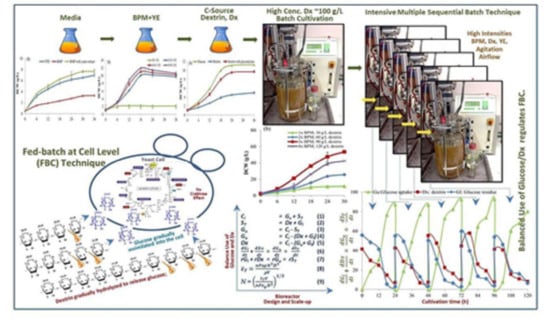
1. Introduction
Since the end of the 19th century to 2020, the addition of exogenous yeast biomass to produce bread, beer, wine, and industrial ethanol has become a common practice. Wineries started using exogenous yeast inoculants in the 1950s. In the 1960s, yeast biomass-producing plants contributed to the technology of producing large amounts of active dry yeast (ADY) or yeast cell biomass, and its use rapidly spread to European, North America, Asian, and other countries. Nowadays, modern industries require very large amounts of selected yeasts to obtain high-quality reproducible products and to ensure fast, complete cultivations. Efficient and profitable factory-scale processes have been developed to produce yeast biomass. The standard process was empirically optimized to obtain the highest yield by increasing biomass and decreasing production costs [
1]. The ADY or yeast cell biomass is a yeast product that is dehydrated and dried from the fresh yeast. The dry yeast products are mainly used in the bakery, brewery, winery, feeds, pharmaceuticals, and ethanol industries, etc. The global production of ADY increased from 589.70 MT (metric tons) in 2010 to 862.50 MT in 2014, with an annual average growth rate of 7.91% [
2]. It is estimated to a value of 1544.74 MT in 2024.
Saccharomyces cerevisiae is the most prominent in the yeast group.
S. cerevisiae is a species of the budding yeasts in a group of unicellular fungi. It is a free-living organism with rapid growth and is simple for cultivation under a defined condition. As profound knowledge of its genetics, functional genomics, biochemistry, physiology, etc. of this yeast is presently available, it is widely used as a model eukaryote for certain studies [
3], e.g., fermentation, aerobic cell cultivation, and molecular biology. It is a particularly suitable organism for biological studies and is generally regarded as safe status (GRAS). Moreover, it can produce a wide range of biological products of both wild types, e.g., single cell proteins and β-glucans [
4], and recombinant products, e.g., viral vaccines and certain proteins [
5]. More than 47 natural bioproducts have been produced in yeasts [
6]. For those reasons, it was used herein as the model yeast for this study.
High cell density cultivation (HCDC) is a potential technique that has been developed to produce microbial cell biomasses at high concentrations and subsequently to gain relatively high product concentrations. It is used in various bioprocesses to achieve the cost-effective production of desired products. HCDC can be applied to produce a variety of biological products from microbial cells, such as cell biomasses, probiotics, single cell proteins, amino acids, organic acids, bioplastics, biochemicals, pharmaceuticals, etc. HCDC is a relative term with no exact concentration of dry cell weight value. In general, a cell concentration of more than those achieved in conventional cultivation has been considered as the high cell density. Most experiments have dry cell weights ranging from 20 to 40 g/L, and if they are higher than 100 g/L they are designated as having a high cell density concentration (modified from [
7]). They are normally operated with the fed-batch mode. There has not been any batch cultivation to achieve a high cell concentration at a high productivity by using high concentration of dextrin or oligomer as a carbon source (C-source), but we have successfully done this. This is unlike an anaerobic SSF (simultaneous saccharification and fermentation), a fermentation method using maltodextrin as a substrate at high titer without the problem of oxygen requirement. The HCDC is principally designed as the fed-batch mode to control the carbon substrate feeding in order to avoid cell growth inhibition due to too high a concentration of substrate and the accumulation of toxic by-products. The substrate feeding strategies are implemented to eliminate the problems of using high concentrations of substrates, especially glucose and sucrose, that effect to the biosynthesis of growth inhibitory by-products due to the Crabtree effect [
8]. The fed-batch HCDC development often studies the optimum conditions of two main factors, i.e., medium feeding and cultivation strategies.
A defined medium is generally used to obtain a high cell density concentration because the nutrient amounts are known and can be controlled during cultivation while complex medium can vary in composition and quality making fermentation less reproducible. However, semi-defined or complex media are sometimes necessary to boost cell growth and product formation. The use of a defined medium with a single or a few amino acids to achieve high cell biomass and/or recombinant proteins would be attractive [
9]. Although the fed-batch method is complicated, the results of some research works using fed-batch cultivations to produce yeast cell biomass have been reported. The dry cell weights and productivities of 187.63 g/L and 1.71 g/L/h were obtained from [
10], 120 g/L and 1.84 g/L/h [
11], 95.7 g/L and 1.20 g/L/h [
12], and 63.30–96.10 g/L and 1.33–1.99 g/L/h [
13].
For aerobic cultivation, using sugar as a substrate at a high concentration usually causes the Crabtree effect. Although glycerol is generally used to minimize this effect, it can be utilized by microorganisms at a limited concentration, though its advantage generates a higher degree of reduction per carbon atom when compared to other sugars [
14]. As a result, in this study, dextrin, an oligomer of glucose from cassava starch liquefaction was used as the major C-source substrate. It can efficiently be used at a high concentration. So far, there have not been any other research reports using dextrin or other oligomers as the potential C-source to prevent the system from oxygen limitation, especially for the aerobic batch cultivation operation to achieve a high cell density. Our new finding herein essentially explains that the dextrin oligomer regulates gradual glucose assimilation into the yeast cells, as so-called the “fed-batch at cell level” (FBC). It also plays a key role in a reserved C-source in the cultivation system when it is in the mixture of glucose.
Both fed-batch and continuous techniques usually: (i) require more types and units of equipment and (ii) complicated process steps; (iii) entail a high cost of operation, (iv) long time cultivation and time-consuming both in up-stream and down-stream processes, and (v) induce the Crabtree effect problem to inhibit cell growth because of using glucose and sucrose at high concentrations. Here, in order to achieve a high cell density concentration at a high production rate, these problems can be overcome by: (i) the use of aerobic batch mode with (ii) the use of a simple-defined minimal medium, (iii) the ability to use high concentrations of C-source substrate, (iv) the cultivation with a short time course, (v) with the intensive multiple sequential batches, (vi) the use of dextrin, oligomer of glucose from cassava starch liquefaction as the major substrate; (vii) the control of “fed-batch at cell level”, and (viii) the prevention of the yeast cultivation system from the Crabtree effect to achieve these system balances, operating at the high intensities of (ix) aeration rates and (x) impeller agitation speeds, both proportional to the high concentrations of the C-source dextrin and the BPM (batch production medium).
These are the objectives of this research work in order to achieve high cell density cultivation of the model yeast at a high productivity with comprehensive and profound explanations of the details of all aspects, from culture medium design, new cultivation technique, kinetics of cell growth, and biochemical engineering mass balance models of substrate utilizations to regulate the fed-batch at cell level (FBC), through fluid dynamics, and reactor scale-up for the industries.
This study isolates the problem of the efficient operation of S. cerevisiae cultivation in bioreactors focusing only on the need for high cell density cultures. On this basis, the novelty of the FBC technique is proposed. It has been explained in principles and details of the cultivation technique, the potential substrate dextrin, the mechanism of substrate utilization to regulate the FBC, the FBC kinetics, and material balances, through the bioreactor design and scale-up.
