Influence of Granular Activated Carbon on Anaerobic Co-Digestion of Sugar Beet Pulp and Distillers Grains with Solubles
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Biochemical Methane Potential Experiments
2.3. Analytical Methods
2.4. Microbial Community Structure
3. Results and Discussion
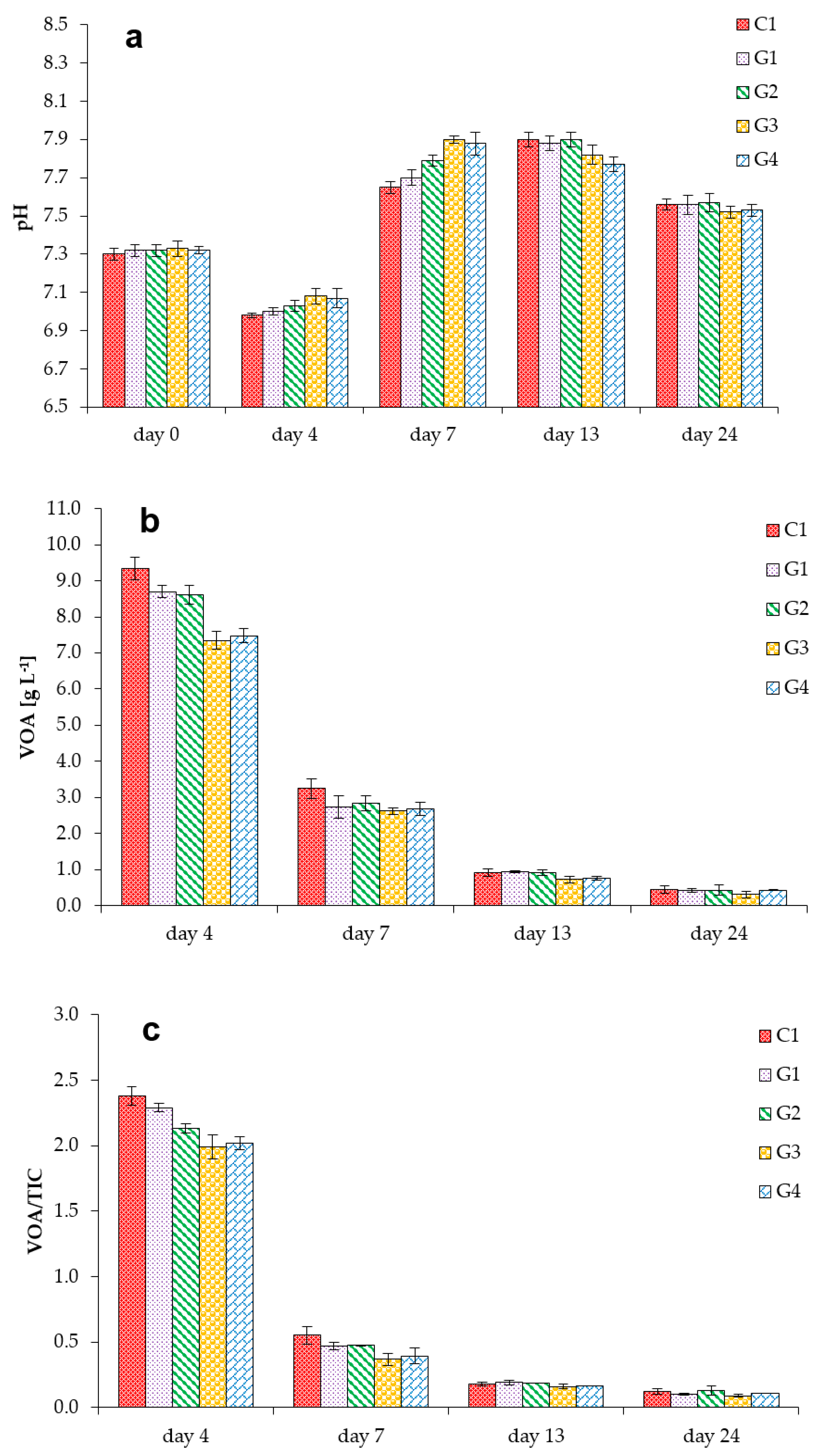
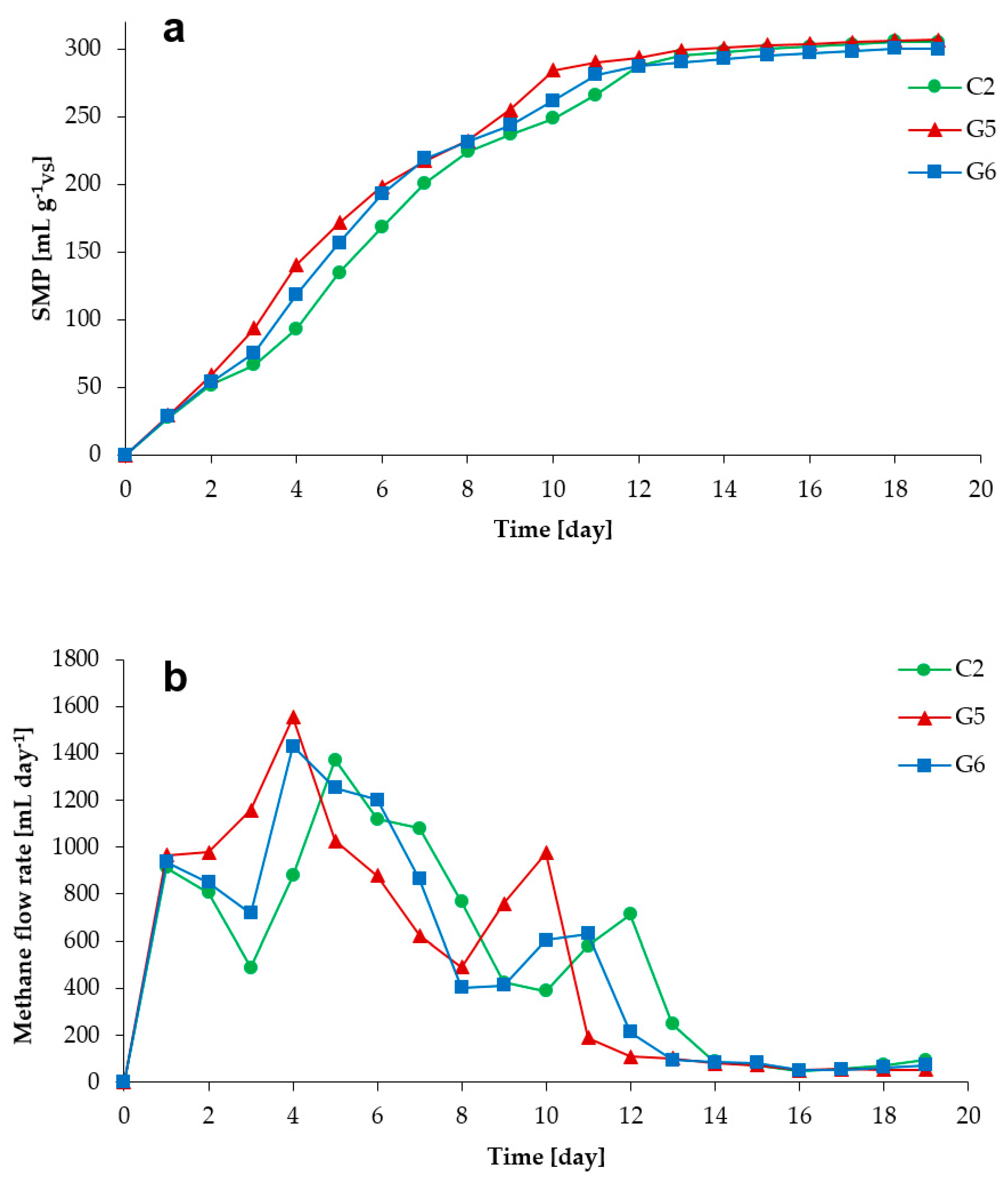
3.1. Process Stability and Methane Production
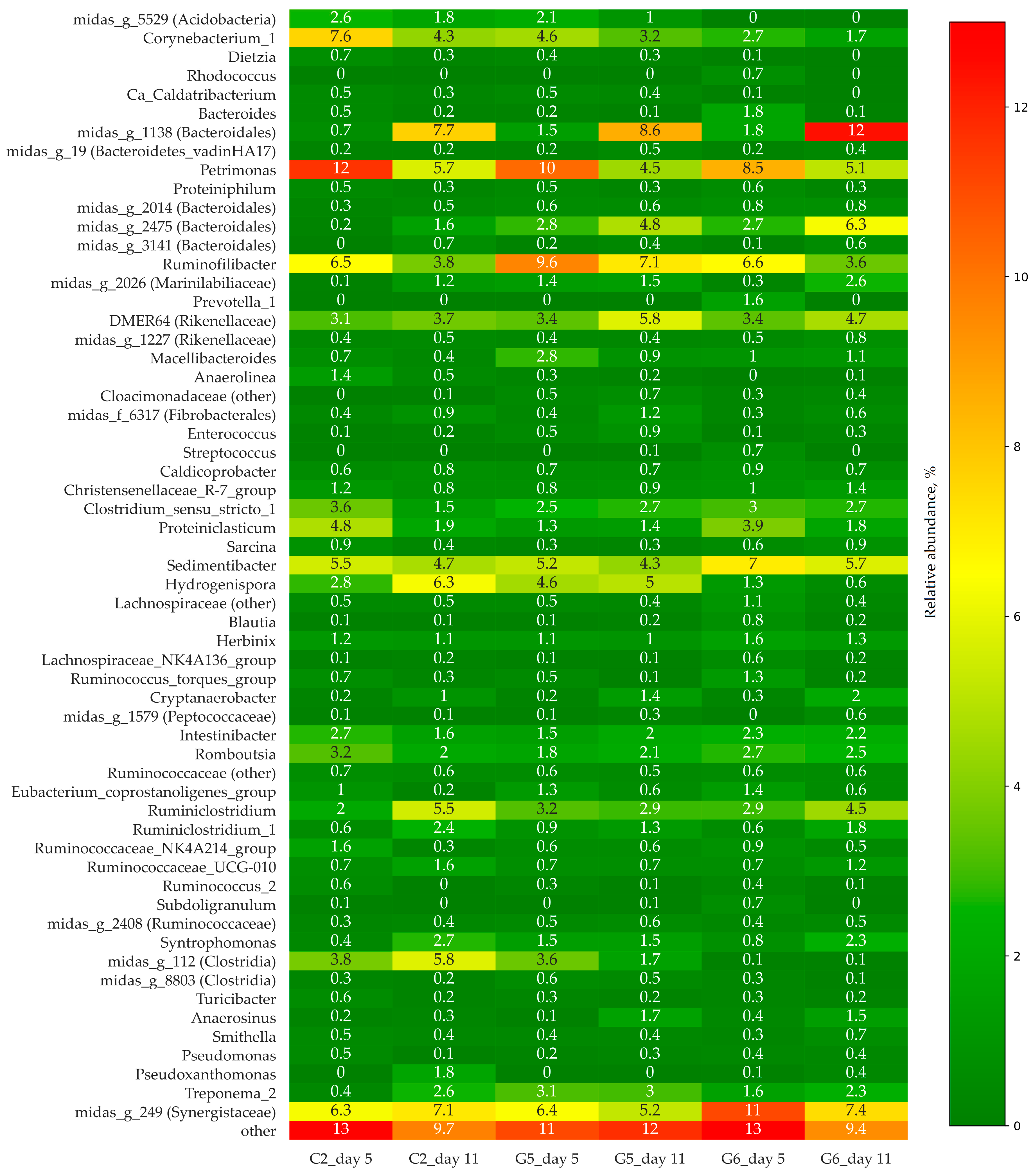
3.2. Bacterial Community Structure
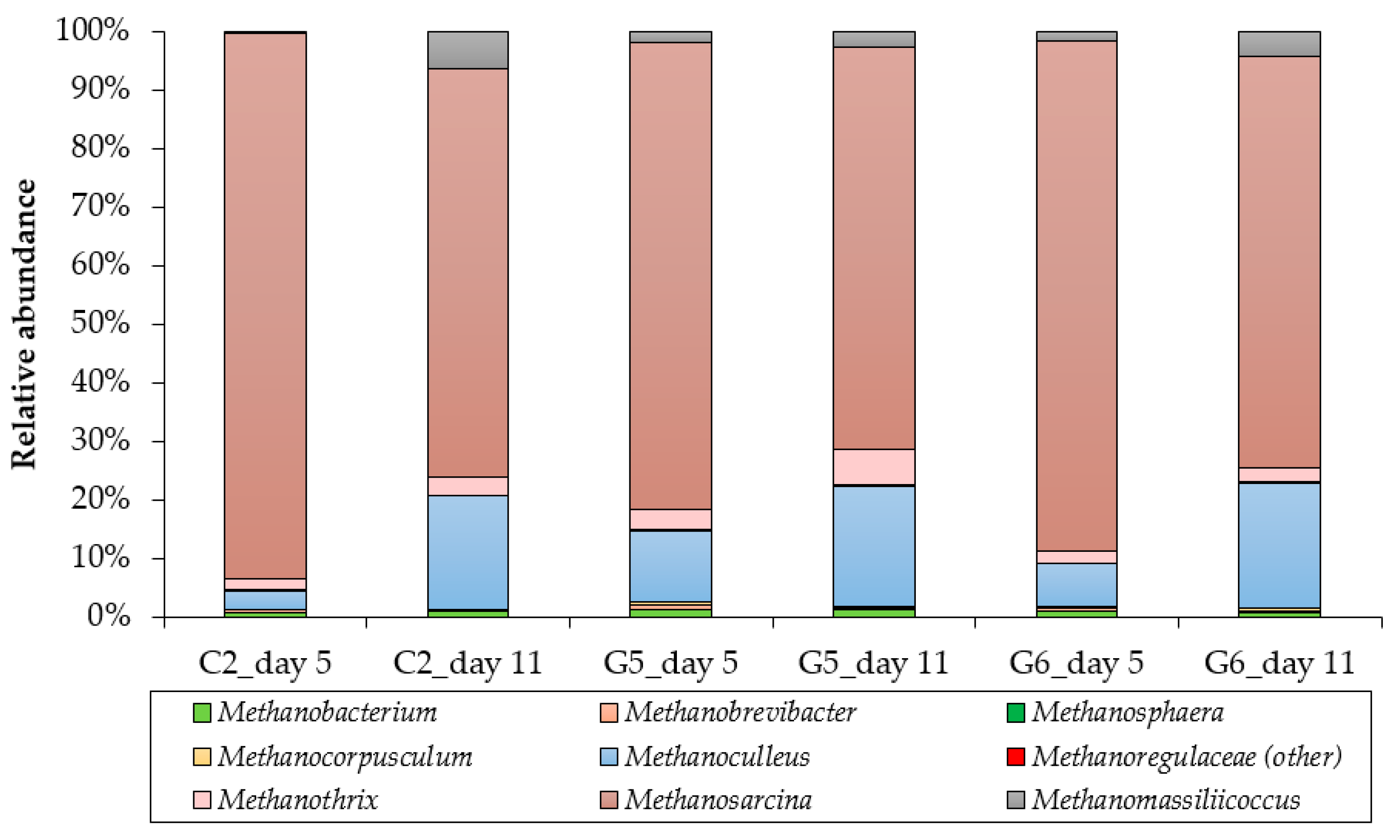
3.3. Archaeal Community Structure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maurya, R.; Tirkey, S.R.; Rajapitamahuni, S.; Ghosh, A.; Mishra, S. Chapter 9—Recent advances and future prospective of biogas production. In Advances in Feedstock Conversion Technologies for Alternative Fuels and Bioproducts, 1st ed.; Hosseini, M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 159–178. [Google Scholar] [CrossRef]
- Siciliano, A.; Limonti, C.; Curcio, G.M.; Calabrò, V. Biogas generation through anaerobic digestion of compost leachate in semi-continuous completely stirred tank reactors. Processes 2019, 7, 635. [Google Scholar] [CrossRef]
- Holder, N.; Mota-Meira, M.; Born, J.; Sutrina, S.L. Bio-methane production via anaerobic co-digestion by optimizing the mixing ratios of river tamarind (Leucaena leucocephala) and dolphin fish (Coryphaena hippurus) offal. Processes 2020, 8, 934. [Google Scholar] [CrossRef]
- Cruz-Salomón, A.; Ríos-Valdovinos, E.; Pola-Albores, F.; Lagunas-Rivera, S.; Cruz-Rodríguez, R.I.; Cruz-Salomón, K.C.; Hernández-Méndez, J.M.E.; Domínguez-Espinosa, M.E. Treatment of cheese whey wastewater using an expanded granular sludge bed (EGSB) bioreactor with biomethane production. Processes 2020, 8, 931. [Google Scholar] [CrossRef]
