Oxygen Transfer Capacity as a Measure of Water Aeration by Floating Reed Plants: Initial Laboratory Studies
Abstract
1. Introduction
2. Materials and Methods
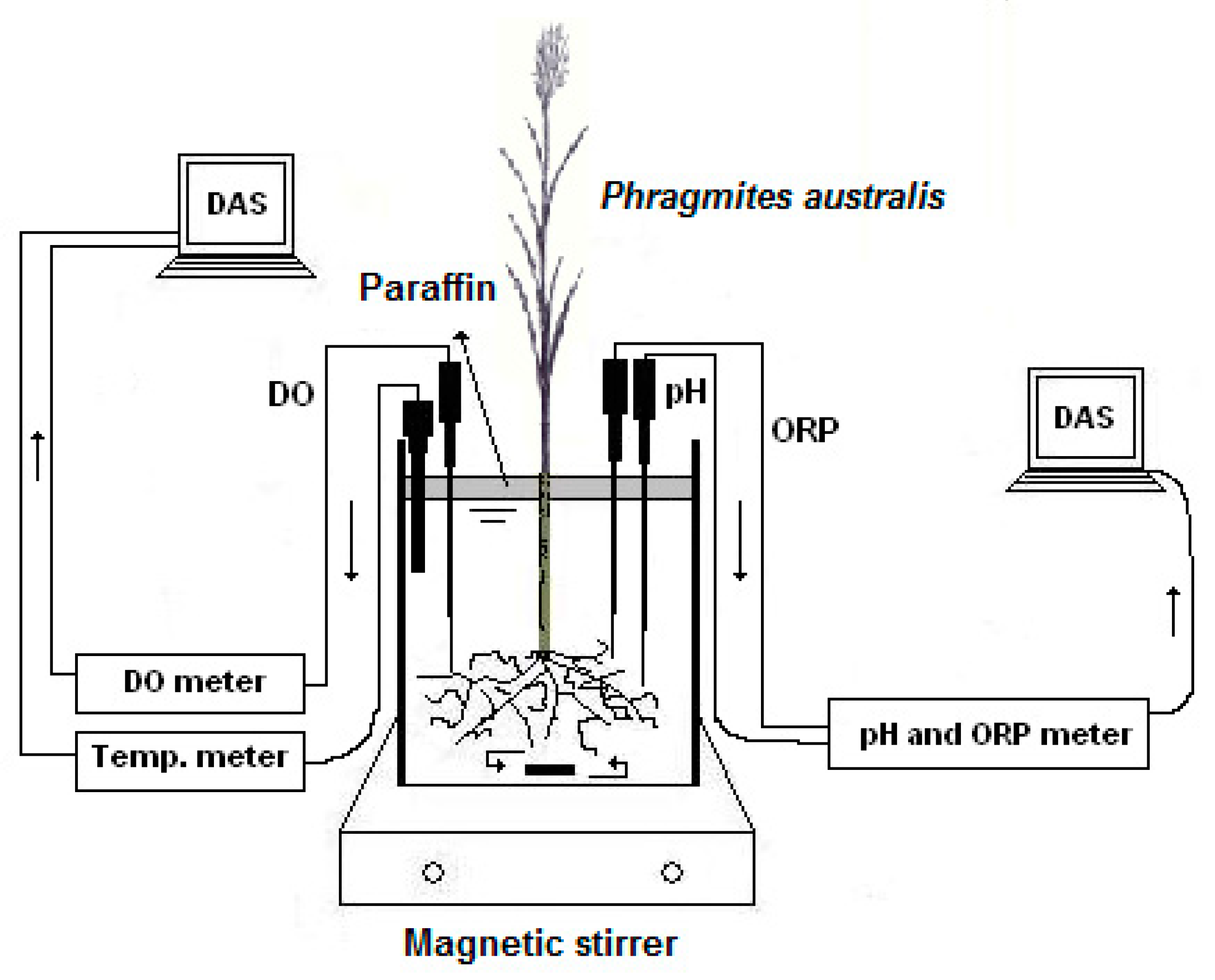
2.1. Experimental Set-Up
2.2. Experimental Procedure
2.3. Oxygen Transfer Capacity (OTC) Determination
3. Results and Discussion
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EPA-United States Environmental Protection Agency. Sources of Greenhouse Gas Emissions. Available online: https://www.epa.gov/ghgemissions/sources-greenhouse-gas-emissions (accessed on 6 October 2020).