2. Materials and Methods
2.1. Yeast Strain and Preparations of Inoculants and Culture Media
The stock of Saccharomyces cerevisiae S3, a strain of model yeasts was from the Microbiology Department, Faculty of Science, Burapha University, Thailand. It was maintained in YPD (Yeast Peptone Dextrose) medium, (composed of 10 g/L of yeast extract, 10 g/L of peptone, and 20 g/L of dextrose) at pH 4.5, then mixed with a cryo-protective agent of glycerol at 15% (v/v) and stored in 2-mL cryo-tubes at −40 °C as the stocks. Before use, they were activated by defrosting at a room temperature of 28–30 °C and then transferred into the 10-mL YPD contained in the 50-mL Erlenmeyer flasks. The cultures were incubated at 30 °C in a rotary incubator shaker at a speed of 200 rpm overnight. Then, 5-mL volumes were transferred into the 20-mL YPD contained in the 50-mL baffled flasks and cultured at the same conditions (i.e., pH, temperature, and shaker speed above) for 18 h. Further, 10-mL volumes were transferred into the 100-mL YPD contained in the 500-mL baffled flasks and again cultured at the same conditions for 18 h to use as the inoculants for further cultivation experiments in the 5-L fermenters of Topic 2.6. They had an optical density (OD600 nm) of about 8–10. To increase the inoculum concentration at an OD600 nm of ~15–18, the cultures were grown for 24 h. This was used for the initial batch of the intensive multiple sequential batch cultivation in Topic 2.7.
The batch production medium (BPM) [
15], the minimal synthetic defined medium containing (L
−1) of 2.2 g (NH
4)
2SO
4, 1.5 g KH
2PO
4, 1.8 g Na
2HPO
4, 0.2 g MgSO
4.7H
2O, and 1.0 mL of trace element solution, was composed of (L
−1) of 10.0 g CaCl
2.2H
2O, 6.0 g (NH
4)
5[Fe(C
6H
4O
7)
2], 0.2 g CoCl
2.6H
2O, 0.3 g H
3BO
3, 0.1 g ZnSO
4.7H
2O, 0.03 g MnCl
2.4H
2O, 0.03 g Na
2MoO
4.2H
2O, 0.02 g NiSO
4.7H
2O, and 0.01 g CuSO
4.5H
2O. The BPM is designed for use as the main medium for high cell density cultivation because of its simplicity, low cost (~0.035–0.045 US
$ per liter), and high potential for industrial applications at the commercial scale of cell cultivation for the productions of varieties of bioproducts, from cell biomasses to fine biochemicals, e.g., pharmaceuticals. When compared to a laboratory medium, it costs ~0.85 US
$ per liter which is ~25 times higher.
2.2. Preparations of Carbon Sources, Dextrin from Cassava Starch Liquefaction and Glucose from Further Saccharification of Dextrin
It is important that an industrial application practice was implemented, in terms of potential raw material use and its preparation with in-house technologies. As plenty of a major C-source substrate, dextrin solution with a DE (dextrose equivalent) value of 25–40 was prepared by our own liquefaction method [
16] using the in-house design and fabrication of a 200-L stirred-tank enzymatic hydrolysis bioreactor in the controlled optimum conditions. A 40% (
w/v) of cassava starch powder (3-Elephant Heads Brand (Chorchiwat Industry Co., Ltd. (CCW), Thailand) was suspended in distilled water and subsequently added with 0.1% (
v/w) of α-amylase (Spezyme Alpha, thermostable α-amylase from
Bacillus licheniformis with a minimum activity of 13,775 Units/g protein; DuPont Industrial Biosciences, Shanghai, China) per dry starch basis. The mixture was controlled at 85–90 °C, pH 6.5, and agitated with 2-Ekato Intermig, high-efficiency impellers at a speed of 125 rpm for 3 h [
17]. The desired concentrations of dextrin were adjusted by dilutions depending on each experimental condition.
A glucose solution with a DE of 95 was prepared by further hydrolysis called saccharification of the above dextrin solution. After liquefaction, the slurry of dextrin solution was cooled to 65 °C and subsequently supplemented with a glucoamylase at 0.2% (v/w) (Distillase ASP, a blend of enzymes produced by B. licheniformis and Trichoderma reesei with a minimum activity of 580 units/g protein; DuPont Industrial Biosciences, Shanghai, China). The mixture was controlled at 65 °C, pH 4.5, and agitated at 100 rpm for 20 h. The desired concentrations of glucose were adjusted by dilutions on each experimental condition.
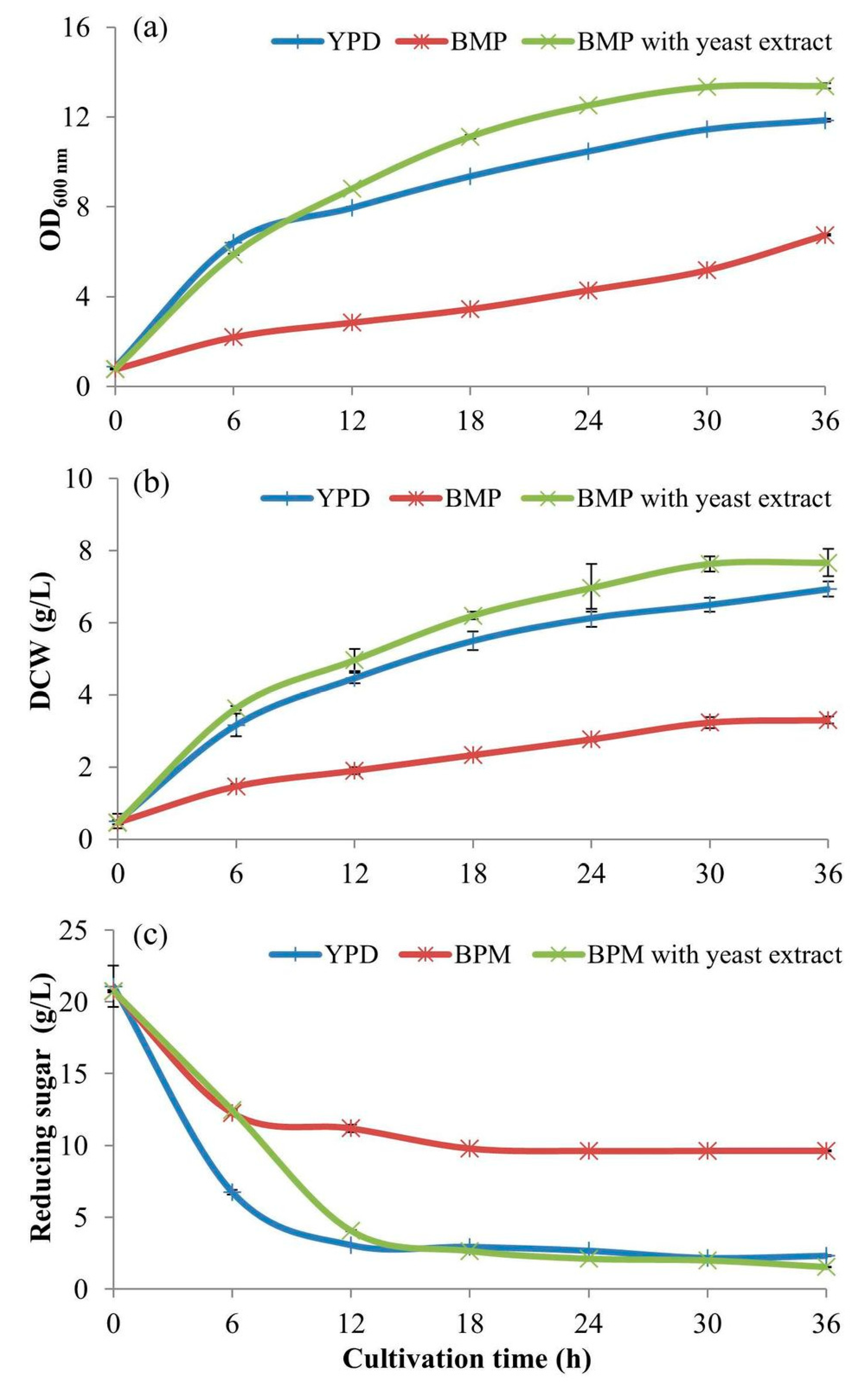
2.3. The Effective BPM for Aerobic Batch Cultivation of S. cerevisiae
The effective BPM for aerobic cultivation of
S. cerevisiae was determined. The cultivations were undertaken in the 500-mL baffled flasks contained 100 mL of BPM with 20 g/L of glucose supplemented with YE at a concentration of 0.3% (
w/v) and another without the YE. They were compared with cultivation in the YPD medium. The initial pH of the three media was adjusted to 4.5 with 4 N NaOH and 4 N HCl. After autoclaving and leaving to a temperature of 30 °C, the 5-mL inoculant volumes (from
Section 2.1) were inoculated into those culture media and incubated at 30 °C in a rotary incubator shaker at a speed of 200 rpm for 36 h. The culture broth samples of 5 mL of each treatment were withdrawn every 6 h, put on ice for 20 min to suddenly drop the temperature to 2 °C to stop cell activities before keeping at 5–10 °C in a refrigerator for further analyses of the OD
600 nm, cell biomass, and reducing sugar concentrations.
