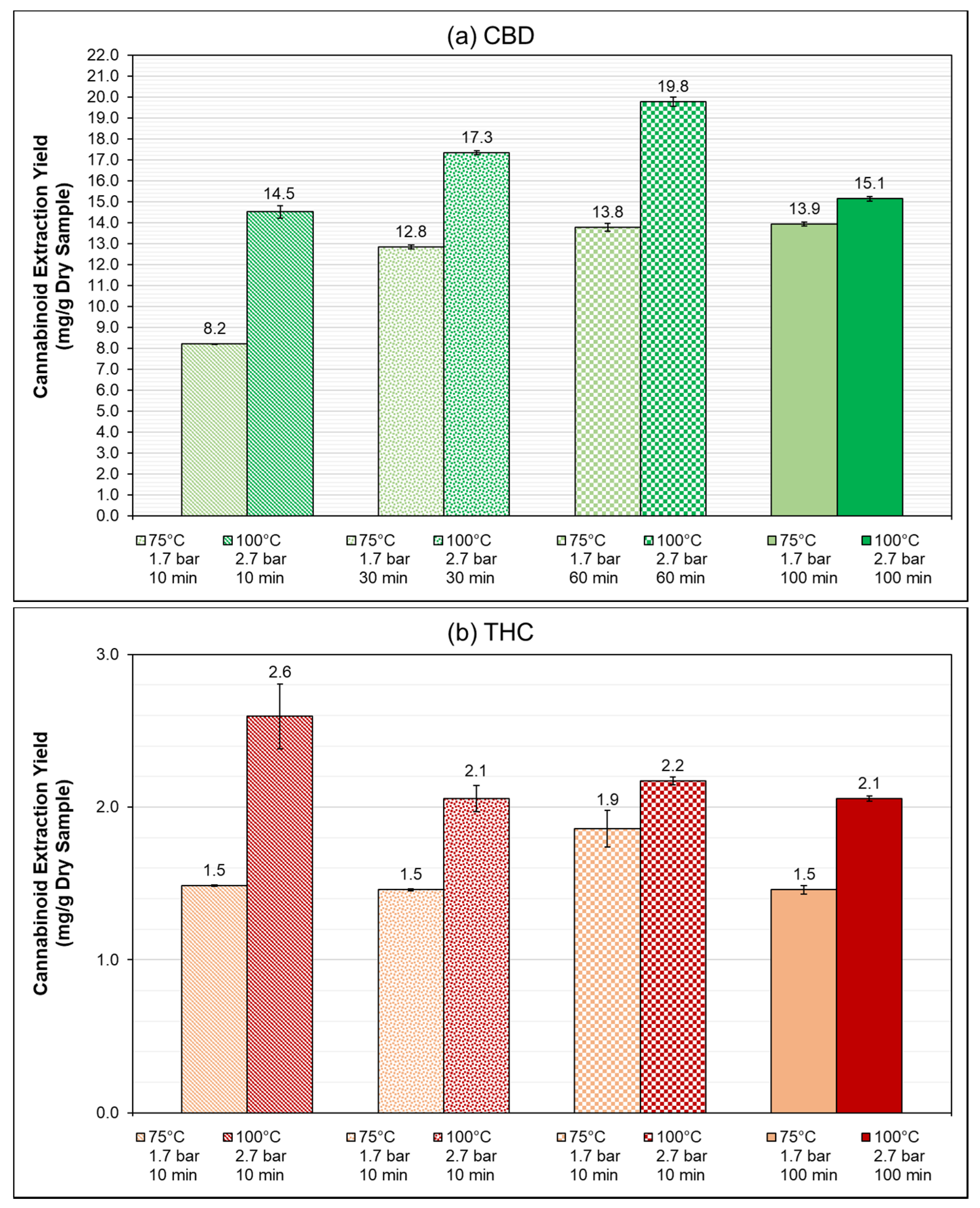
3.4.2. Corroboration: Extraction from Hemp Variety Finola
4. Discussion
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cardona Alzate, C.A.; Serna-Loaiza, S.; Ortiz-Sanchez, M. Sustainable biorefineries: What was learned from the design, analysis and implementation. J. Sustain. Dev. Energy Water Environ. Syst. 2020, 8, 88–117. [Google Scholar] [CrossRef]
- Oreopoulou, V.; Tzia, C. Utilization of plant by-products for the recovery of proteins, dietary fibers, antioxidants, and colorants. In Utilization of By-Products and Treatment of Waste in the Food Industry; Springer: Berlin, Germany, 2007; pp. 209–232. [Google Scholar]
- Guaadaoui, A.; Benaicha, S.; Elmajdoub, N.; Bellaoui, M.; Hamal, A. What is a bioactive compound? A combined definition for a preliminary consensus. Int. J. Food Sci. Nutr. 2014, 3, 17–179. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Vieira, A.R.; Abar, L.; Vingeliene, S.; Chan, D.S.M.; Aune, D.; Navarro-Rosenblatt, D.; Stevens, C.; Greenwood, D.; Norat, T. Fruits, vegetables and lung cancer risk: A systematic review and meta-analysis. Ann. Oncol. 2015, 27, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Tiyaboonchai, W.; Tungpradit, W.; Plianbangchang, P. Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int. J. Pharm. 2007, 337, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kitrytė, V.; Bagdonaitė, D.; Rimantas Venskutonis, P. Biorefining of industrial hemp (Cannabis sativa L.) threshing residues into cannabinoid and antioxidant fractions by supercritical carbon dioxide, pressurized liquid and enzyme-assisted extractions. Food Chem. 2018, 267, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Beisl, S.; Friedl, A.; Miltner, A. Lignin from micro- to nanosize: Applications. Int. J. Mol. Sci. 2017, 18, 2367. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Porto, J.V.; Ramalhosa, M.J.; Švarc-Gajić, J.; Estevinho, L.; Morais, S.; Delerue-Matos, C. Potential of Portuguese vine shoot wastes as natural resources of bioactive compounds. Sci. Total Environ. 2018, 634, 831–842. [Google Scholar] [CrossRef]
- Luque-Rodríguez, J.M.; Pérez-Juan, P.; Luque De Castro, M.D. Extraction of polyphenols from vine shoots of Vitis vinifera by superheated ethanol-water mixtures. J. Agric. Food Chem. 2006, 54, 8775–8781. [Google Scholar] [CrossRef]
- Mintz, C.S.; Nison, E.; Fabrizio, A.J. Cannabis-derived pharmaceuticals. J. Commer. Biotechnol. 2015, 21, 16–31. [Google Scholar] [CrossRef]
