Intracellular Sodium Changes in Cancer Cells Using a Microcavity Array-Based Bioreactor System and Sodium Triple-Quantum MR Signal
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Solutions
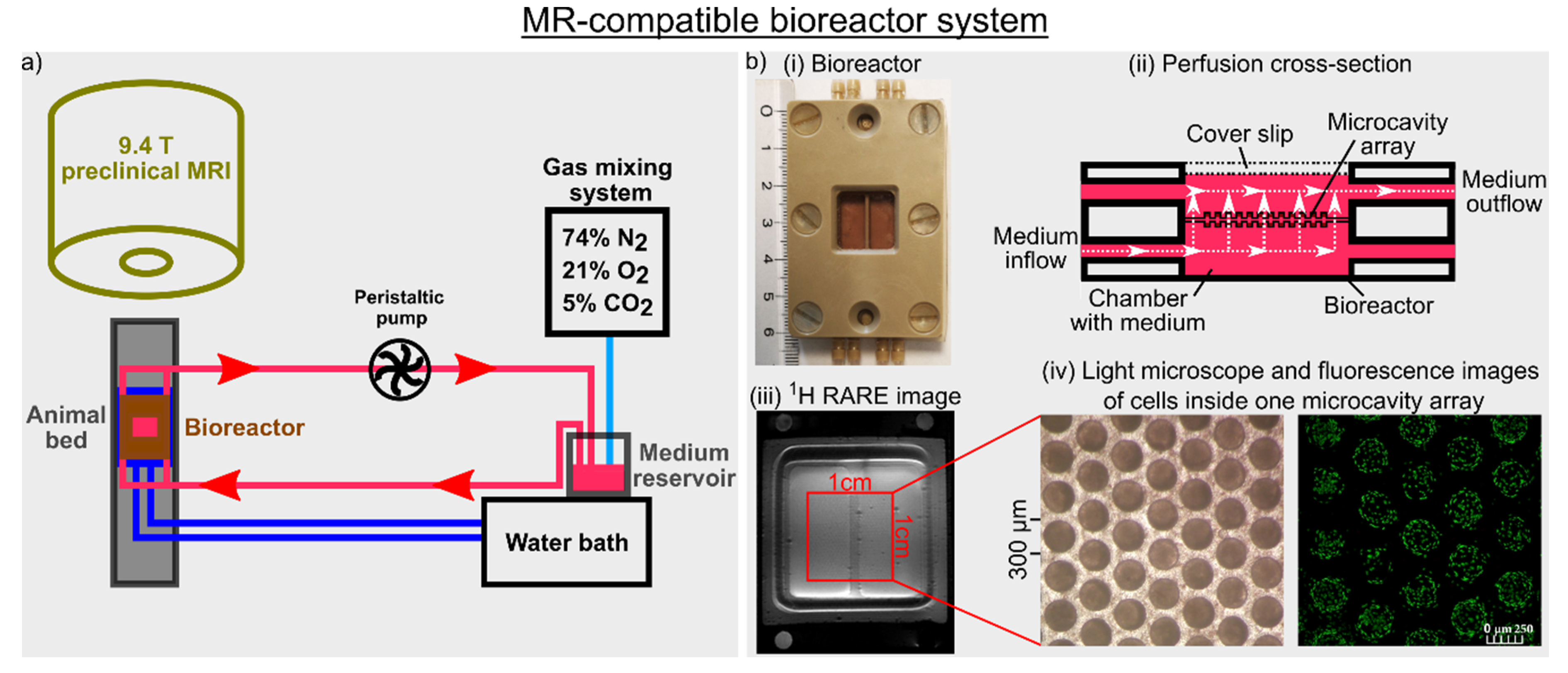
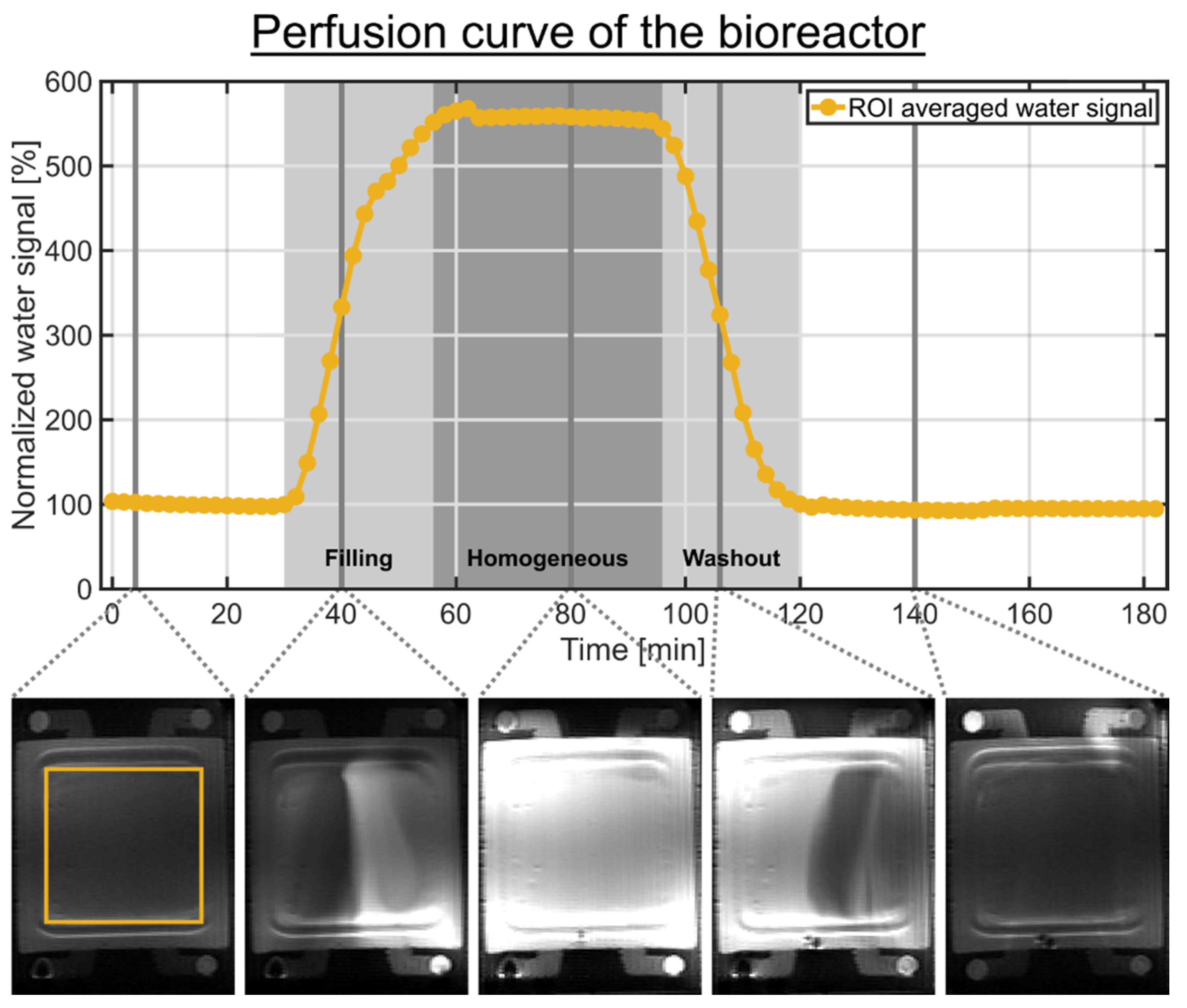
2.2. MR-Compatible Microcavity Array-Based Bioreactor System
2.3. Sodium TQ MR Spectroscopy
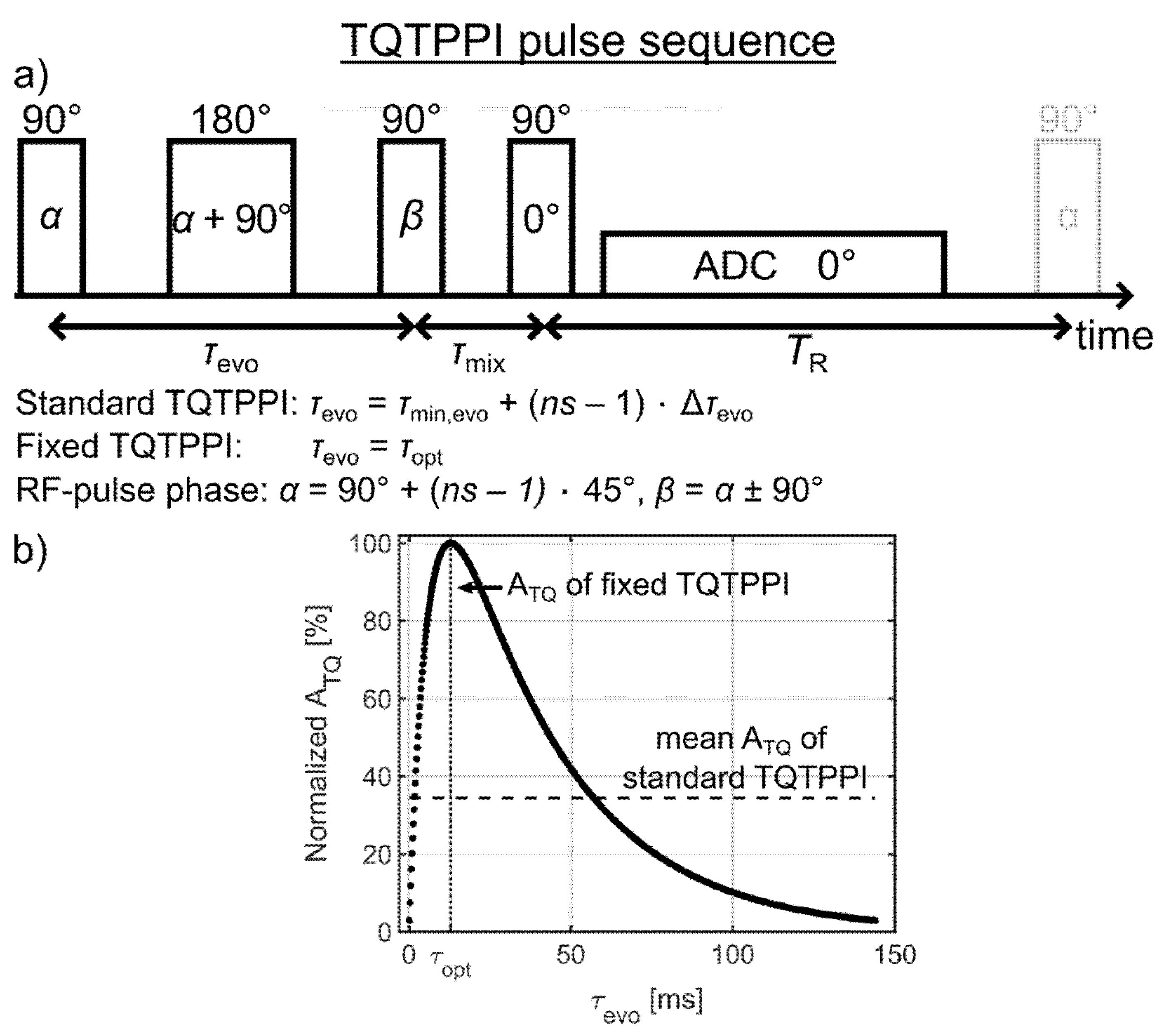
2.3.1. Standard TQTPPI Pulse Sequence
sin(3ωτevo + φ2)(exp(−τevo/T2F) − exp(−τevo/T2S)) + DC,
2.3.2. Fixed TQTPPI Pulse Sequence
2.4. Statistical Analysis
3. Results
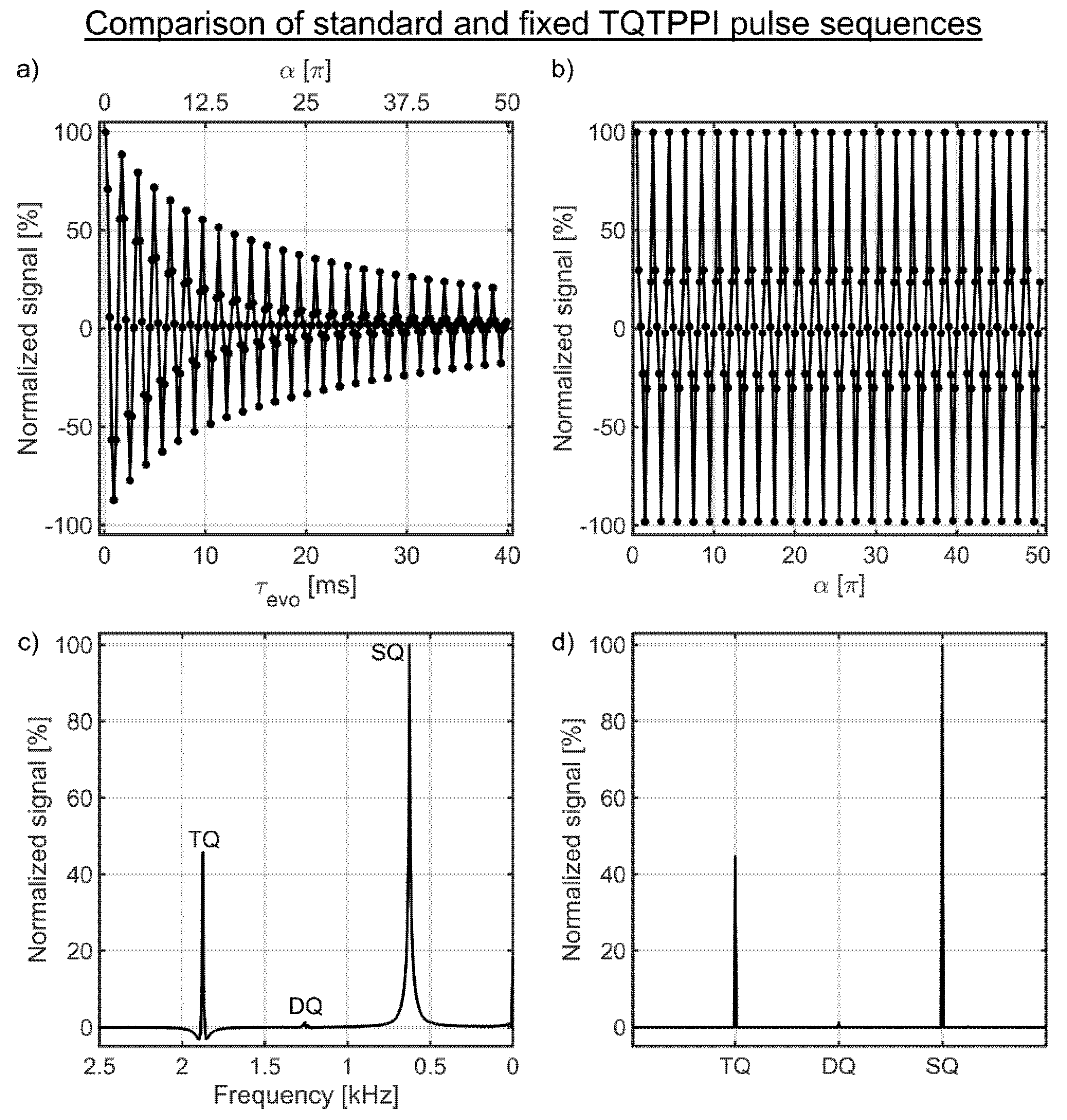
3.1. Comparison of the Standard and the Fixed TQTPPI Pulse Sequences
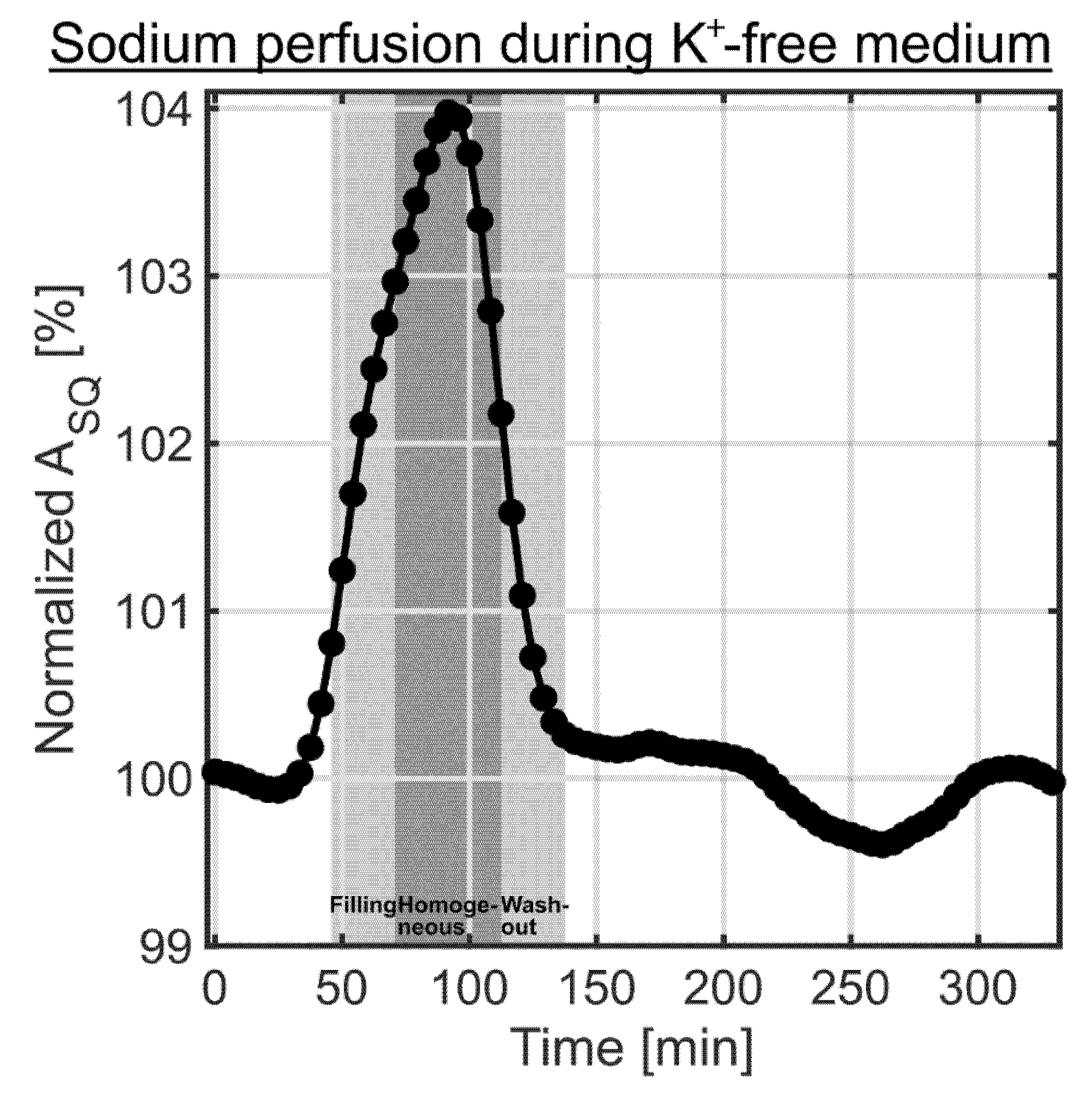
3.2. Contributions to the Sodium TQ Signal from the Bioreactor
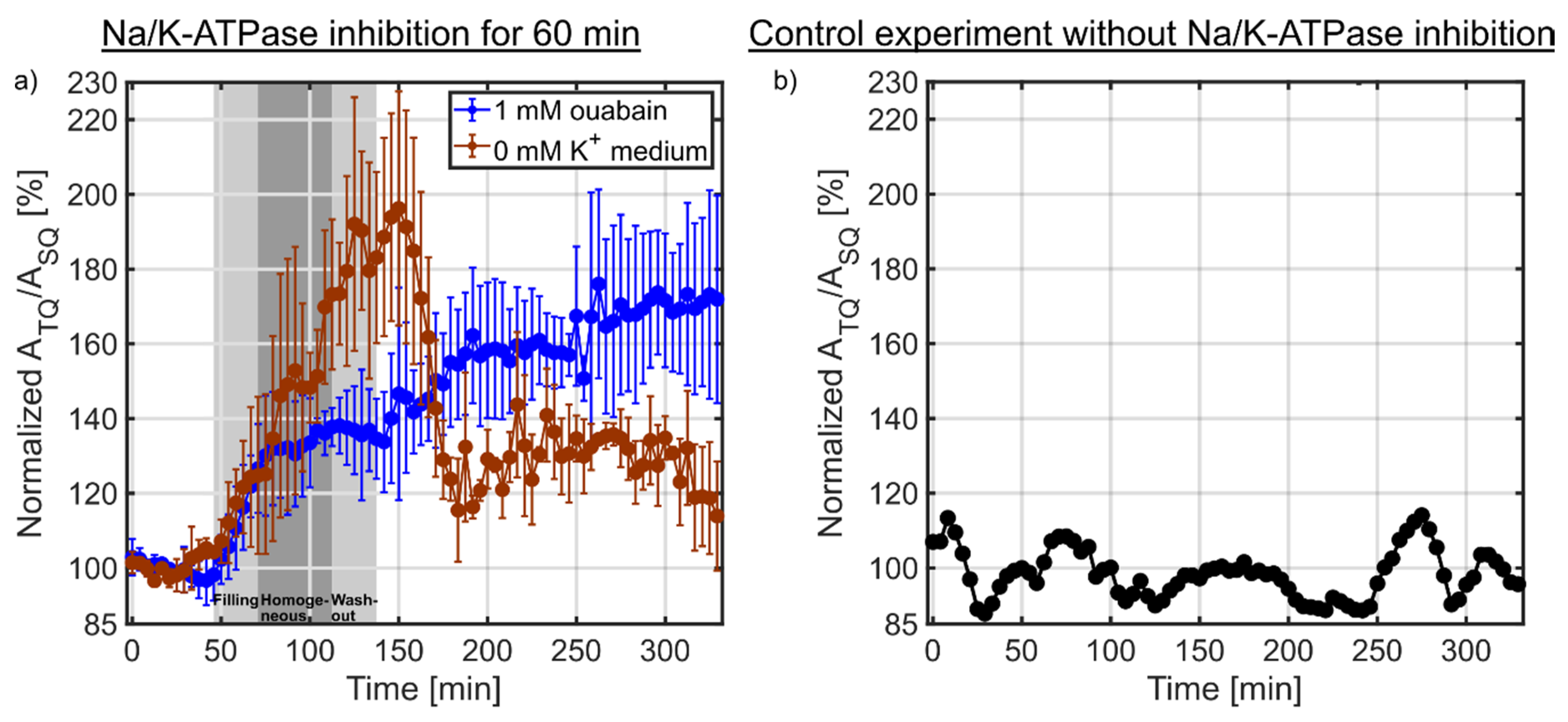
3.3. Na/K-ATPase Inhibition
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clausen, M.V.; Hilbers, F.; Poulsen, H. The structure and function of the Na, K-ATPase isoforms in health and disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2017. [Google Scholar]
- Madelin, G.; Regatte, R.R. Biomedical applications of sodium MRI in vivo. J. Magn. Reson. Imaging 2013, 38, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Kleimaier, D.; Malzacher, M.; Hoesl, M.A.U.; Paschke, N.K.; Schad, L.R. X-nuclei imaging: Current state, technical challenges, and future directions. J. Magn. Reson. Imaging 2020, 51, 355–376. [Google Scholar] [CrossRef] [PubMed]
- Madelin, G.; Lee, J.S.; Regatte, R.R.; Jerschow, A. Sodium MRI: Methods and applications. Prog. Nucl. Magn. Reason Spectrosc. 2014, 79, 14–47. [Google Scholar] [CrossRef]
- Thulborn, K.R. Quantitative sodium MR imaging: A review of its evolving role in medicine. Neuroimage 2018, 168, 250–268. [Google Scholar] [CrossRef]
- Malzacher, M.; Chacon-Caldera, J.; Paschke, N.; Schad, L.R. Feasibility study of a double resonant 1 H/23 Na abdominal RF setup at 3T. Zeitschrift für Medizinische Physik 2019, 29, 359–367. [Google Scholar] [CrossRef]
- Lachner, S.; Ruck, L.; Niesporek, S.C.; Utzschneider, M.; Lott, J.; Hensel, B.; Dorfler, A.; Uder, M.; Nagel, A.M. Comparison of optimized intensity correction methods for 23 Na MRI of the human brain using a 32-channel phased array coil at 7 Tesla. Zeitschrift für Medizinische Physik 2020, 30, 104–115. [Google Scholar] [CrossRef]
- Neumaier-Probst, E.; Konstandin, S.; Ssozi, J.; Groden, C.; Hennerici, M.; Schad, L.R.; Fatar, M.A. Double-tuned 1 H/23 Na resonator allows 1 H-guided 23 Na -MRI in ischemic stroke patients in one session. Int. J. Stroke 2015, 10, 56–61. [Google Scholar] [CrossRef]