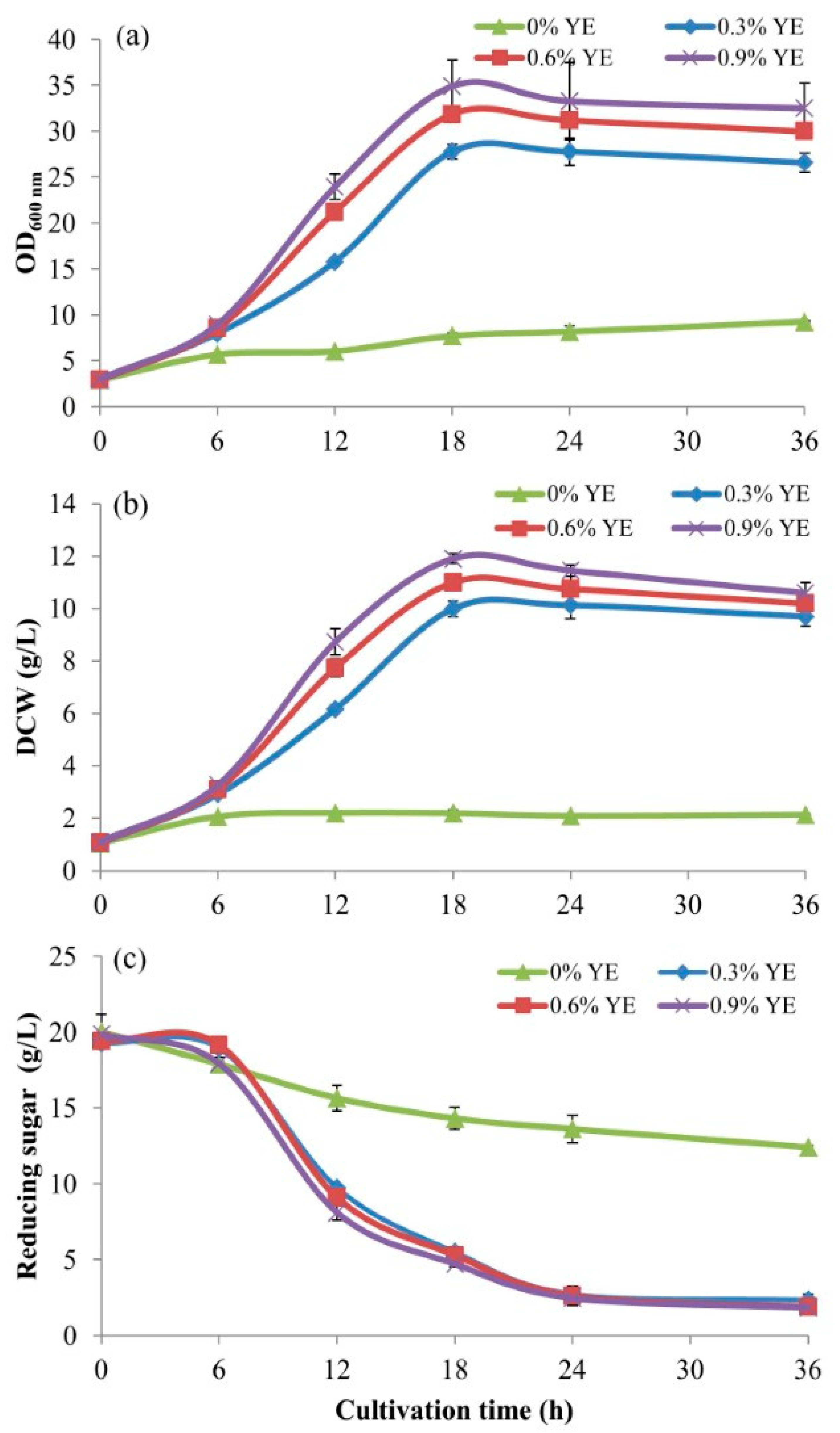
2.4. The Addition of Yeast Extract as the Supplement in BPM
From the above experimental result, in order to enhance more efficient utilization of BPM by
S. cerevisiae, yeast extract (Hi-media, India), the major source of complex nutrients was supplemented into the BPM of 20 g/L of glucose. The experiment was conducted using 500-mL baffled flasks contained 100 mL of BPM supplemented with YE at concentrations of 0, 0.3, 0.6, and 0.9% (
w/v). The initial pH of the media was adjusted to 4.5 with 4 N NaOH and 4 N HCl. After autoclaving and leaving to a temperature of 30 °C, the 5-mL inoculant volumes (from
Section 2.1) were inoculated into the four cultivation media and incubated at 30 °C in a rotary incubator shaker at a speed of 200 rpm for 36 h. The culture broth samples of 5 mL were withdrawn every 6 h for the first 24 h and after that every 12 h. They were put on ice for 20 min to suddenly drop the temperature to 2 °C to stop cell activities before keeping them at 5–10 °C for further analyses.