- Maurer, D.L.; Koziel, J.A.; Kalus, K.; Andersen, D.S.; Opaliński, S. Pilot-Scale Testing of Non-Activated Biochar for Swine Manure Treatment and Mitigation of Ammonia, Hydrogen Sulfide, Odorous Volatile Organic Compounds (VOCs), and Greenhouse Gas Emissions. Sustainability 2017, 9, 929. [Google Scholar] [CrossRef]
- Headley, T.R.; Tanner, C. Constructed Wetlands with Floating Emergent Macrophytes: An Innovative Stormwater Treatment Technology. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2261–2310. [Google Scholar] [CrossRef]
- Wießner, A.; Kuschk, P.; Stottmeister, U. Oxygen Release by Roots of Typha latifolia and Juncus effusus in Laboratory Hydroponic Systems. Acta Biotechnol. 2002, 22, 209–216. [Google Scholar] [CrossRef]
- Pezeshki, S.R.; Delaune, R.D. Soil Oxidation-Reduction in Wetlands and Its Impact on Plant Functioning. Biology 2012, 1, 196–221. [Google Scholar] [CrossRef] [PubMed]
- Randerson, P.F.; Moran, C.; Białowiec, A. Oxygen transfer capacity of willow (Salix viminalis L.). Biomass-Bioenergy 2011, 35, 2306–2309. [Google Scholar] [CrossRef]
- Allen, W.; Hook, P.B.; Biederman, J.A.; Stein, O.R. Temperature and Wetland Plant Species Effects on Wastewater Treatment and Root Zone Oxidation. J. Environ. Qual. 2002, 31, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.; Armstrong, W.; Beckett, P.M. Phragmites australis: Venturi- and humidity-induced pressure flows enhance rhizome aeration and rhizosphere oxidation. New Phytol. 1992, 120, 197–207. [Google Scholar] [CrossRef]
- Armstrong, J.; Armstrong, W. Light-enhanced convective throughflow increases oxygenation in rhizomes and rhizosphere of Phragmites australis (Cav.) Trin. ex Steud. New Phytol. 1990, 114, 121–128. [Google Scholar] [CrossRef]
- Randerson, P.F. Constructed wetlands and vegetation filters: An ecological approach to wastewater treatment. Environ. Biotechnol. 2006, 2, 78–89. [Google Scholar]
- Williams, H.G.; Białowiec, A.; Slater, F.; Randerson, P.F. Diurnal cycling of dissolved gas concentrations in a willow vegetation filter treating landfill leachate. Ecol. Eng. 2010, 36, 1680–1685. [Google Scholar] [CrossRef]