- Thulborn, K.R.; Davis, D.; Snyder, J.; Yonas, H.; Kassam, A. Sodium MR imaging of acute and subacute stroke for assessment of tissue viability. Neuroimaging Clin. N. Am. 2005, 15, 639–653. [Google Scholar] [CrossRef]
- Jacobs, M.A.; Ouwerkerk, R.; Wolff, A.C.; Gabrielson, E.; Warzecha, H.; Jeter, S.; Bluemke, D.A.; Wahl, R.; Stearns, V. Monitoring of neoadjuvant chemotherapy using multiparametric, 23Na sodium MR, and multimodality (PET/CT/MRI) imaging in locally advanced breast cancer. Breast Cancer Res. Treat. 2011, 128, 119–126. [Google Scholar] [CrossRef]
- Schepkin, V.D.; Ross, B.D.; Chenevert, T.L.; Rehemtulla, A.; Sharma, S.; Kumar, M.; Stojanovska, J. Sodium magnetic resonance imaging of chemotherapeutic response in a rat glioma. Magn. Reson. Med. 2005, 53, 85–92. [Google Scholar] [CrossRef]
- Zaric, O.; Pinker, K.; Zbyn, S.; Strasser, B.; Robinson, S.; Minarikova, L.; Gruber, S.; Farr, A.; Singer, C.; Helbich, T.H.; et al. Quantitative sodium MR imaging at 7 T: Initial results and comparison with diffusion-weighted imaging in patients with breast tumors. Radiology 2016, 280, 39–48. [Google Scholar] [CrossRef]
- Rooney, W.D.; Li, X.; Sammi, M.K.; Bourdette, D.N.; Neuwelt, E.A.; Springer, C.S., Jr. Mapping human brain capillary water lifetime: High-resolution metabolic neuroimaging. NMR Biomed. 2015, 28, 607–623. [Google Scholar] [CrossRef]
- Schepkin, V.D. Statistical tensor analysis of the MQ MR signals generated by weak quadrupole interactions. Zeitschrift für Medizinische Physik 2019, 29, 326–336. [Google Scholar] [CrossRef]
- Schepkin, V.D.; Neubauer, A.; Nagel, A.M.; Budinger, T.F. Comparison of potassium and sodium binding in vivo and in agarose samples using TQTPPI pulse sequence. J. Magn. Reson. 2017, 277, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Dizon, J.M.; Tauskela, J.S.; Wise, D.; Burkhoff, D.; Cannon, P.J.; Katz, J. Evaluation of triple-quantum-filtered 23 Na NMR in monitoring of Intracellular Na content in the perfused rat heart: Comparison of intra-and extracellular transverse relaxation and spectral amplitudes. Magn. Reson. Med. 1996, 35, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Eykyn, T.R.; Aksentijevic, D.; Aughton, K.L.; Southworth, R.; Fuller, W.; Shattock, M.J. Multiple quantum filtered 23 Na NMR in the Langendorff perfused mouse heart: Ratio of triple/double quantum filtered signals correlates with [Na]i. J. Mol. Cell. Cardiol. 2015, 86, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Jelicks, L.A.; Gupta, R.K. On the extracellular contribution to multiple quantum filtered 23 Na NMR of perfused rat heart. Magn. Reson. Med. 1993, 29, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Knubovets, T.; Shinar, H.; Navon, G. Quantification of the contribution of extracellular sodium to Na-23 multiple-quantum-filtered NMR spectra of suspensions of human red blood cells. J. Magn. Reson. 1998, 131, 92–96. [Google Scholar] [CrossRef]