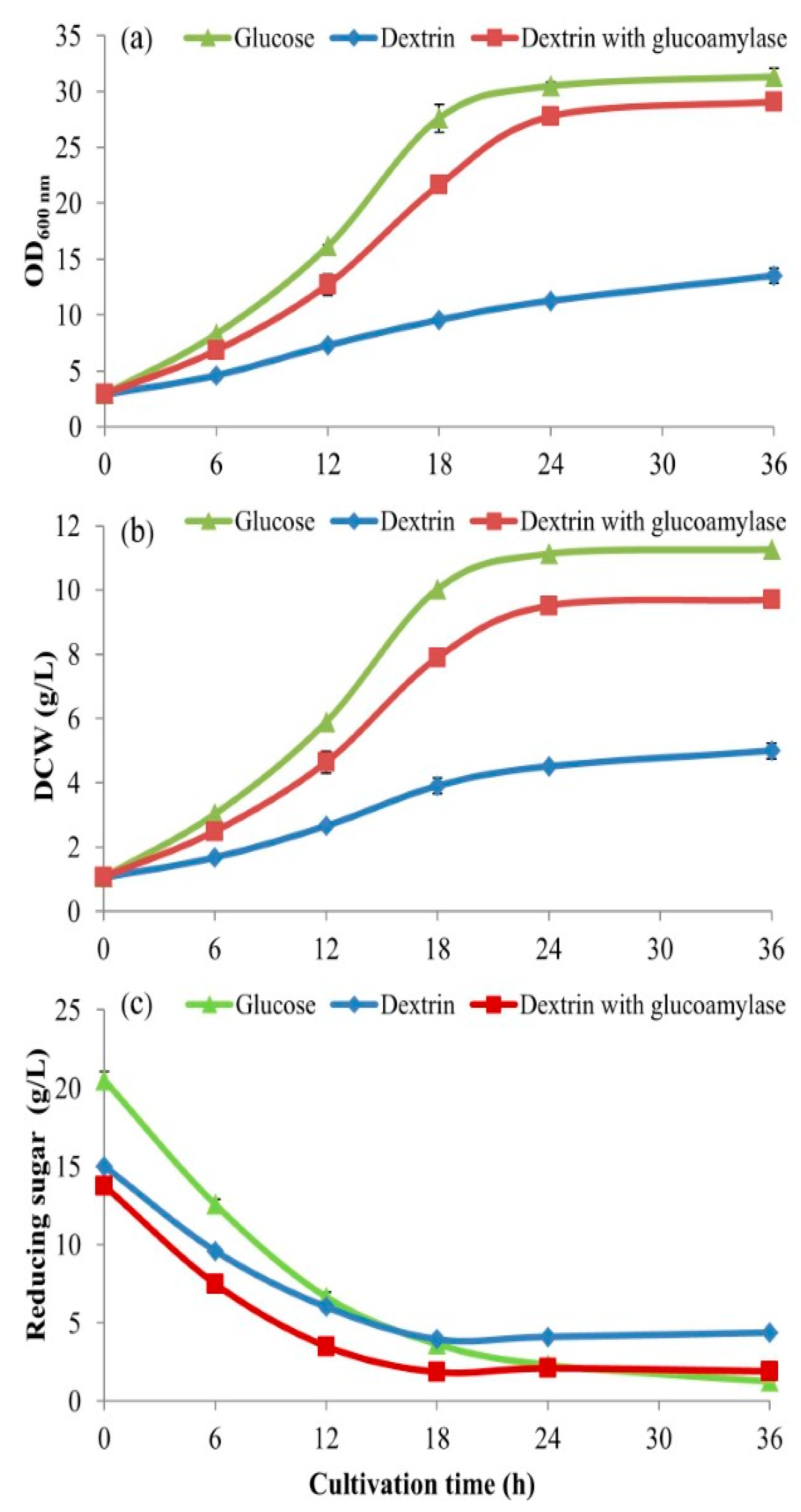
2.5. The Comparison of Different Carbon Sources
This was to compare the efficient utilization, by
S. cerevisiae, of different C-sources, i.e., dextrin from cassava starch liquefaction, glucose from further dextrin saccharification (from
Section 2.2), and the commercial pure glucose (D-glucose, Sigma-Aldrich). The experiment was conducted using 500-mL baffled flasks contained with 100 mL of BPM added with those C-sources at the same concentration of 20 g/L. The initial pH of the media was adjusted to 4.5. After autoclaving and leaving to a temperature of 30 °C, the 5-mL inoculant volumes (from
Section 2.1) were inoculated into those three media. The cultivation conditions and the sample collections were undertaken with the same as above in
Section 2.4. Noted, the concentrations of dextrin and glucose from dextrin saccharification were calculated from the initial concentration of starch hydrolysis at 40% (
w/v). For stoichiometry, one gram of starch produces 1.11 g of glucose, thus the factor of 1.11 is used for the calculation of using glucose to meet the equimolar concentration of using dextrin at 20 g/L. So, glucose at a concentration of 22.22 g/L should be used. When the starch hydrolysis is considered at 90% (based on 90% (
w/w) pure starch content in cassava starch), the equimolar concentration of glucose to use is 19.98 g/L. This means that a 20 g/L of dextrin from starch when completely hydrolyzed will produce 19.98 g/L of glucose. This proves that the 20 g/L of the three C-sources are comparable as they are all the same equimolar concentration of 111 mM (millimolar) as the glucose at 20 g/L (calculated from (20/180.156) × 1000 = 111 mM).
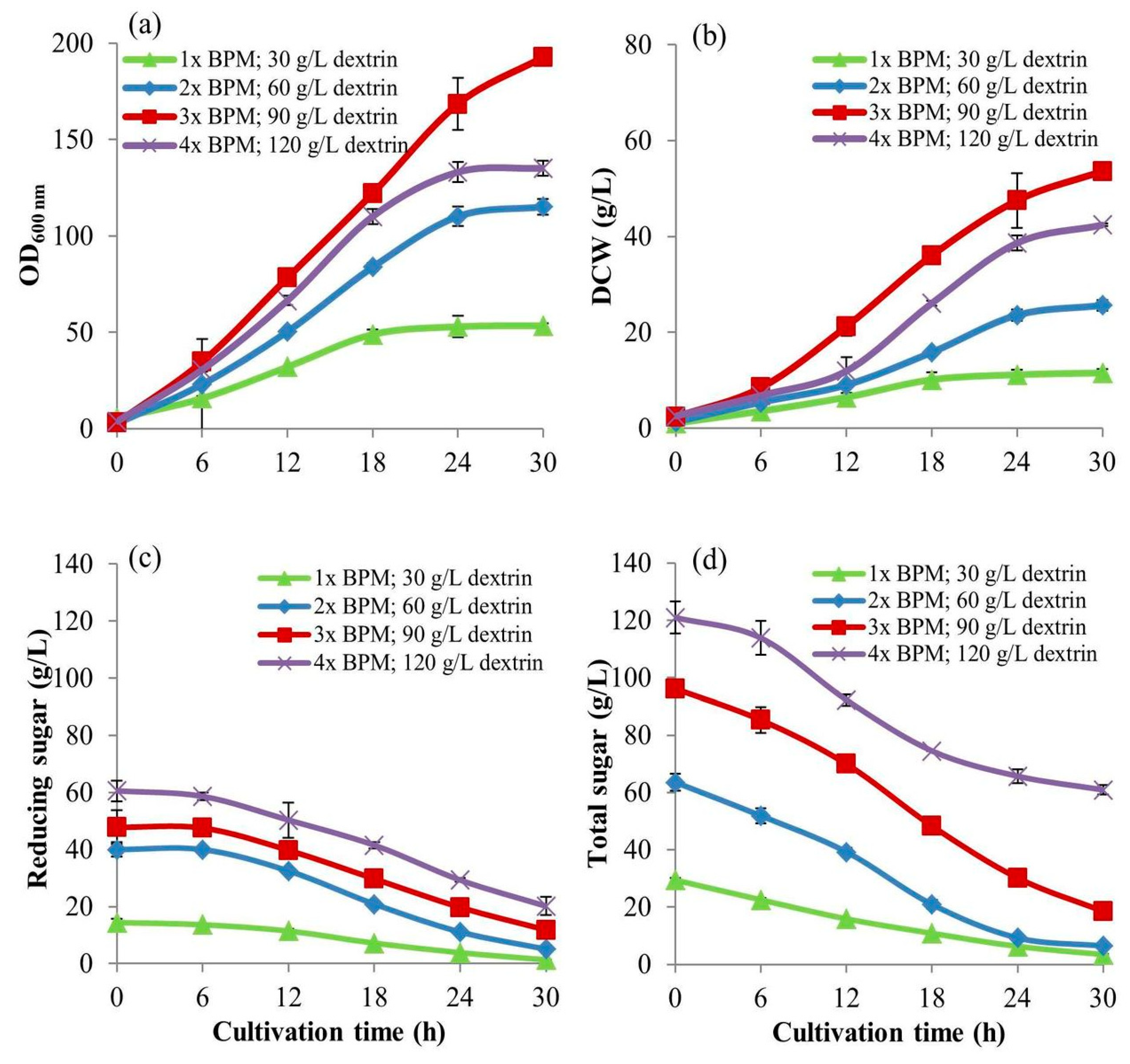
2.6. The High Concentrations of Dextrin for S. cerevisiae Cultivation in the 5-L Bioreactors
The larger-scale cell cultivation in the accurate control conditions using bioreactor makes the results approaching more scalability. The 5-L bioreactors of 0.15 m in diameter and 0.35 m height fit with 2 Rushton turbine impellers of 0.065 m were operated. This experiment was carried out to investigate the optimal-high concentration of dextrin to use as the C-source instead of glucose by
S. cerevisiae cultivation in the 5-L bioreactor with 3-L working volume. This experiment was carefully developed from a normal batch procedure to the intensive batch cultivation technique using four intense parameters: (i) high concentrations of BMP, (ii) high concentrations of dextrin, (ii) high agitation rates, and (iv) high aeration rates. They were proportionally increased when the concentrations of dextrin used were increasingly changed (
Table 1). This experiment was designed to achieve high cell biomass yield related to stoichiometry. The higher the concentrations of BPM and dextrin, the higher the intensities of aeration and agitation were operated with the optimum cultivation conditions that could produce the higher biomass yields. Those parameters effect efficient mass transfers and balance the uses of substrates, i.e., carbon and nitrogen sources; O