- Albert, D.A.; Cox, D.T.; Lemein, T.; Yoon, H.-D. Characterization of Schoenoplectus pungens in a Great Lakes Coastal Wetland and a Pacific Northwestern Estuary. Wetlands 2013, 33, 445–458. [Google Scholar] [CrossRef]
- Li, S.; Pezeshki, S.R.; Shields, F.D. Partial flooding enhances aeration in adventitious roots of black willow (Salix nigra) cuttings. J. Plant Physiol. 2006, 163, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, W.; Armstrong, J. Stem Photosynthesis not Pressurized Ventilation is Responsible for Light-enhanced Oxygen Supply to Submerged Roots of Alder (Alnus glutinosa). Ann. Bot. 2005, 96, 591–612. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grosse, W.; Frye, J.; Lattermann, S. Root aeration in wetland trees by pressurized gas transport. Tree Physiol. 1992, 10, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Grosse, W.; Schulte, A.; Fujita, H. Pressurized gas transport in two Japanese alder species in relation to their natural habitats. Ecol. Res. 1993, 8, 151–158. [Google Scholar] [CrossRef]
- Grosse, W.; Jovy, K.; Tiebel, H. Influence of plants on redox potential and methane production in water-saturated soil. Hydrobiologia 1996, 340, 93–99. [Google Scholar] [CrossRef]
- Armstrong, W.; Armstrong, J.; Beckett, P. Measurement and Modelling of Oxygen Release from Roots of Phragmites Australis. In Constructed Wetlands in Water Pollution Control; Elsevier BV: Amsterdam, The Netherlands, 1990; pp. 41–51. [Google Scholar]
- Barco, A.; Bona, S.; Borin, M. Plant species for floating treatments wetlands: A decade of experiments in North Italy. Sci. Total Environ. 2020, 751. [Google Scholar] [CrossRef]
- Albuquerque, A.; Oliveira, J.; Semitela, S.; Amaral, L.M.M. Influence of bed media characteristics on ammonia and nitrate removal in shallow horizontal subsurface flow constructed wetlands. Bioresour. Technol. 2009, 100, 6269–6277. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, E. Oxygen Transport during Aeration Processes; Chemical technology manual; Gdansk University of Technology, Department of Chemical Technology: Gdansk, Poland, 1998. (In Polish) [Google Scholar]
- Kadlec, R.; Knight, R.; Vymazal, J.; Brix, H.; Cooper, P.; Haberl, R. Constructed Wetlands for Pollution Control: Processes, Performance, Design and Operation; Report No. 8; IWA Publishing: London, UK, 2000. [Google Scholar]
