Abstract
Flavonoids have a broad spectrum of established positive effects on human and animal health. They find an application in medicine for disease therapy and chemoprevention, whence the interest in flavonoids increases. In addition, they are used in food and cosmetic industries as pigments and biopreservatives. Plants are an inexhaustible source of flavonoids. The most important step of plant raw material processing is extraction and isolation of target compounds. The quality of an extract and efficiency of a procedure are influenced by several factors: Plant material and pre-extracting sample preparation, type of solvent, extraction technique, physicochemical conditions, etc. The present overview discusses the common problems and key challenges of the extraction procedures and the different mechanisms for selective extraction of flavonoids from different plant sources. In summary, there is no universal extraction method and each optimized procedure is individual for the respective plants. For an extraction technique to be selective, it must combine an optimal solvent or mixture of solvents with an appropriate technique. Last but not least, its optimization is important for a variety of applications. Moreover, when the selected method needs to be standardized, it must achieve acceptable degree of repeatability and reproducibility.
1. Introduction
Flavonoids are small molecules, produced de novo by plants as secondary metabolites in response to diverse biotic and abiotic factors. These chemical compounds have a broad spectrum of established health-promoting effects [1,2,3,4]. They are due to their antioxidative, anti-inflammatory, anti-mutagenic, and anti-carcinogenic properties coupled with their capacity to modulate key cellular enzyme functions. They are also known as potent inhibitors of several enzymes, such as xanthine oxidase (XO), cyclo-oxygenase (COX), lipoxygenase, and phosphoinositide 3-kinase [5,6]. The antioxidant potential of flavonoids is due to their ability to scavenge free radicals, as indicated by Korkina and Afanasev [7]. Kerry and Abbey [8] reported that by scavenging radicals, flavonoids can inhibit low-density lipoprotein (LDL) oxidation in in vitro studies. They further mentioned that this action protects the LDL particles and, theoretically, flavonoids may have preventive action against atherosclerosis. Flavonoids can build complex by chelation metal ions, which can be crucial in the prevention of radical generation, which damages some target biomolecules [2,9]. In a review, Cushnie and Lamb (2006) described their antibacterial, antifungal, and antiviral activity, as well as the synergy between the antimicrobial activity of several groups of flavonoids and between flavonoids and existing chemotherapeutics [10]. Because of their positive effects on human and animal health and medical application for disease therapy and chemoprevention, interest in flavonoids is increasing [1,3,4]. Furthermore, they are used in the food and cosmetic industries as pigments and biopreservatives [8].
Flavonoids are widely distributed chemical compounds in the plant kingdom. Plants are therefore an inexhaustible source of flavonoids. The most important step in plant raw materials processing is the extraction and isolation of target compounds. The quality of an extract and the efficiency of a procedure are influenced by several factors: Plant material and pre-extracting sample preparation, solvent type, extraction technique, physicochemical conditions, etc. The classical methods (maceration and Soxhlet extraction) are generally used in research laboratories or in small manufacturing companies. Microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), and supercritical fluid extraction (SFE) belong to a group of modern methods. They are very promising: The yield is increased at a lower cost [11].
A lot of research teams compared classical modern methods for the extraction of flavonoids from plant materials and discussed their advantages and disadvantages [4,11,12,13,14,15,16]. But the focus was not on the selectivity of the reviewed methods. Moreso, the plant matrix is complex, and the extraction produces a cocktail of substances. When the goal is to produce an extract with a narrow range of physiological effects, the first step of sample preparation and extraction is critical. Therefore, the solvents and the technique for extracting must be carefully selected. The potential role of selective extraction methods has a significant importance for the subsequent steps of the extract production [17]. The selectivity of this first extraction step contributes to the effectivity, profitability, and simplicity of the whole process of extract producing. This overview discusses common problems and key challenges in extraction procedures, as well as various mechanisms for selective extraction of flavonoids from different plant sources.