2 and inorganic salts to produce cell biomass at high-density concentrations. This is superior to the conventional fed-batch technique in many ways as mentioned before.
A 3 L of BPM was prepared at a concentration of 1×, put into a 5-L bioreactor, and sterilized at 121 °C under 15 lb/in
2 pressure for 25 min in an autoclave. A dextrin solution of 40% (
w/v) from cassava starch liquefaction was separately sterilized at 110 °C under 15 lb/in
2 for 10 min. Its 225 mL was added into the bioreactor to the designed concentration of 30 g/L (equivalent to a concentration of 150 mM of glucose content) and YE was added at a concentration of 3 g/L. The cultivation conditions were set and controlled at 30 °C, pH of 4.5 by 4 N HCl and 4 N NH
4OH solutions, 1 vvm airflow rate, and 1 W/kg of power input,
at an impeller speed of 500 rpm throughout the run. After they were well controlled and reached the designed conditions, a 150 mL of
S. cerevisiae inoculum (from
Section 2.1) was inoculated into the bioreactor (at a concentration of 5%
v/v). For other treatments, the concentrations of dextrin in the cultures were at 60, 90, and 120 g/L (equivalent to concentrations of 300, 450, and 600 mM of glucose content, respectively). The YE was added at the concentrations of 6, 9, and 12 g/L, respectively giving the same YE/dextrin fraction with a value of 0.1. They were proportional to the increase in BPM concentration, agitation speed, and aeration rate as in
Table 1. to achieve higher biomass yields. During cultivations, the culture broth samples of 10 mL of each treatment were withdrawn every 6 h for 30 h. Again, they were put on ice for 20 min to suddenly drop the temperature to 2 °C and stop cell activities before keeping at −20 °C for further analyses.
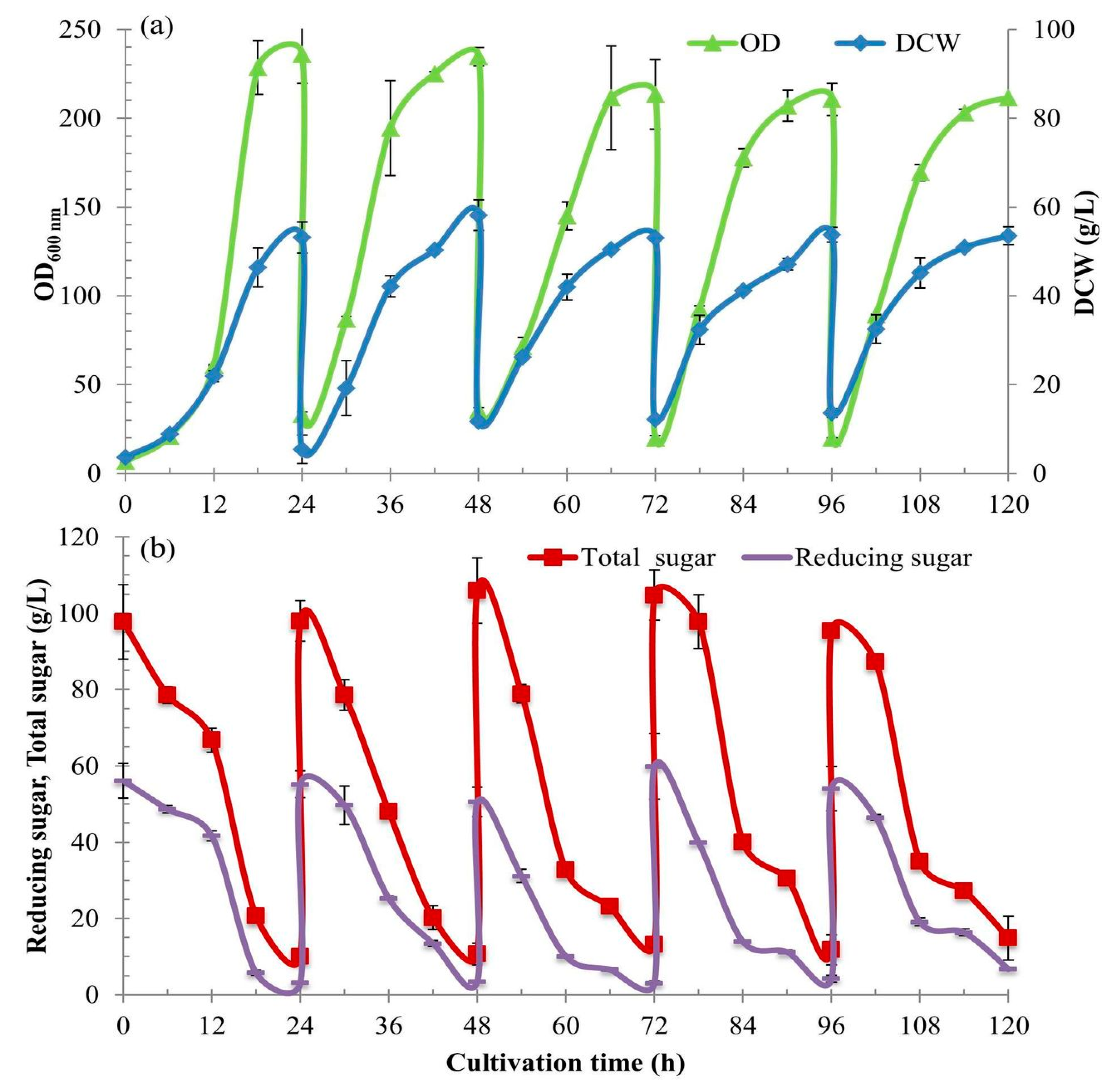
2.7. The Development from Batch to the Intensive Multiple Sequential Batch (IMSB) Technique to Achieve High Cell Density Concentration
This experiment was developed from a single batch to the intensive multiple sequential batches (see [
17]) to achieve a high cell biomass with a high productivity. A 3 L of BPM was prepared at a concentration of 3× put into a 5-L bioreactor and sterilized at 121 °C under 15 lb/in
2 pressure for 25 min. A dextrin solution of 40% (
w/v) was separately sterilized as above and its 675 mL was added into the bioreactor to the designed concentration of 90 g/L for 3-L working volume. The cultivation conditions were controlled at 30 °C and pH 4.5; 3 vvm airflow rate and 3 W/kg
at the impeller speed of 700 rpm. After they were well controlled and reached the designed conditions. To increase the growth rate higher than the experiment in
Section 2.6 (30 h of cultivation time course), a higher concentration of inoculum culture was used. A 150 mL of
S. cerevisiae inoculum of an OD
600 nm of ~15–20 from 24 h culture was inoculated into the bioreactor (at a concentration of 5%
v/v) and grown for 24 h of the initial (first) batch.