- Schepkin, V.D.; Choy, I.O.; Budinger, T.F. Sodium alterations in isolated rat heart during cardioplegic arrest. J. Appl. Physiol. 1996, 81, 2696–2702. [Google Scholar] [CrossRef][Green Version]
- Schepkin, V.D.; Choy, I.O.; Budinger, T.F.; Obayashi, D.Y.; Taylor, S.E.; DeCampli, W.M.; Amartur, S.C.; Young, J.N. Sodium TQF NMR and intracellular sodium in isolated crystalloid perfused rat heart. Magn. Reson. Med. 1998, 39, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Seshan, V.; Sherry, A.D.; Bansal, N. Evaluation of triple quantum-filtered 23 Na NMR spectroscopy in the in situ rat liver. Magn. Reson. Med. 1997, 38, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Winter, P.M.; Bansal, N. Triple-quantum-filtered 23 Na NMR spectroscopy of subcutaneously implanted 9 L gliosarcoma in the rat in the presence of TmDOTP(5–1). J. Magn. Reson. 2001, 152, 70–78. [Google Scholar] [CrossRef]
- Choy, I.O.; Schepkin, V.D.; Budinger, T.F.; Obayashi, D.Y.; Young, J.N.; DeCampli, W.M. Effects of specific sodium/hydrogen exchange inhibitor during cardioplegic arrest. Ann. Thorac. Surg. 1997, 64, 94–99. [Google Scholar] [CrossRef][Green Version]
- Schepkin, V.D.; Choy, I.O.; Budinger, T.F.; Young, J.N.; DeCampli, W.M. Multi-dose crystalloid cardioplegia preserves intracellular sodium homeostasis in myocardium. J. Mol. Cell. Cardiol. 1999, 31, 1643–1651. [Google Scholar] [CrossRef][Green Version]
- Tauskela, J.S.; Dizon, J.M.; Whang, J.; Katz, J. Evaluation of multiple-quantum-filtered 23 Na NMR in monitoring intracellular Na content in the isolated perfused rat heart in the absence of a chemical-shift reagent. J. Magn. Reson. 1997, 127, 115–127. [Google Scholar] [CrossRef]
- LaVerde, G.; Nemoto, E.; Jungreis, C.A.; Tanase, C.; Boada, F.E. Serial triple quantum sodium MRI during non-human primate focal brain ischemia. Magn. Reson. Med. 2007, 57, 201–205. [Google Scholar] [CrossRef]
- Babsky, A.M.; Zhang, H.; Hekmatyar, S.K.; Hutchins, G.D.; Bansal, N. Monitoring chemotherapeutic response in RIF-1 tumors by single-quantum and triple-quantum-filtered 23 Na MRI, 1 H diffusion-weighted MRI and PET imaging. Magn. Reson. Imaging 2007, 25, 1015–1023. [Google Scholar] [CrossRef]
- Winter, P.M.; Poptani, H.; Bansal, N. Effects of chemotherapy by 1,3-bis(2-chloroethyl)-1-nitrosourea on single-quantum-and triple-quantum-filtered 23 Na and 31 P nuclear magnetic resonance of the subcutaneously implanted 9 L glioma. Cancer Res. 2001, 61, 2002–2007. [Google Scholar]