- Berlowska, J.; Binczarski, M.; Dziugan, P.; Wilkowska, A.; Kregiel, D.; Witonska, I. Chapter 13—Sugar beet pulp as a source of valuable biotechnological products. In Advances in Biotechnology for Food Industry, Handbook of Food Bioengineering; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: London, UK, 2018; pp. 359–392. [Google Scholar] [CrossRef]
- Murphy, J.D.; Power, N.M. How can we improve the energy balance of ethanol production from wheat? Fuel 2008, 87, 1799–1806. [Google Scholar] [CrossRef]
- Wirth, R.; Kovacs, E.; Maroti, G.; Bagi, Z.; Rakhely, G.; Kovacs, K.L. Characterization of a biogas-producing microbial community by short-read next generation DNA sequencing. Biotechnol. Biofuels. 2012, 5, 41–57. [Google Scholar] [CrossRef]
- Li, Y.F.; Chen, P.H.; Yu, Z. Spatial and temporal variations of microbial community in a mixed plug-flow loop reactor fed with dairy manure. J. Microb. Biotechnol. 2014, 7, 332–346. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Ibragimov, E.M.; Vankov, P.Y.; Miluykov, V.A.; Ziganshin, A.M. Comparison of anaerobic digestion strategies of nitrogen-rich substrates: Performance of anaerobic reactors and microbial community diversity. Waste Manag. 2017, 59, 160–171. [Google Scholar] [CrossRef]
- Ziganshin, A.M.; Wintsche, B.; Seifert, J.; Carstensen, M.; Born, J.; Kleinsteuber, S. Spatial separation of metabolic stages in a tube anaerobic baffled reactor: Reactor performance and microbial community dynamics. Appl. Microbiol. Biotechnol. 2019, 103, 3915–3929. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Li, Z.; Zhao, Z.; Quan, X.; Zhao, Z. Adding granular activated carbon into anaerobic sludge digestion to promote methane production and sludge decomposition. J. Clean. Prod. 2017, 149, 1101–1108. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Moscoviz, R.; Ruiz, D.; Santa-Catalina, G.; Trably, E.; Rouez, M.; Crest, M.; Steyer, J.P.; Bernet, N.; Delgenes, J.P.; et al. Addition of granular activated carbon and trace elements to favor volatile fatty acid consumption during anaerobic digestion of food waste. Bioresour. Technol. 