Following the first batch, the intensive multiple sequential batch operations were further continually repeated for four more cycles without newly batch up and sterilization. When each batch was completed, a major culture broth was discharged using a peristaltic pump, leaving only a 150 mL to be the starter inoculum for the next batch (at 5% v/v). Unlike the initial batch, the inoculants of the batch cycles 2–5 were the high cell density starters (the culture broths left from the prior batches).
During the time of the first batch cultivation, a further 3000 mL of the fresh medium of the same components and concentration was prepared. Dextrin solution was again prepared. It was sterilized separately and then newly added into the same bioreactor after the first culture broth withdrawal. The second cultivation process was again restarted under the same controlled conditions. The cultivation process was continually repeated for five cycles. Along with the five cultivations, the culture broth samples of 10 mL were withdrawn every 6 h for 24 h. They were put on ice for 20 min for the same reason above and kept at −20 °C for further analyses.
2.8. Cell Growth Determination by Measuring Optical Density (OD) and Dry Cell Weight (DCW)
The growth during the cultivations of S. cerevisiae was determined by measuring absorbance and cell biomass concentration. For the former, a 1 mL of each culture broth sample was diluted 10 or 100 times with distilled water at an appropriate concentration and measured the absorbance at an optical density of 600 nm (OD600 nm) using a spectrophotometer. For the latter, a 1.5 mL of each culture broth sample was put into the micro-centrifuge tube and centrifuged at 10,000 rpm for 10 min. The supernatant was decanted and then the tube was added with a more 1.0 mL distilled water and re-centrifuged to wash the cells. The cell samples were dried at 80 °C for 24 h and weighed. Both methods were used in order to recheck each other for confident reliability.
2.9. Reducing Sugar Analysis
Glucose concentrations of the culture broth samples during cultivations were analyzed as the reducing (residual) sugar (G
l) quantities in the culture using the DNS method [
18]. The 1-mL volumes of DNS reagent were added to test tubes each contained a 1 mL of 1/10 diluted culture broth sample. The mixtures were incubated in a boiling water bath at 95 °C for 5 min and then transferred into a cold bath at 5 °C for 5 min to stop the reaction. Then, each tube was added with a 10 mL of distilled water and mixed well before measuring the absorbance at 520 nm by a spectrophotometer. The quantities of reducing sugar were measured as the glucose concentration by comparison with the standard plot of known glucose concentrations of 0, 0.2, 0.4, 0.6, 0.8, and 1.0 g/L reacted with the DNS reagent and following the same procedure to measure the absorbance.
To verify the DNS assay method is significantly accurate. The concentrations of glucose standard solutions from 0 to 1.0 g/L as above were analyzed both by the DNS assay and by the High Performance Liquid Chromatography (HPLC) method. The HPLC (KNAUER Smartline, Berlin, Germany) with a refractive index (RI) detector (KNAUER Smartline 2300, Berlin, Germany) and a Eurokat H vertex column (KNAUER, Berlin, Germany) was used. Eluent of 0.01 N sulfuric acid solution at a flow rate of 0.8 mL/min was utilized. The analyses were performed at 60 °C. The samples were 10-fold diluted, filtered through 0.45-µm filters, and injected into the column with an amount of 20 µL. The results of both methods were compared and given no significant difference. The two might deviate from each other with a value of ≤5% deviation. Thus, the DNS method was used to quantify the concentration of glucose along with the yeast cell cultivations.
2.10. Total Sugar Analysis
Total sugars (S
T) mean all the concentrations of C-sources, i.e., free residual glucose (G
l) plus dextrin (Dx) left in the culture of which dextrin in the mixture of cell culture was completely hydrolyzed by a strong acid to release all glucose molecules, and then glucose from both sources in the same mixture was measured as the total sugar or total glucose. Thus S
T is the glucose from hydrolyzed Dx plus G
l. The total sugar of culture broth during cell growth was analyzed by sulfuric acid hydrolysis method modified from [
16]. The 1-mL samples of culture broth in micro-tubes were centrifuged at 10,000 rpm for 10 min. The supernatants of 0.5 mL were transferred into 20-mL test tubes. Then 2-mL volumes of 2 N H
2SO
4 solution were added, mixed well, and capped. Test tubes with the mixture were boiled in a water bath at 95 °C for 30 min. The neutralizations were done with the addition of 4 N NaOH and re-centrifugation to precipitate the residues. The supernatants were used to measure the total sugar by the DNS method.