- Gottwald, E.; Kleintschek, T.; Giselbrecht, S.; Truckenmuller, R.; Altmann, B.; Worgull, M.; Dopfert, J.; Schad, L.; Heilmann, M. Characterization of a chip-based bioreactor for three-dimensional cell cultivation via magnetic resonance imaging. Zeitschrift für Medizinische Physik 2013, 23, 102–110. [Google Scholar] [CrossRef]
- Kleimaier, D.; Goerke, S.; Nies, C.; Zaiss, M.; Kunz, P.; Bachert, P.; Ladd, M.E.; Gottwald, E.; Schad, L.R. The cellular heat shock response monitored by chemical exchange saturation transfer MRI. Sci. Rep. 2020, 10, 11118. [Google Scholar] [CrossRef] [PubMed]
- Hoesl, M.A.U.; Kleimaier, D.; Hu, R.; Malzacher, M.; Nies, C.; Gottwald, E.; Schad, L.R. 23 Na triple-quantum signal of in vitro human liver cells, liposomes, and nanoparticles: Cell viability assessment vs. separation of intra-and extracellular signal. J. Magn. Reson. Imaging 2019, 50, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Hoesl, M.A.U.; Wuestemann, T.; Malzacher, M.; Nies, C.; Gottwald, E.; Schad, L. Non-invasive assessment of myocardial cell viability based on 23 Na triple-quantum signal. Proc. Int. Soc. Magn. Reson. Med. 2019, 27, 4202. [Google Scholar]
- Neubauer, A.; Nies, C.; Schepkin, V.D.; Hu, R.; Malzacher, M.; Chacon-Caldera, J.; Thiele, D.; Gottwald, E.; Schad, L.R. Tracking protein function with sodium multi quantum spectroscopy in a 3D-tissue culture based on microcavity arrays. Sci. Rep. 2017, 7, 3943. [Google Scholar] [CrossRef] [PubMed]
- Gottwald, E.; Nies, C.; Wuchter, P.; Saffrich, R.; Truckenmuller, R.; Giselbrecht, S. A Microcavity Array-based 3D model system of the hematopoietic stem cell niche. Methods Mol. Biol. 2019, 2017, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Altmann, B.; Giselbrecht, S.; Weibezahn, K.F.; Welle, A.; Gottwald, E. The three-dimensional cultivation of the carcinoma cell line HepG2 in a perfused chip system leads to a more differentiated phenotype of the cells compared to monolayer culture. Biomed. Mater. 2008, 3, 034120. [Google Scholar] [CrossRef]
- Gottwald, E.; Giselbrecht, S.; Augspurger, C.; Lahni, B.; Dambrowsky, N.; Truckenmuller, R.; Piotter, V.; Gietzelt, T.; Wendt, O.; Pfleging, W.; et al. A chip-based platform for the in vitro generation of tissues in three-dimensional organization. Lab Chip 2007, 7, 777–785. [Google Scholar] [CrossRef]
- Biechele, P.; Busse, C.; Solle, D.; Scheper, T.; Reardon, K. Sensor systems for bioprocess monitoring. Eng. Life Sci. 2015, 15, 469–488. [Google Scholar] [CrossRef]
- Jaccard, G.; Wimperis, S.; Bodenhausen, G. Multiple-quantum NMR spectroscopy of S = 3/2 spins in isotropic phase: A new probe for multiexponential relaxation. J. Chem. Phys. 1986, 85, 6282–6293. [Google Scholar] [CrossRef]