2018, 260, 157–168. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, Y.; Tan, D.; Zhao, Z.; Zhao, H.; Quan, X. Roles of magnetite and granular activated carbon in improvement of anaerobic sludge digestion. Bioresour. Technol. 2018, 249, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.M.; Choi, Y.K.; Kan, E. Effects of dairy manure-derived biochar on psychrophilic, mesophilic and thermophilic anaerobic digestions of dairy manure. Bioresour. Technol. 2018, 250, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Wambugu, C.W.; Rene, E.R.; Vossenberg, J.; Dupont, C.; Hullebusch, E.D. Role of biochar in anaerobic digestion based biorefinery for food waste. Front. Energy Res. 2019, 7, 14. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Dai, L.; Dong, B.; Dai, X. Magnetite triggering enhanced direct interspecies electron transfer: A scavenger for the blockage of electron transfer in anaerobic digestion of high-solids sewage sludge. Environ. Sci. Technol. 2018, 52, 7160–7169. [Google Scholar] [CrossRef] [PubMed]
- Marsh, H.; Reinoso, F.R. Activated Carbon, 1st ed.; Elsevier Science: London, UK, 2006; pp. 1–554. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Chen, S.; Rotaru, A.E.; Shrestha, P.M.; Malvankar, N.S.; Liu, F.; Fan, W.; Nevin, K.P.; Lovley, D.R. Promoting interspecies electron transfer with biochar. Sci. Rep. 2014, 4, 5019. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Wang, J.; Chen, T.; Tan, J.; Wang, G. Methanogenic and carbon sequestration process facilitated by goethite and hematite in the presence of dissimilatory iron-reducing bacteria. Fresen. Environ. Bull. 2016, 25, 1883–1891. [Google Scholar]