- de Ceballos, M.L. Cannabinoids for the treatment of neuroinflammation. In Cannabinoids in Neurologic and Mental Disease; Fattore, L., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 3–14. ISBN 978-0-12-417041-4. [Google Scholar]
- Fagan, S.G.; Campbell, V.A. Endocannabinoids and Alzheimer’s disease. In Cannabinoids in Neurologic and Mental Disease; Fattore, L., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 15–33. ISBN 978-0-12-417041-4. [Google Scholar]
- Concannon, R.; Finn, D.P.; Dowd, E. Cannabinoids in Parkinson’s disease. In Cannabinoids in Neurologic and Mental Disease; Fattore, L., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 35–39. ISBN 978-0-12-417041-4. [Google Scholar]
- Luís, Â.; Marcelino, H.; Rosa, C.; Domingues, F.; Pereira, L.; Cascalheira, J.F. The effects of cannabinoids on glioblastoma growth: A systematic review with meta-analysis of animal model studies. Eur. J. Pharmacol. 2020, 876, 173055. [Google Scholar] [CrossRef] [PubMed]
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key cultivation techniques for hemp in Europe and China. Ind. Crops Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Messina, F.; Rosati, O.; Curini, M.; Marcotullio, M.C. Cannabis and Bioactive Cannabinoids; Elsevier: Amsterdam, The Netherlands, 2015; Volume 45, ISBN 9780444634733. [Google Scholar]
- Fathordoobady, F.; Singh, A.; Kitts, D.D.; Singh, A.P. Hemp (Cannabis Sativa L.) extract: Anti-Microbial properties, methods of extraction, and potential oral delivery. Food Rev. Int. 2019, 35, 664–684. [Google Scholar] [CrossRef]
- Felipe Osorio-Tobón, J.; Angela, A.; Meireles, M. Recent applications of pressurized fluid extraction: Curcuminoids extraction with pressurized liquids. Food Public Health 2013, 3, 289–303. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Solana, M.; Boschiero, I.; Dall’Acqua, S.; Bertucco, A. A comparison between supercritical fluid and pressurized liquid extraction methods for obtaining phenolic compounds from Asparagus officinalis L. J. Supercrit. Fluids 2015, 100, 201–208. [Google Scholar] [CrossRef]
- Gul, W.; Gul, S.W.; Radwan, M.M.; Wanas, A.S.; Mehmedic, Z.; Khan, I.I.; Sharaf, M.H.M.; ElSohly, M.A. Determination of 11 cannabinoids in biomass and extracts of different varieties of cannabis using high-performance liquid chromatography. J. AOAC Int. 2015, 98, 1523–1528. [Google Scholar] [CrossRef]
- Yang, Y.; Lewis, M.M.; Bello, A.M.; Wasilewski, E.; Clarke, H.A.; Kotra, L.P. Cannabis sativa (Hemp) seeds, Δ(9)-tetrahydrocannabinol, and potential overdose. Cannabis Cannabinoid Res. 2017, 2, 274–281. [Google Scholar] [CrossRef]