- Marion, D.; Wüthrich, K. Application of phase sensitive two-dimensional correlated spectroscopy (COSY) for measurements of 1 H-1 H spin-spin coupling constants in proteins. Biochem. Biophys. Res. Commun. 1983, 113, 967–974. [Google Scholar] [CrossRef]
- Kleimaier, D.; Schepkin, V.; Hu, R.; Schad, L.R. Protein conformational changes affect the sodium triple-quantum MR signal. NMR Biomed. 2020, 33, e4367. [Google Scholar] [CrossRef] [PubMed]
- Van der Maarel, J.R.C. Thermal relaxation and coherence dynamics of spin 3/2. I. Static and fluctuating quadrupolar interactions in the multipole basis. Concepts Magn. Reason. 2003, 19, 97–116. [Google Scholar] [CrossRef]
- Johnstone, I.M.; Silverman, B.W. Needles and straw in haystacks: Empirical Bayes estimates of possibly sparse sequences. Ann. Stat. 2004, 32, 1594–1649. [Google Scholar] [CrossRef]
- Reinsch, C.H. Smoothing by spline functions. Numer. Math. 1967, 10, 177–183. [Google Scholar] [CrossRef]
- Zheng, X.; Baker, H.; Hancock, W.S.; Fawaz, F.; McCaman, M.; Pungor, E., Jr. Proteomic analysis for the assessment of different lots of fetal bovine serum as a raw material for cell culture. Part IV. Application of proteomics to the manufacture of biological drugs. Biotechnol. Prog. 2006, 22, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Deitmer, J.W.; Ellis, D. The intracellular sodium activity of cardiac Purkinje fibres during inhibition and re-activation of the Na-K pump. J. Physiol. 1978, 284, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J. Physiol. 1977, 273, 211–240. [Google Scholar] [CrossRef]
- Pike, M.M.; Frazer, J.C.; Dedrick, D.F.; Ingwall, J.S.; Allen, P.D.; Springer, C.S.; Smith, T.W. 23 Na and 39 K nuclear magnetic resonance studies of perfused rat hearts. Discrimination of intra- and extracellular ions using a shift reagent. Biophys. J. 1985, 48, 159–173. [Google Scholar] [CrossRef]
- Cameron, I.L.; Smith, N.K.; Pool, T.B.; Sparks, R.L. Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Res. 1980, 40, 1493–1500. [Google Scholar]
- Amorino, G.P.; Fox, M.H. Intracellular Na + measurements using sodium green tetraacetate with flow cytometry. Cytometry 1995, 21, 248–256. [Google Scholar] [CrossRef]
- Gao, G.; Cao, Y.; Liu, W.; Li, D.; Zhou, W.; Liu, J. Fluorescent sensors for sodium ions. Anal. Methods 2017, 9, 5570–5579. [Google Scholar] [CrossRef]
- Yin, J.; Hu, Y.; Yoon, J. Fluorescent probes and bioimaging: Alkali metals, alkaline earth metals and pH. Chem. Soc. Rev. 2015, 44, 4619–4644. [Google Scholar] [CrossRef] [PubMed]