- Ziganshina, E.E.; Belostotskiy, D.E.; Shushlyaev, R.V.; Miluykov, V.A.; Vankov, P.Y.; Ziganshin, A.M. Microbial community diversity in anaerobic reactors digesting turkey, chicken, and swine wastes. J. Microbiol. Biotechnol. 2014, 24, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, W.S.; Ziganshina, E.E.; Shagimardanova, E.I.; Gogoleva, N.E.; Ziganshin, A.M. Comparison of intestinal bacterial and fungal communities across various xylophagous beetle larvae (Coleoptera: Cerambycidae). Sci. Rep. 2018, 8, 10073. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Mohammed, W.S.; Shagimardanova, E.I.; Vankov, P.Y.; Gogoleva, N.E.; Ziganshin, A.M. Fungal, bacterial, and archaeal diversity in the digestive tract of several beetle larvae (Coleoptera). Biomed Res. Int. 2018, 2018, 6765438. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Nierychlo, M.; Andersen, K.S.; Xu, Y.; Green, N.; Jiang, C.; Albertsen, M.; Dueholm, M.S.; Nielsen, P.H. MiDAS 3: An ecosystem-specific reference database, taxonomy and knowledge platform for activated sludge and anaerobic digesters reveals species-level microbiome composition of activated sludge. Water Res. 2020, 182, 115955. [Google Scholar] [CrossRef] [PubMed]
- Miroshnichenko, I.; Lindner, J.; Lemmer, A.; Oechsner, H.; Vasilenko, I. Anaerobic digestion of sugar beet pulp in Russia. Landtechnik 2016, 71, 175–185. [Google Scholar] [CrossRef]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 1–192. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Biotechnol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Fagbohungbe, M.O.; Herbert, B.M.J.; Hurst, L.; Ibeto, C.N.; Li, H.; Usmani, S.Q.; Semple, K.T. The challenges of anaerobic digestion and the role of biochar in optimizing anaerobic digestion. Waste Manag. 2017, 61, 236–249. [Google Scholar] [CrossRef]