2. Flavonoids—Classification and Natural Sources
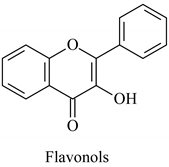
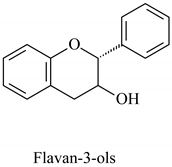
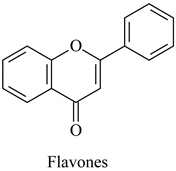
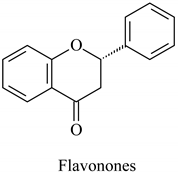
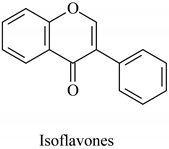
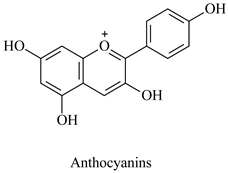
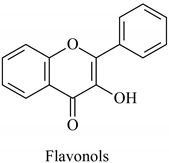
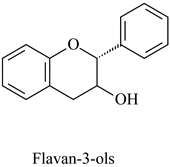
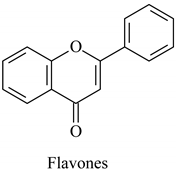
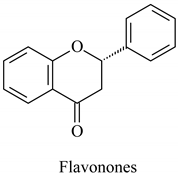
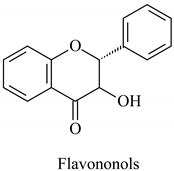
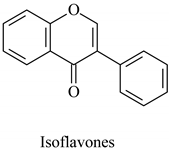
The flavonoid family includes more than 6000 low-molecular-weight phenolic compounds [18], derivatives of flavan (Figure 1). The main subgroups are flavones, flavonols, flavonones, flavononols, flavan-3-ols, anthocyanines, isoflavones, and chalcones.
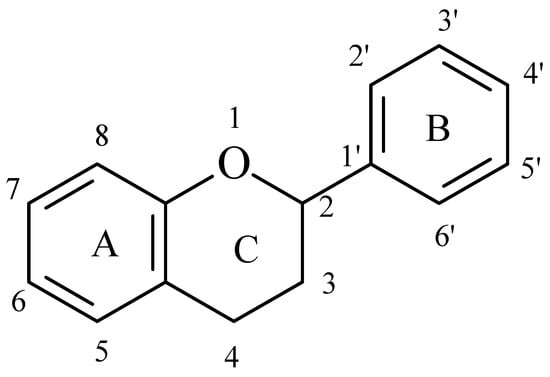
Figure 1.
Flavan—basic structure of flavonoids.
Their biochemical roles are multifarious: From flower pigmentation to taking part in the growing processes of the plant organism, and the defense against diseases [19]. Each flavonoid group plays a unique biochemical role and has a particular distribution in plants [20]: Anthocyanins are responsible for the coloring of the plants, and are therefore found in high amounts in the plant flowers and the skins of colored fruits; flavan-3-ols, such as catechin, epicatechin gallate, etc., are found in high concentrations in green tea; isoflavones are found in legumes (e.g., soybean); flavanones, in citrus fruits; flavones, in green leafy spices (e.g., parsley); and flavonols, in most plants. Chalcones are a subclass of flavonoids. They are characterized by the absence of “ring C” of the basic skeleton structure. Chalcones can be referred to as open-chain flavonoids. Major examples of chalcones include phloridzin, arbutin, phloretin, and chalconaringenin. Chalcones occur in significant amounts in tomatoes, pears, strawberries, bearberries, and certain wheat products [3]. The most popular edible and medicinal plants rich in flavonoids, categorized in subgroups, are systematized in Table 1 and Table 2.

Table 1.
Food plants rich in flavonoids.

Table 2.
Medicinal plants rich in flavonoids.
3. Sample Preparation
Before the beginning of the extraction procedure, the following issues have to be clarified: First—the plant material type, and second—the potential application of the flavonoid extract (Figure 2). In case the plant material is used to produce a standard extract—an extract with established biochemical activity and pharmaceutical application—the standard extraction procedure has to be followed, in order to imitate the traditional “herbal” drug [75]. When the plant is not well known, or the aim of the procedure is the selective separation of the flavonoid fraction—a fraction free of fats, terpenoids, pigments, saponines, alcaloids, etc.—the pre-existing extraction procedure has to be modulated into new one. In this respect, scientific knowledge in this area can be very helpful.