- Iamshanova, O.; Mariot, P.; Lehen’Kyi, V.; Prevarskaya, N. Comparison of fluorescence probes for intracellular sodium imaging in prostate cancer cell lines. Eur. Biophys. J. 2016, 45, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Naritomi, H.; Kanashiro, M.; Sasaki, M.; Kuribayashi, Y.; Sawada, T. In vivo measurements of intra-and extracellular Na + and water in the brain and muscle by nuclear magnetic resonance spectroscopy with shift reagent. Biophys. J. 1987, 52, 611–616. [Google Scholar] [CrossRef]
- Navon, G. Complete elimination of the extracellular 23 Na NMR signal in triple quantum filtered spectra of rat hearts in the presence of shift reagents. Magn. Reson. Med. 1993, 30, 503–506. [Google Scholar] [CrossRef]
- Simor, T.; Lóránd, T.; Szöllösy, Á.; Gaszner, B.; Digerness, S.B.; Elgavish, G.A. 23 Na NMR shift reagents enhance cardiac staircase effect in isolated perfused rat hearts. NMR Biomed. 1999, 12, 267–274. [Google Scholar] [CrossRef]
- Fiege, D.P.; Romanzetti, S.; Mirkes, C.C.; Brenner, D.; Shah, N.J. Simultaneous single-quantum and triple-quantum-filtered MRI of 23 Na (SISTINA). Magn. Reson. Med. 2013, 69, 1691–1696. [Google Scholar] [CrossRef]
- Hoesl, M.A.U.; Schad, L.R.; Rapacchi, S. Efficient 23 Na triple-quantum signal imaging on clinical scanners: Cartesian imaging of single and triple-quantum 23 Na (CRISTINA). Magn. Reson. Med. 2020, 84, 2412–2428. [Google Scholar] [CrossRef]
- Worthoff, W.A.; Shymanskaya, A.; Shah, N.J. Relaxometry and quantification in simultaneously acquired single and triple quantum filtered sodium MRI. Magn. Reson. Med. 2018, 81, 303–315. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Aebisher, D.; Czmil, A.; Mazur, D. Evaluation of MR relaxation times following trastuzumab treatment of breast cancer cells in a 3D bioreactor. Acta Pol. Pharm. 2020, 77, 35–41. [Google Scholar] [CrossRef]
- Carvalho, J.; Alves, S.; Castro, M.; Geraldes, C.; Queiroz, J.A.; Fonseca, C.P.; Cruz, C. Development of a bioreactor system for cytotoxic evaluation of pharmacological compounds in living cells using NMR spectroscopy. J. Pharmacol. Toxicol. Methods 2019, 95, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.L.; Erickson-Bhatt, S.; Szulczewski, J.M.; Squirrell, J.M.; Ludwig, K.D.; Macdonald, E.B.; Swader, R.; Ponik, S.M.; Eliceiri, K.W.; Fain, S.B.; et al. A novel bioreactor for combined magnetic resonance spectroscopy and optical imaging of metabolism in 3D cell cultures. Magn. Reson. Med. 2019, 81, 3379–3391. [Google Scholar] [CrossRef]
- Grivet, J.-P.; Delort, A.-M. NMR for microbiology: In vivo and in situ applications. Prog. Nucl. Magn. Reason. Spectrosc. 2009, 54, 1–53. [Google Scholar] [CrossRef]
- Hemminga, M.A.; Visser, J. NMR in biotechnology. J. Biotechnol. 2000, 77, 1–3. [Google Scholar] [CrossRef]