- Rechtsinformationssystem des Bundes. Bundesgesetz über Suchtgifte, Psychotrope Stoffe und Drogenausgangsstoffe (Suchtmittelgesetz–SMG); Federal Chancellery of Austria: Vienna, Austria, 2020. [Google Scholar]
- Khan, S.A.; Aslam, R.; Makroo, H.A. High pressure extraction and its application in the extraction of bioactive compounds: A review. J. Food Process Eng. 2019, 42, e12896. [Google Scholar] [CrossRef]
- Büchi. SpeedExtractor E-916/E-916XL/E-914 Technical Datasheet; Büchi: Flawil, Switzerland, 2019. [Google Scholar]
- Namdar, D.; Mazuz, M.; Ion, A.; Koltai, H. Variation in the compositions of cannabinoid and terpenoids in Cannabis sativa derived from inflorescence position along the stem and extraction methods. Ind. Crops Prod. 2018, 113, 376–382. [Google Scholar] [CrossRef]
- Wianowska, D.; Dawidowicz, A.L.; Kowalczyk, M. Transformations of tetrahydrocannabinol, tetrahydrocannabinolic acid and cannabinol during their extraction from Cannabis sativa L. J. Anal. Chem. 2015, 70, 920–925. [Google Scholar] [CrossRef]
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Grijó, D.R.; Osorio, I.A.V.; Cardozo-Filho, L. Supercritical extraction strategies using CO2 and ethanol to obtain cannabinoid compounds from Cannabis hybrid flowers. J. CO2 Util. 2018, 28, 174–180. [Google Scholar] [CrossRef]
- Serna-Loaiza, S.; Miltner, A.; Miltner, M.; Friedl, A. A Review on the feedstocks for the sustainable production of bioactive compounds in Biorefineries. Sustainability 2019, 11, 6765. [Google Scholar] [CrossRef]
| Extraction Setup | Solid-to-Liquid Ratio * | Solvent Volume | Constant Conditions | Variables | |||
|---|---|---|---|---|---|---|---|
| Section 2.3 | Schott Flasks | 0.045–0.046 | 10 mL | Pressure: 1 bar | Temperature {25, 50} °C | Extraction Time {10, 100, 1000} min | |
| Section 2.4 | Speed Extractor | 0.2–0.3 | 22 mL | Extraction time: 15 min | Temperature {50, 75, 100} °C | Pressure {50, 100, 150} bar | No. Cycles 2 |
| Section 2.5 | Autoclave | 0.1 | 500 mL | Pressure: Vapor pressure of the solvent | Temperature {50, 75, 100} °C | Extraction Time {10, 30, 60, 100} min | |
| Hemp Variety | Total Extraction Yield (mg/g of Dry Sample) | |||
|---|---|---|---|---|
| CBD | THC | CBN | CBG | |
| KC Virtus | 11.51 ± 0.28 | 0.37 ± 0.003 | 0.27 ± 0.004 | 0.18 ± 0.01 |
| Finola | 19.91 ± 0.23 | 2.62 ± 0.22 | 1.46 ± 0.17 | 0.14 ± 0.001 |
| Temperature (°C) | Time (min) | Extraction Efficiency () * | |
|---|---|---|---|
| CBD | THC | ||
| 25 | 10 | 42.4 ± 5.9 | 32.6 ± 8.3 |
| 100 | 82.0 ± 0.2 | 74.8 ± 1.7 | |
| 1000 | 85.4 ± 0.1 | 77.6 ± 0.4 | |
| 50 | 10 | 80.4 ± 2.3 | 66.5 ± 2.6 |
| 100 | 84.9 ± 1.4 | 72.6 ± 0.5 | |
| 1000 | 85.9 ± 0.8 | 72.6 ± 4.8 | |