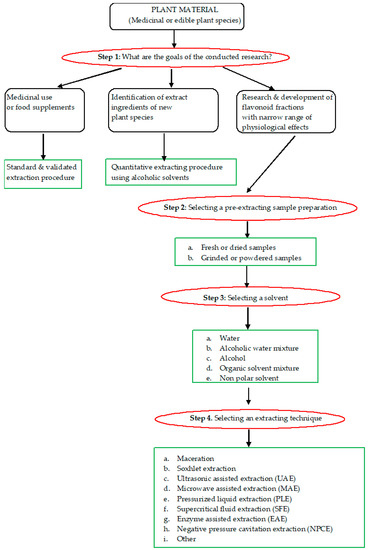
Figure 2.
Flavonoid extraction scheme.
Sample preparation is the first step in the extraction procedure and usually includes two or sometimes three steps: Drying, grinding, and defatting.
Flavonoids, particularly glycosides, can be degraded by enzymes when the plant material is fresh and undried [76]. Later on, the first step is to dry, lyophilize, or freeze the sample. Often the yield of flavonoid conjugates is reduced by the usage of dried plant material. For example, acylated flavonoid glycosides are particularly thermally unstable and are degraded during the process of drying at high temperature, and this first step of the sample utilization is very important for the profiling of this class of natural products in studies of their physiological and biochemical roles in plants [12]. Plant samples, such as leaves, barks, roots, fruits, and flowers, can be extracted from fresh or dried plant material. Often a dried sample is preferred because of the experimental design timing [77].
There are several methods of drying the plant material—air-, microwave-, oven-, and freeze-drying (lyophilisation). Because of their effectivity and efficiency air- and freeze-drying are preferred. Using lyophilization, the phytochemicals are preserved, but this technique is complex and expensive [11]. Air-drying usually takes a long time but does not need any lab equipment, and the heat-labile compounds are preserved. For that reason, shade-drying was preferred by many researchers [68,78,79]. Microwave- and oven-drying require a short time but sometimes cause degradation of plant products and can be applied for drying of no conjugated and thermo-stable flavonoids. Oven-drying at 40–45 °C is considered as a compromising plant material preparation. It is easy to be carried out, and is time consuming [80]. However, even a short time of exposure to high temperatures can destroy some thermo-unstable compounds, and the extract profile is changed [57].
After drying, the plant material must be ground, whereby the cells are destroyed, and the ingredients are able to leach out. Decreasing the particle size enhances surface contact between samples and extraction solvents. A particle size smaller than 0.5 mm is optimal for efficient extraction [81]. The usage of powdered dried plant material leads to effective extraction of flavonoids [67,74,79,82,83].
Often, the target compounds of extracting are unconjugated flavonoids, which are the active compounds [84]. In this case, fresh plant material can be used, which is directly ground and suspended in the selected solvent or mixture of solvents. Flavonoids are extracted without drying and grinding from fruits, such as citrus [85], or from plants, whose pre-extracting preparation includes enzymatic treatment [86].
The presence of lipophilic substances can affect the profile of the composition of flavonoids and their derivatives in the extracts obtained, and in many cases an additional cleaning procedure (e.g., solid-phase extraction) is required [12]. Many researchers started with removing lipophilic compounds from the plant material by using n-hexane [87,88,89] or petroleum ether [67]. The non-polar flavonoids are passed into the liquid fraction. Next follows the extraction with a polar, alcoholic solvent to mobilize the polar flavonoid fractions. The extraction of flavonones and anthocyanes depends on the pH values [76].
4. Extraction Techniques
The choice of the extraction procedure for flavonoids from plant material is crucial and depends on the goals of the conducted research [14]. Some questions have to be answered: Is this fraction polar or non-polar? Are the flavonoids thermally stable or unstable? What amount of extract should be made? What equipment is available and accessible?
Extraction of flavonoids is designed using the similar principles of polyphenols extraction. The process is usually carried out with methanol, ethanol, acetonitrile, acetone, or their mixtures with water [90]. The required solvent polarity depends on the type of the flavonoid [12]. For less polar flavonoid structures such as isoflavons, flavonones, and flavones, the right choice can be acetone, chloroform, methylene chloride, and diethyl ether; for more polar flavonoid fractions, the solvent is usually alcohol or an alcohol–water mixture [76].
The last step is the selection of the extraction technique. There are several options divided into two groups: Conventional techniques (maceration, reflux, and Soxhlet extraction) and modern techniques (extraction by ultrasound, microwaves, pressurized liquid, supercritical fluid, enzyme assistance, matrix solid-phase dispersion, etc).