2.11. Quantitative Analyses of Total Sugar and Reducing Sugar (glucose) during Cell Cultivations
Again, total sugar (S
T) means the amount of dextrin (Dx) plus residual glucose or glucose left (G
l),
ST =
Dx +
Gl in the cultures during cell cultivation. Thus, the concentration of dextrin in culture broth is S
T minus G
l, as
Dx =
ST −
Gl. As we know the initial concentration of C-source, (C
i), thus we know the concentration and the utilization rate of G
l, and also know those of the S
T. As a result, we know the concentration and the degradation rates Dx. So, we know the rates of change of all the carbon components in the culture system through the cultivation time course (
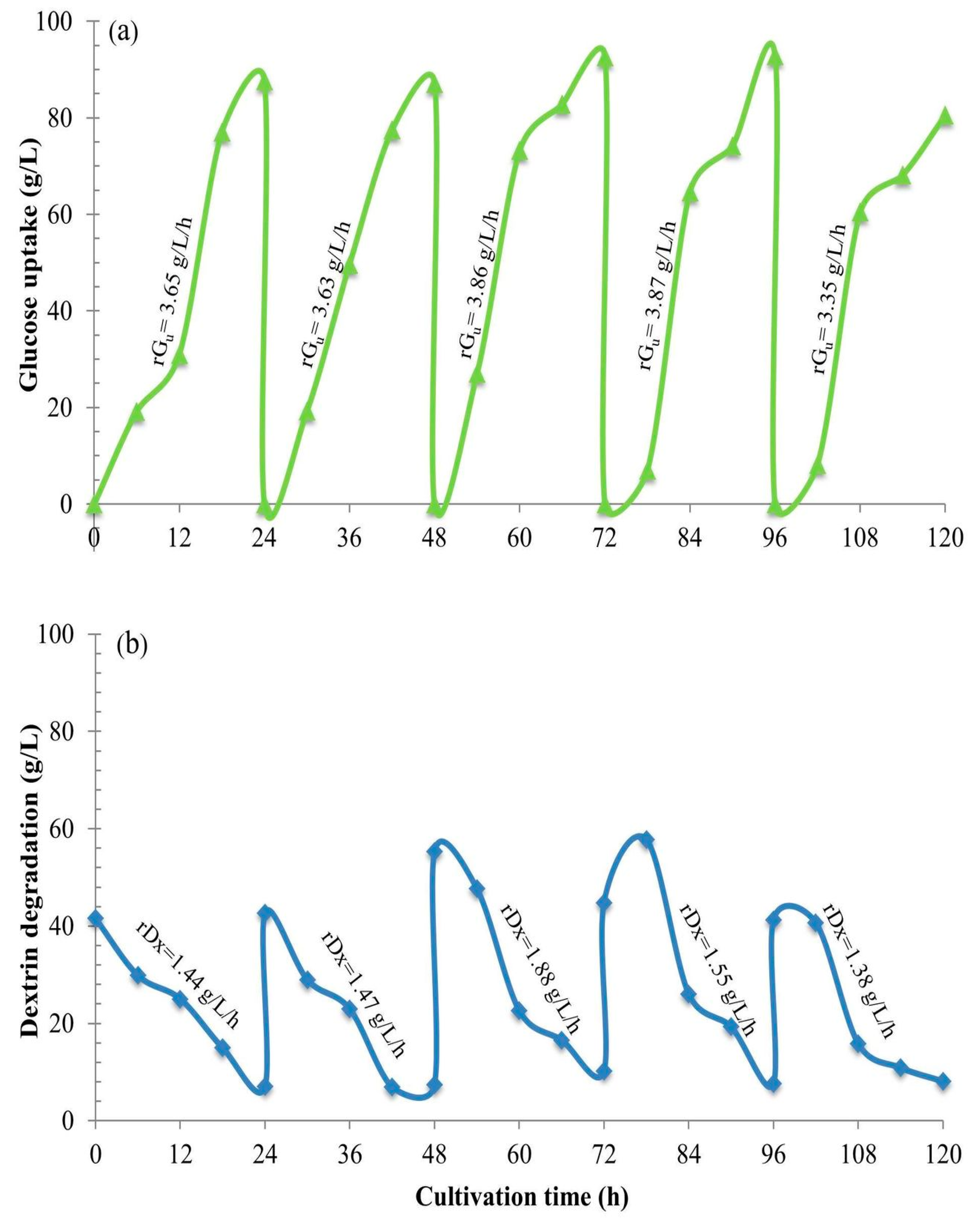
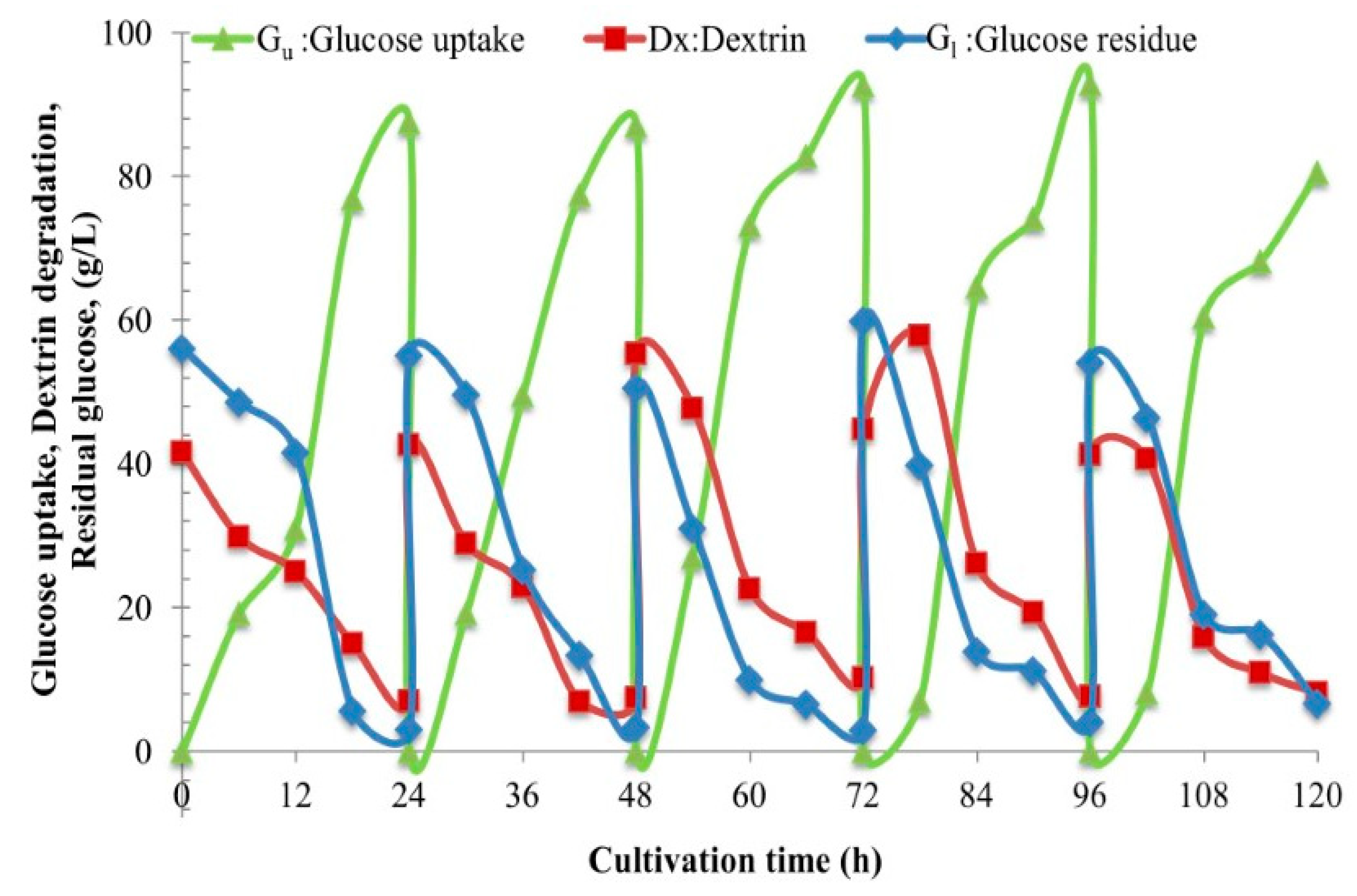
Section 2.12). We obviously see gradual hydrolysis or degradation of the dextrin oligomer to release free glucose molecules continuously into the culture and subsequently gradual assimilation of glucose into the yeast cells. This phenomenon essentially regulates the fed-batch strategy at the cell level. So, we have herein invented the new technical term “fed-batch at cell level” (FBC). The FBC importantly prevents the cell cultivation system from the Crabtree effect. As a result, it promotes cell growth at a high rate, and enables the use of a very high concentration of C-source with just the aerobic batch mode whilst in principle this cannot be done using glucose and/or sucrose. In the laboratories to the pilot scales, many researchers increased the nutrients, i.e., proteins, minerals, growth factors, etc. in the media and increased the impeller speeds and the airflow rates, but they exceeded the economically scalable values.