- Hertig, D.; Maddah, S.; Memedovski, R.; Felser, A.; Moreno, A.; Pennestri, M.; Nuoffer, J.-M.; Vermathen, P. Life monitoring of cellular metabolism and mitochondrial respiration in 3D cell culture system using NMR Spectroscopy. Proc. Int. Soc. Magn. Reson. Med. 2020, 28, 2994. [Google Scholar]
- Keshari, K.R.; Kurhanewicz, J.; Jeffries, R.E.; Wilson, D.M.; Dewar, B.J.; Van Criekinge, M.; Zierhut, M.; Vigneron, D.B.; Macdonald, J.M. Hyperpolarized 13 C spectroscopy and an NMR-compatible bioreactor system for the investigation of real-time cellular metabolism. Magn. Reson. Med. 2010, 63, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.M.; Grillo, M.; Schmidlin, O.; Tajiri, D.T.; James, T.L. NMR spectroscopy and MRI investigation of a potential bioartificial liver. NMR Biomed. 1998, 11, 55–66. [Google Scholar] [CrossRef]
- Macdonald, J.M.; Kurhanewicz, J.; Dahiya, R.; Espanol, M.T.; Chang, L.H.; Goldberg, B.; James, T.L.; Narayan, P. Effect of glucose and confluency on phosphorus metabolites of perfused human prostatic adenocarcinoma cells as determined by 31 P MRS. Magn. Reson. Med. 1993, 29, 244–248. [Google Scholar] [CrossRef]
- Majors, P.D.; McLean, J.S.; Scholten, J.C. NMR bioreactor development for live in-situ microbial functional analysis. J. Magn. Reson. 2008, 192, 159–166. [Google Scholar] [CrossRef]
- Mancuso, A.; Fernandez, E.J.; Blanch, H.W.; Clark, D.S. A nuclear magnetic resonance technique for determining hybridoma cell concentration in hollow fiber bioreactors. Biotechnology 1990, 8, 1282–1285. [Google Scholar] [CrossRef]
- Narayan, K.S.; Moress, E.A.; Chatham, J.C.; Barker, P.B. 31 P NMR of mammalian cells encapsulated in alginate gels utilizing a new phosphate-free perfusion medium. NMR Biomed. 1990, 3, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Siegal, G.; Selenko, P. Cells, drugs and NMR. J. Magn. Reson. 2019, 306, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Thelwall, P.E.; Brindle, K.M. Analysis of CHO-K1 cell growth in a fixed bed bioreactor using magnetic resonance spectroscopy and imaging. Cytotechnology 1999, 30, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Trouard, T.P.; Harkins, K.D.; Divijak, J.L.; Gillies, R.J.; Galons, J.P. Ischemia-induced changes of intracellular water diffusion in rat glioma cell cultures. Magn. Reson. Med. 2008, 60, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Giusti, S.; Mazzei, D.; Cacopardo, L.; Mattei, G.; Domenici, C.; Ahluwalia, A. Environmental control in flow bioreactors. Processes 2017, 5, 16. [Google Scholar] [CrossRef]
- Lundervold, A.S.; Lundervold, A. An overview of deep learning in medical imaging focusing on MRI. Zeitschrift für Medizinische Physik 2019, 29, 102–127. [Google Scholar] [CrossRef]
- Madelin, G.; Poidevin, F.; Makrymallis, A.; Regatte, R.R. Classification of sodium MRI data of cartilage using machine learning. Magn. Reson. Med. 2015, 74, 1435–1448. [Google Scholar] [CrossRef]