- Dang, Y.; Holmes, D.E.; Zhao, Z.; Woodard, T.L.; Zhang, Y.; Sun, D.; Wang, L.-Y.; Nevin, K.P.; Lovley, D.R. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour. Technol. 2016, 220, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Syntrophy goes electric: Direct interspecies electron transfer. Annu. Rev. Microbiol. 2017, 71, 643–664. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Zakaria, B.S.; Lin, L.; Dhar, B.R. Magnetite doped granular activated carbon as an additive for high-performance anaerobic digestion. Mater. Sci. Energy Technol. 2019, 2, 377–384. [Google Scholar] [CrossRef]
- Bernard, K.A.; Wiebe, D.; Burdz, T.; Reimer, A.; Ng, B.; Singh, C.; Schindle, S.; Pacheco, A.L. Assignment of Brevibacterium stationis (ZoBell and Upham 1944) Breed 1953 to the genus Corynebacterium, as Corynebacterium stationis comb. nov., and emended description of the genus Corynebacterium to include isolates that can alkalinize citrate. Int. J. Syst. Evol. Microbiol. 2010, 60, 874–879. [Google Scholar] [CrossRef]
- Grabowski, A.; Tindall, B.J.; Bardin, V.; Blanchet, D.; Jeanthon, C. Petrimonas sulfuriphila gen. nov., sp. nov., a mesophilic fermentative bacterium isolated from a biodegraded oil reservoir. Int. J. Syst. Evol. Microbiol. 2005, 55, 1113–1121. [Google Scholar] [CrossRef]
- Kröber, M.; Bekel, T.; Diaz, N.N.; Goesmann, A.; Jaenicke, S.; Krause, L.; Miller, D.; Runte, K.J.; Viehöver, P.; Pühler, A.; et al. Phylogenetic characterization of a biogas plant microbial community integrating clone library 16S-rRNA sequences and metagenome sequence data obtained by 454-pyrosequencing. J. Biotechnol. 2009, 142, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Zankel, A.; Lebuhn, M.; Petrak, S.; Somitsch, W.; Guebitz, G.M. Investigation of microorganisms colonising activated zeolites during anaerobic biogas production from grass silage. Bioresour. Technol. 2011, 102, 4353–4359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Song, L.; Dong, X. Proteiniclasticum ruminis gen. nov., sp. nov., a strictly anaerobic proteolytic bacterium isolated from yak rumen. Int. J. Syst. Evol. Microbiol. 2010, 60, 2221–2225. [Google Scholar] [CrossRef] [PubMed]