| Temperature (°C) | Pressure (bar) | Extraction Efficiency () * | |||
|---|---|---|---|---|---|
| CBD | THC | ||||
| Cycle 1 | Cycle 2 | Cycle 1 | Cycle 2 | ||
| 50 | 50 | 92.6 ± 2.5 | 2.9 ± 0.4 | 96.8 ± 2.4 | 3.1 ± 1.0 |
| 100 | 77.2 ± 2.9 | 5.3 ± 0.4 | 58.2 ± 0.7 | 4.2 ± 0.3 | |
| 150 | 77.1 ± 2.9 | 5.2 ± 0.3 | 59.3 ± 3.8 | 4.3 ± 0.2 | |
| 75 | 50 | 94.2 ± 0.6 | 2.4 ± 0.1 | 99.9 ± 3.9 | 2.5 ± 0.2 |
| 100 | 82.7 ± 2.2 | 4.3 ± 0.2 | 60.6 ± 0.4 | 3.3 ± 0.1 | |
| 150 | 80.2 ± 2.1 | 5.3 ± 0.3 | 61.9 ± 1.7 | 3.8 ± 0.2 | |
| Extraction Time (min) | 10 | 30 | 60 | 100 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 50 | 75 | 100 | 50 | 75 | 100 | 50 | 75 | 100 | 50 | 75 | 100 | |
| Extraction Efficiency () * | CBD | 51.0 ± 1.9 | 58.3 ± 2.6 | 61.7 ± 0.4 | 60.0 ± 1.0 | 63.9 ± 0.7 | 63.5 ± 2.0 | 79.8 ± 1.0 | 84.3 ± 0.2 | 90.2 ± 0.0 | 76.6 ± 2.5 | 79.6 ± 1.1 | 78.2 ± 0.8 |
| THC | 64.2 ± 2.4 | 63.0 ± 0.6 | 56.0 ± 3.6 | 32.9 ± 0.4 | 34.2 ± 0.1 | 44.0 ± 0.6 | 35.6 ± 1.4 | 40.0 ± 0.7 | 47.3 ± 0.0 | 61.7 ± 1.8 | 61.3 ± 0.7 | 54.3 ± 0.7 | |
| Extraction Time (min) | 10 | 30 | 60 | 100 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 75 | 100 | 75 | 100 | 75 | 100 | 75 | 100 | |
| Extraction Efficiency () * | CBD | 41.2 ± 0.0 | 72.9 ± 1.5 | 64.4 ± 0.5 | 87.1 ± 0.4 | 69.2 ± 1.0 | 99.3 ± 1.2 | 69.9 ± 0.5 | 76.0 ± 0.6 |
| THC | 56.6 ± 0.1 | 98.9 ± 8.1 | 55.6 ± 0.2 | 78.3 ± 3.3 | 70.9 ± 4.6 | 82.8 ± 1.0 | 99.2 ± 1.1 | 86.8 ± 0.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serna-Loaiza, S.; Adamcyk, J.; Beisl, S.; Kornpointner, C.; Halbwirth, H.; Friedl, A. Pressurized Liquid Extraction of Cannabinoids from Hemp Processing Residues: Evaluation of the Influencing Variables. Processes 2020, 8, 1334. https://doi.org/10.3390/pr8111334
Serna-Loaiza S, Adamcyk J, Beisl S, Kornpointner C, Halbwirth H, Friedl A. Pressurized Liquid Extraction of Cannabinoids from Hemp Processing Residues: Evaluation of the Influencing Variables. Processes. 2020; 8(11):1334. https://doi.org/10.3390/pr8111334
Chicago/Turabian StyleSerna-Loaiza, Sebastián, Johannes Adamcyk, Stefan Beisl, Christoph Kornpointner, Heidi Halbwirth, and Anton Friedl. 2020. "Pressurized Liquid Extraction of Cannabinoids from Hemp Processing Residues: Evaluation of the Influencing Variables" Processes 8, no. 11: 1334. https://doi.org/10.3390/pr8111334
APA StyleSerna-Loaiza, S., Adamcyk, J., Beisl, S., Kornpointner, C., Halbwirth, H., & Friedl, A. (2020). Pressurized Liquid Extraction of Cannabinoids from Hemp Processing Residues: Evaluation of the Influencing Variables. Processes, 8(11), 1334. https://doi.org/10.3390/pr8111334