- Wang, S.; Su, Z.; Ying, L.; Peng, X.; Zhu, S.; Liang, F.; Feng, D.; Liang, D. Accelerating magnetic resonance imaging via deep learning. In Proceedings of the 2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI), Prague, Czech Republic, 13–16 April 2016. [Google Scholar] [CrossRef]
- Lustig, M.; Donoho, D.; Pauly, J.M. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn. Reson. Med. 2007, 58, 1182–1195. [Google Scholar] [CrossRef]
- Madelin, G.; Chang, G.; Otazo, R.; Jerschow, A.; Regatte, R.R. Compressed sensing sodium MRI of cartilage at 7T: Preliminary study. J. Magn. Reson. 2012, 214, 360–365. [Google Scholar] [CrossRef]

| Agar. [%] | TQTPPI Sequence | ASQ [106 a.u.] | ATQ [106 a.u.] | ATQ/ASQ [%] | TQ SNR Gain Theoretical | TQ SNR Gain Measured | T2S [ms] | T2F [ms] | τopt [ms] |
|---|---|---|---|---|---|---|---|---|---|
| 2 | standard | 28.92 ± 0.39 | 7.84 ± 0.12 | 27.1 ± 0.6 | 41.1 ± 1.7 | 10.3 ± 0.1 | 19.0 ± 0.3 | ||
| fixed | 28.64 ± 0.03 | 7.67 ± 0.03 | 26.8 ± 0.1 | 2.4 ± 0.1 | 3.2 ± 0.4 | 43.2 ± 1.1 | 9.9 ± 0.2 | 18.8 ± 0.3 | |
| 4 | standard | 20.02 ± 0.34 | 9.01 ± 0.08 | 45.1 ± 1.2 | 34.2 ± 0.8 | 5.8 ± 0.1 | 12.4 ± 0.1 | ||
| fixed | 20.18 ± 0.02 | 8.95 ± 0.02 | 44.3 ± 0.1 | 2.9 ± 0.1 | 3.6 ± 0.1 | 33.9 ± 0.7 | 5.8 ± 0.1 | 12.3 ± 0.2 | |
| 6 | standard | 22.38 ± 0.27 | 11.66 ± 0.15 | 52.1 ± 0.9 | 30.0 ± 0.4 | 4.4 ± 0.1 | 9.8 ± 0.1 | ||
| fixed | 22.58 ± 0.03 | 11.71 ± 0.03 | 51.9 ± 0.1 | 3.2 ± 0.1 | 3.8 ± 0.2 | 32.2 ± 1.5 | 4.2 ± 0.2 | 9.9 ± 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleimaier, D.; Schepkin, V.; Nies, C.; Gottwald, E.; Schad, L.R. Intracellular Sodium Changes in Cancer Cells Using a Microcavity Array-Based Bioreactor System and Sodium Triple-Quantum MR Signal. Processes 2020, 8, 1267. https://doi.org/10.3390/pr8101267
Kleimaier D, Schepkin V, Nies C, Gottwald E, Schad LR. Intracellular Sodium Changes in Cancer Cells Using a Microcavity Array-Based Bioreactor System and Sodium Triple-Quantum MR Signal. Processes. 2020; 8(10):1267. https://doi.org/10.3390/pr8101267
Chicago/Turabian StyleKleimaier, Dennis, Victor Schepkin, Cordula Nies, Eric Gottwald, and Lothar R. Schad. 2020. "Intracellular Sodium Changes in Cancer Cells Using a Microcavity Array-Based Bioreactor System and Sodium Triple-Quantum MR Signal" Processes 8, no. 10: 1267. https://doi.org/10.3390/pr8101267
APA StyleKleimaier, D., Schepkin, V., Nies, C., Gottwald, E., & Schad, L. R. (2020). Intracellular Sodium Changes in Cancer Cells Using a Microcavity Array-Based Bioreactor System and Sodium Triple-Quantum MR Signal. Processes, 8(10), 1267. https://doi.org/10.3390/pr8101267