- Breitenstein, A.; Wiegel, J.; Haertig, C.; Weiss, N.; Andreesen, J.R.; Lechner, U. Reclassification of Clostridium hydroxybenzoicum as Sedimentibacter hydroxybenzoicus gen. nov., comb. nov., and description of Sedimentibacter saalensis sp. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 801–807. [Google Scholar] [CrossRef]
- Imachi, H.; Sakai, S.; Kubota, T.; Miyazaki, M.; Saito, Y.; Takai, K. Sedimentibacter acidaminivorans sp. nov., an anaerobic, amino-acid-utilizing bacterium isolated from marine subsurface sediment. Int. J. Syst. Evol. Microbiol. 2016, 66, 1293–1300. [Google Scholar] [CrossRef]
- Chung, K.T. Inhibitory effects of H2 on growth of Clostridium cellobioparum. Appl. Environ. Microbiol. 1976, 31, 342–348. [Google Scholar] [CrossRef]
- Lee, J.; Koo, T.; Yulisa, A.; Hwang, S. Magnetite as an enhancer in methanogenic degradation of volatile fatty acids under ammonia-stressed condition. J. Environ. Manag. 2019, 241, 418–426. [Google Scholar] [CrossRef]
- Roy, F.; Samain, E.; Dubourguier, H.C.; Albagnac, G. Synthrophomonas sapovorans sp. nov., a new obligately proton reducing anaerobe oxidizing saturated and unsaturated long chain fatty acids. Arch. Microbiol. 1986, 145, 142–147. [Google Scholar] [CrossRef]
- McInerney, M.J.; Bryant, M.P.; Hespell, R.B.; Costerton, J.W. Syntrophomonas wolfei gen. nov. sp. nov., an anaerobic, syntrophic, fatty acid-oxidizing bacterium. Appl. Environ. Microbiol. 1981, 41, 1029–1039. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Liu, P.; Fu, L.; Ding, D.; Lu, Y. Direct interspecies electron transfer accelerates syntrophic oxidation of butyrate in paddy soil enrichments. Environ. Microbiol. 2015, 17, 1533–1547. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, Y. Conductive Fe3O4 nanoparticles accelerate syntrophic methane production from butyrate oxidation in two different lake sediments. Front. Microbiol. 2016, 7, 1316. [Google Scholar] [CrossRef]