2.12. Engineering Mass Balances’ Method to Quantify the Unknown Values of Dextrin and Glucose Uptake
This is the “state-of-the-art” method. As a limitation of the HPLC method to analyze the quantities of dextrin oligomer at a time of such a wide range of Dp (Degree of polymerization of carbon atoms), e.g., from C
18, C
17, C
16…C
10, C
9, C
8, C
7… to C
1, thus we use the Biochemical Chemical Engineering mass balances’ technique. In
Section 2.11, with the use of the mass balances’ model from the overall mass balance equation,
Ci = Gu + ST…. (Equation (1)), where G
u is the total glucose uptake, and the component mass balances of
ST =
Dx +
Gl…. (Equation (2)), the concentrations of all the components can be calculated. Furthermore, the utilization rates,
r, of each component can also be calculated as,
rST =
is the rate of total sugar degradation;
rGu =
is the rate of overall glucose uptake;
rGl =
, is the rate of residual glucose utilization;
rDx =
is the rate of dextrin degradation. Significantly, this biochemical engineering mass balance model,
rST =
rGu =
rGl +
rDx… (Equation (3)). This clearly again proves and confirms the “fed-batch at cell level”, the FBC phenomenon.
2.13. Statistical Analysis
The statistical analyses were done with the one-way analysis of variance (ANOVA) and the differences of the treatment mean values from three replications (each experiment was done in 3 replicates) were compared with the Tukey’s range test method at p-value ≤0.05 using a software Minitab version 17. All the experimental result data were presented as the graphical plots using the mean values together with the error bars of standard deviations. The kinetic results are presented in the tables.
2.14. Growth Kinetic Parameters’ Calculations
The five growth kinetic parameters were calculated using experimental data, i.e., cultivation time, t (h), cell biomass concentration,
x (g/L), and substrate use,
s (g/L). (i) The specific growth rate,
μ (h
−1) is calculated from,
where the differential natural log of
x is divided by the time change. (ii) The productivity or production rate of cell biomass,
rx (g/L/h) is
(iii) The utilization rate of substrate,
rs (g/L/h) is from
, where
s can be all types of the sugar components, i.e., total sugar (S
T), glucose uptake (G
u), glucose residual (G
l), and dextrin (Dx), (iv) The cell yield coefficient,
Yx/s (g/g) is from
, where Δ
x is the cell biomass produced (g/L) and Δ
s is the substrate utilized (g/L). (v) The production efficiency,
Ef (%) is from
where
Yx/s is from the experiment and
Y’x/s is from the theoretical yield coefficient or stoichiometry [
15,
19]. One gram of commercial glucose or glucose from dextrin could produce a cell biomass of 0.53 g, (
) and one gram of YE could additionally produce a cell biomass of ~0.6–0.7 g, (
). When they are mixed in the medium, the
was calculated based on the fraction of their components.
2.15. Calculation of the Designed Impeller Speeds
For fluid dynamics in a stirred-tank bioreactor [
19,
20,
21], the power P (W) is calculated from
, where
n is the number of impellers of 2 for 5-L bioreactor,
Pog is the power number of 2.4, (no unit or dimensionless) for fluid with air sparging of Rushton turbine impeller in the bioreactor,
is the culture broth density (kg/m
3),
N is the impeller speed (rps), and
D is the impeller diameter of 0.065 (m). The power input or energy dissipation rate per unit mass,
(W/kg) is calculated from
or
, where
V is the culture broth volume (m
3). Thus, the designed impeller speeds,
N of the power inputs 1, 2, 3, and 4 (W/kg) are calculated from
N =
. These both kinetic and fluid dynamic parameters from the laboratory experiments are very crucial for the scale-up of further microbial cell cultivations both at the pilot and especially at the industrial scales. That is the foreseen reason why we designed the impeller speeds based on the power input rather than just the speed in rpm (
Table 1).
4. Conclusions
The BPM with 90 g/L of dextrin C-source supplemented with YE at 0.9% (w/v) as a source of nutrients is very effective for the high cell density cultivation of S. cerevisiae. This medium enhances excellent cell growth for the production of yeast biomasses and/or their products. As the BPM is very simple and cheap, it is suitable for industrial applications. S. cerevisiae can efficiently use glucose from the gradual hydrolysis of dextrin. Dextrin from cassava starch liquefaction, the major C-source in BPM is more efficient than using commercial pure glucose for the production of yeast cell biomass at high density. It can substitute the major popular C-sources like glucose and/or sucrose which often cause the Crabtree effect inhibiting cell growth. Dextrin can be added into the BPM at a high concentration of ≥90 g/L for aerobic batch cultivation without the problem of too high a C-source concentration, as it regulates the assimilation of glucose at the cell level (FBC). The single 5-L batch cultivation provided the highest values of cell concentration (x), specific growth rate (μ), cell yield coefficient (Yx/s), cell productivity (rx), and efficiency (Ef), at 55.17 g/L, 0.21 h−1, 0.54 g/g, 2.30 g/L/h, and 98.18%, respectively. To achieve higher yields, the intensive multiple sequential batches (IMSB) cultivation was designed and operated for five sequential batch runs. It showed very good results of those average values at 53.72 g/L, 0.16 h−1, 0.50 g/g, 2.24g/L/h, and 90.55%, respectively. With the studies of cell growth kinetics, biochemical engineering mass balances, and fluid dynamics in the bioreactors during cultivations, the production of yeast cell biomass at high density using dextrin at high concentrations can be scaled-up to the industries.
5. The Advantages
Again, we concluded that the advantages of this research work are as follows: (i) BPM, a minimal defined medium with YE, has a very high potential in terms of cost and efficient use for the production of S. cerevisiae biomass at a high density concentration with just an aerobic batch mode rather than using the fed-batch technique with a more complicated operation and longer time course. (ii) Dextrin, an oligomer of glucose from cassava starch liquefaction, is one of the best C-sources for aerobic batch cultivation to produce yeast cell biomass at a high concentration other than using the commercial glucose or/and sucrose. (iii) Dextrin can be used at a very high concentration ≥100 g/L with an aerobic batch mode without the Crabtree effect whereas glucose and sucrose cannot be done. With gradual hydrolysis, it regulates the gradual assimilation of glucose into the yeast cells like “fed-batch at cell level” (FBC), so no glucose accumulation and congestion in the cells will cause O2 deficiency, enhancing the production of toxic by-products, i.e., ethanol in yeasts and acetate in bacteria, to inhibit cell growth. (iv) The intensive multiple sequential batch technique (IMSB) achieves high cell density concentration superior to the fed-batch technique, but it has fewer complications. (v) The kinetics of cell growth, substrate use, and product formation can be utilized to monitor, predict, validate, and control the cultivation to achieve high cell biomass. (vi) As a limitation of HPLC, the biochemical engineering mass balances of our work here are a key model of a very powerful tool implemented to quantify the unknown C-source components, i.e., dextrin and glucose uptake, both in terms of concentration and rate. (vii) Using fluid dynamics in biochemical engineering principles, the stirred-tank bioreactors at every scale can be designed and scaled-up to the industries. (viii) All (v), (vi), and (vii) are very crucial for the production of yeast and other microbial cell biomasses and their products at high concentrations in all scales of operations throughout the industries.