4.1. Conventional Extraction Techniques
The conventional methods are easy to perform, need no special equipment, are applicable for extraction of a large number of samples, and lead to high yield of the extract obtained. However, they are not selective. Some selectivity can be reached with the right solvent, according to the polarity of the target flavonoid fraction.
4.1.1. Maceration
Maceration is carried out by soaking the ground plant materials in a stoppered container with a proper solvent. The sample is allowed to stand for at least 3 days at room temperature and is shaken frequently. During the process, the solvent softens and breaks the plant’s cell wall and the soluble phytochemicals are released. Afterwards, a filtration of the mixture follows. Table 3 presents a number of studies in which flavonoid fractions were successfully extracted by conventional techniques, including maceration.

Table 3.
Conventional techniques used in the recent years for extraction of flavonoids.
In the conventional methods, the choice of the solvents determines the type of the target compounds and the extraction rate [14]. Cowan [105] pointed out acetone as the most selective solvent for extracting flavonoids. Often alcohol–water mixtures are applied for the extraction of flavonoids and their conjugates from plant material. Seventy percent methanol is efficient solvent for extracting flavonoids by maceration [77,92,93,99]. Maceration is the preferred method for extraction of citrus flavonoids: Raw material can be used either fresh or dry; solvents, such as acidified water, methanol, ethanol, acetone, ethyl acetate, and n-hexane are applied for the extraction, of which methanol is frequently used [85].
The selection of suitable operational conditions for a new developed method of extraction is of great importance. For example, the intensity of temperature and light must be evaluated for extraction of thermo-labile compounds. Another risk factor is pH value. Vankar and Srivastava used a slightly acidic solvent (0.1% HCl in methanol v/v) to extract anthocyanin from different colored flowers and described the effect of pH on the extraction procedure [106]. Compared to the acetic acid, the hydrochloric acid in ethanol was found to be more efficient for the extraction of anthocyanin [107]. Similar results were obtained using 70% ethanol during total flavonoids extraction from Moringa oliefera [77]. The most influential extraction factors in the newly developed methods are the solvent type. However, it is reported that the variation of the solvent–sample ratio does not significantly change the extraction effect and the superfluous solvent amount can be saved. Each optimized method is individual for the respective plants. All factors (temperature, solvent type, duration, etc.) can have a negative impact on the extraction procedure, and the lack of proper parameter optimization may cause degradation of the phytochemicals.
The extracts obtained by maceration are complex mixtures and it is necessary to carry out a further purification by re-extrication [61,79,94], column chromatography [85,89,100], or a combination of both methods [95,96].
The technique of maceration is the easiest and no special lab equipment is required. The main disadvantages of this method are the large volume of solvents, the long processing time, and the need for future purification. In case purity is an issue, advanced extraction technology should be considered.
4.1.2. Reflux and Soxhlet Extraction
Reflux and Soxhlet extraction are high-temperature continuous extraction procedures. Soxhlet extraction is carried out using Soxhlet apparatus: A ground sample is placed in a porous “thimble”, which is located in a chamber. Extraction solvents are heated in the bottom flask, vaporized into the sample thimble, condensed in the condenser, and dripped back. Compared to maceration, these methods require a smaller volume of solvent [11] and shorter processing time. The following general disadvantages of Soxhlet extraction are: The extracted flavonoids must be thermostable, the plant sample should be dried, and toxic and flammable liquid organic solvents are used, which is a potential risk factor. A few factors (temperature, solvent–sample ratio, and agitation speed) must be evaluated [108].
A few examples of successful flavonoid extraction from plant material by reflux and Soxhlet methods are given in Table 3. A solvent mixture of methanol, 0.1% butylated hydroxytoluene, and hydrochloric acid (4:1) was used for quantitative extraction of two major isoflavones from quinoa seeds by reflux [101]. Mun and Mun [102] extracted isoflavonoids from Pueraria lobata (Willd.) by reflux in methanol. The Soxhlet extraction of Moringa oliefera leaves led to a lower yield of phenolic amount, including flavonoids [77]. Pluempanupat et al. [104] extracted isoflavonoids from air-dried and powdered Dalbergia oliveri heartwood by Soxhlet. The researcher used solvents with different polarity (hexane, dichloromethane, ethyl acetate, and methanol). The extraction started with the low polar solvent and finished with the high polar solvent. Thus, selectivity of some thermally stable flavonoids can be attained by Soxhlet extraction. However, different solvents have to be used for several successive extractions. Powdered Clitorea ternate flowers were defatted by Soxhlet using petroleum ether at 60–80 °C. After further extraction with ethanol, alkaloids, and saponins were determined, but the major component, anthocyanin, was absent. The researchers explained this with its oxidation and degradation during the extraction process [109].