- Salvador, A.F.; Martins, G.; Mellefranco, M.; Serpa, R.; Ajm, S.; Cavaleiro, A.J.; Pereira, M.A.; Alves, M.M. Carbon nanotubes accelerate methane production in pure cultures of methanogens and in a syntrophic coculture. Environ. Microbiol. 2017, 19, 2727–2739. [Google Scholar] [CrossRef] [PubMed]
- Sowers, K.R.; Baron, S.F.; Ferry, J.G. Methanosarcina acetivorans sp. nov., an acetotrophic methane-producing bacterium isolated from marine sediments. Appl. Environ. Microbiol. 1984, 47, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Ganzert, L.; Schirmack, J.; Alawi, M.; Mangelsdorf, K.; Sand, W.; Hillebrand-Voiculescu, A.; Wagner, D. Methanosarcina spelaei sp. nov., a methanogenic archaeon isolated from a floating biofilm of a subsurface sulphurous lake. Int. J. Syst. Evol. Microbiol. 2014, 64, 3478–3484. [Google Scholar] [CrossRef]
- Dianou, D.; Miyaki, T.; Asakawa, S.; Morii, H.; Nagaoka, K.; Oyaizu, H.; Matsumoto, S. Methanoculleus chikugoensis sp. nov., a novel methanogenic archaeon isolated from paddy field soil in Japan, and DNA-DNA hybridization among Methanoculleus species. Int. J. Syst. Evol. Microbiol. 2001, 51, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.B.; Sprott, G.D. Methanosaeta concilii gen. nov., sp. nov. Methanothrix concilii and Methanosaeta thermoacetophila nom. rev., comb. nov. Int. J. Syst. Evol. Microbiol. 1990, 40, 79–82. [Google Scholar] [CrossRef]
- Kröninger, L.; Gottschling, J.; Deppenmeier, U. Growth characteristics of Methanomassiliicoccus luminyensis and expression of methyltransferase encoding genes. Archaeans 2017, 2017, 2756573. [Google Scholar] [CrossRef]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, K.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef]



| Reactor | Day 4 | Day 24 | Reactor | Day 5 | Day 19 |
|---|---|---|---|---|---|
| C1 | 0.950 ± 0.02 | 1.205 ± 0.10 | C2 | 0.970 ± 0.04 | 1.020 ± 0.05 |