- Vymazal, J.; Kröpfelová, L. Wastewater Treatment in Constructed Wetlands with Horizontal Sub-Surface Flow. Environ. Pollut. 2008, 14. [Google Scholar] [CrossRef]
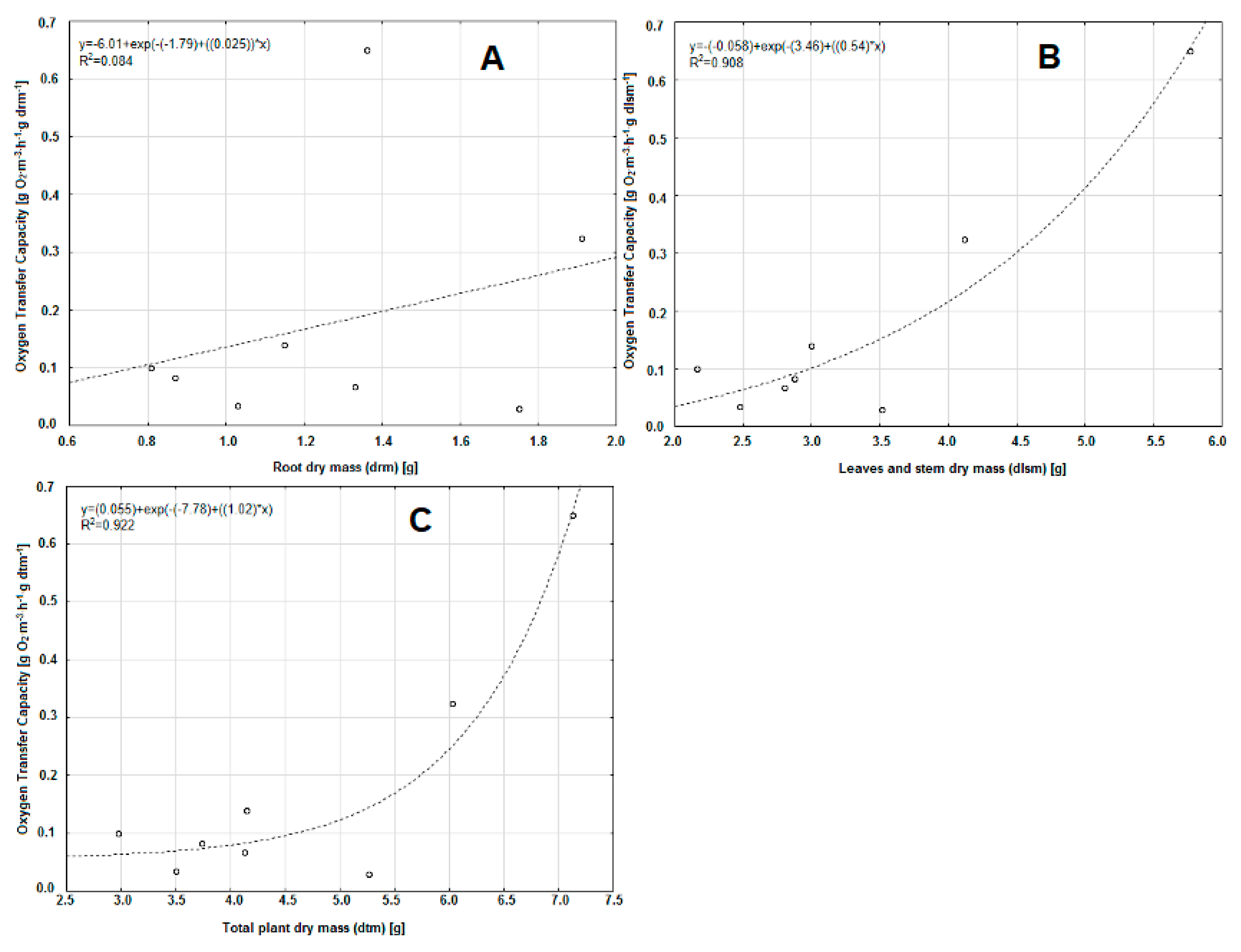
| Plant Number | Oxygen Transfer Capacity | Root Dry Mass (drm) | Leaves and Stem Dry Mass (dlsm) | Total Plant Dry Mass (dtm) | |||
|---|---|---|---|---|---|---|---|
| (g O2·m−3· h−1·plant−1) | (g O2·m−3· h−1·g drm−1) | (g O2·m−3· h−1·g dlsm−1) | (g O2·m−3· h−1·g dtm−1) | (g) | (g) | (g) | |
| 1 | 0.65 | 477.49 | 112.55 | 91.08 | 1.36 | 5.77 | 7.13 |
| 2 | 0.10 | 122.68 | 46.01 | 33.46 | 0.81 | 2.16 | 2.97 |
| 3 | 0.08 | 95.63 | 28.99 | 22.24 | 0.87 | 2.87 | 3.74 |
| 4 | 0.03 | 33.66 | 14.03 | 9.90 | 1.03 | 2.47 | 3.50 |
| 5 | 0.32 | 169.39 | 78.53 | 53.65 | 1.91 | 4.12 | 6.03 |
| 6 | 0.14 | 121.74 | 46.67 | 33.73 | 1.15 | 3.00 | 4.15 |
| 7 | 0.07 | 50.39 | 23.94 | 16.23 | 1.33 | 2.80 | 4.13 |
| 8 | 0.03 | 17.17 | 8.56 | 5.71 | 1.75 | 3.51 | 5.26 |
| Mean | 0.18 | 136.02 | 44.91 | 33.25 | 1.28 | 3.34 | 4.61 |
| Standard deviation | 0.21 | 147.19 | 35.21 | 27.97 | 0.40 | 1.15 | 1.41 |
| Variation coefficient | 119.62 | 108.22 | 78.40 | 84.11 | 30.99 | 34.57 | 30.55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albuquerque, A.; Randerson, P.; Białowiec, A. Oxygen Transfer Capacity as a Measure of Water Aeration by Floating Reed Plants: Initial Laboratory Studies. Processes 2020, 8, 1270. https://doi.org/10.3390/pr8101270
Albuquerque A, Randerson P, Białowiec A. Oxygen Transfer Capacity as a Measure of Water Aeration by Floating Reed Plants: Initial Laboratory Studies. Processes. 2020; 8(10):1270. https://doi.org/10.3390/pr8101270
Chicago/Turabian StyleAlbuquerque, Antonio, Peter Randerson, and Andrzej Białowiec. 2020. "Oxygen Transfer Capacity as a Measure of Water Aeration by Floating Reed Plants: Initial Laboratory Studies" Processes 8, no. 10: 1270. https://doi.org/10.3390/pr8101270
APA StyleAlbuquerque, A., Randerson, P., & Białowiec, A. (2020). Oxygen Transfer Capacity as a Measure of Water Aeration by Floating Reed Plants: Initial Laboratory Studies. Processes, 8(10), 1270. https://doi.org/10.3390/pr8101270