| G1 | 0.972 ± 0.02 | 1.244 ± 0.07 | G5 | 0.977 ± 0.02 | 1.001 ± 0.08 |
| G2 | 0.988 ± 0.04 | 1.218 ± 0.11 | G6 | 0.974 ± 0.03 | 1.005 ± 0.09 |
| G3 | 1.002 ± 0.03 | 1.269 ± 0.06 | |||
| G4 | 0.996 ± 0.04 | 1.239 ± 0.08 |
| Sample | Bacteria | Archaea | ||||||
|---|---|---|---|---|---|---|---|---|
| OTUs | Chao1 | Shannon | Simpson | OTUs | Chao1 | Shannon | Simpson | |
| C2 (day 5) | 508 | 538 | 6.79 | 0.98 | 82 | 94 | 1.63 | 0.56 |
| C2 (day 11) | 417 | 479 | 6.65 | 0.98 | 92 | 96 | 2.88 | 0.76 |
| G5 (day 5) | 523 | 552 | 6.77 | 0.98 | 93 | 99 | 2.51 | 0.69 |
| G5 (day 11) | 513 | 571 | 6.74 | 0.98 | 93 | 93 | 2.91 | 0.76 |
| G6 (day 5) | 558 | 576 | 7.00 | 0.98 | 88 | 88 | 2.12 | 0.63 |
| G6 (day 11) | 502 | 534 | 6.47 | 0.97 | 85 | 87 | 2.78 | 0.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziganshina, E.E.; Belostotskiy, D.E.; Bulynina, S.S.; Ziganshin, A.M. Influence of Granular Activated Carbon on Anaerobic Co-Digestion of Sugar Beet Pulp and Distillers Grains with Solubles. Processes 2020, 8, 1226. https://doi.org/10.3390/pr8101226
Ziganshina EE, Belostotskiy DE, Bulynina SS, Ziganshin AM. Influence of Granular Activated Carbon on Anaerobic Co-Digestion of Sugar Beet Pulp and Distillers Grains with Solubles. Processes. 2020; 8(10):1226. https://doi.org/10.3390/pr8101226
Chicago/Turabian StyleZiganshina, Elvira E., Dmitry E. Belostotskiy, Svetlana S. Bulynina, and Ayrat M. Ziganshin. 2020. "Influence of Granular Activated Carbon on Anaerobic Co-Digestion of Sugar Beet Pulp and Distillers Grains with Solubles" Processes 8, no. 10: 1226. https://doi.org/10.3390/pr8101226
APA StyleZiganshina, E. E., Belostotskiy, D. E., Bulynina, S. S., & Ziganshin, A. M. (2020). Influence of Granular Activated Carbon on Anaerobic Co-Digestion of Sugar Beet Pulp and Distillers Grains with Solubles. Processes, 8(10), 1226. https://doi.org/10.3390/pr8101226