The extracts obtained by Soxhlet extraction have a complex composition and the flavonoid fraction must be re-extracted to be purified [82] or could be purified by liquid chromatography [67,68]. Compared to soaking, the advantages of Soxhlet extraction are the shorter processing time, and the possibility for automation. Nevertheless, the degradation of thermally instable compounds is a serious problem.
After quantitative extraction by classical solid–liquid methods like Soxhlet or maceration, the purification in one step can lead to the preparation of extract rich in flavonoids. Such selective options use nano-encapsulated processing [110], as well as polymer-based solid-phase extraction [16]. The sorbents should have high selectivity according to the flavonoid substances or membrane extraction.
4.2. Green Extraction Techniques
The major shortcomings of the conventional extraction techniques are a long extraction time, a requirement of costly solvent, evaporation of a huge amount of solvent, low extraction selectivity, and these techniques are difficult to be automated [14]. New and promising techniques are being developed to break through the limitations of classical extraction methods. They are categorized as non-conventional, “green” extraction techniques. They have a lot of advantages: Reduction of organic solvent consumption and sample degradation, pollution prevention, elimination of additional sample clean-up and concentration steps, improvement of extraction efficiency and selectivity, and ability for automation [111].
Some of the most promising techniques are ultrasound-assisted extraction (UAE), enzyme-assisted extraction (EAE), microwave-assisted extraction (MAE), pressurized liquid extraction (PLE), supercritical fluid extraction (SFE), enzyme-assisted extraction (EAE), and matrix solid-phase dispersion (MSPD). There are individual solution cases of flavonoid extraction that demonstrated a promising selectivity. Green extraction techniques concerning the isolation of flavonoids from plant matrices are listed in Table 4.

Table 4.
Green techniques used in the recent years for extraction of flavonoids.
4.2.1. Ultrasound-Assisted Extraction (UAE)
UAE, also called sonication, uses ultrasonic wave energy during the extraction. Ultrasound produces cavitation, which accelerates the dissolution and diffusion of the cell ingredients: The ultrasound wave is propagated in the molecules of the medium and cavitation bubbles are formed at sufficiently high power. The disintegration of these bubbles generates energy, and the jets of solvent towards herbal particles extract the target compounds from them more efficiently [169]. In addition, the heat transfer improves the extraction efficiency. The other advantages of UAE include low solvent and energy consumption and reduction of extraction temperature and time, which make UAE a simple and relatively low-cost technology [170]. The most broadly used extraction apparatus is the ultrasonic bath. Because of the shorter processing time, this technique is relevant to extraction of thermally unstable compounds. This makes UAE an alternative to maceration and Soxhlet extraction. UAE has been employed in extraction of thermolabile compounds, such as anthocyanin from flower parts, in order to reduce extraction time and avoid high-temperature exposure [112]. But high ultrasound waves result in free radical formation and unacceptable changes of extracted components [13]. Therefore, all UAE parameters must be carefully optimized in order to avoid thermal degradation of phenolic compounds, such as flavonoids. The technique parameters are: Temperature, extraction time, polarity and amount of solvent, amount and type of sample, ultrasound frequency and intensity, and number of pulses. Lu et al. [113] optimized the operating conditions of UAE for total flavonoids from Cryptotaenia japonica Hassk using the Box–Behnken design. Their results suggested UAE as a promising technique for this extraction.
Different solvents and mixtures can be used to perform sonication, but most widely applied was ethanol (Table 4). Many reports demonstrated the application of UAE in the extraction of isoflavones. Isoflavones were extracted from Pueraria lobata (Wild.) Ohwi stem using 95% and 50% aqueous solutions of n-butanol and ethanol [114]. The authors established UAE as more efficient for the total yield of isoflavone extracts compared to Soxhlet extraction. The optimal extraction conditions were found to be: 50% ethanol (as extractant), 650 W ultrasonic power at 298.15 K with agitation rate at 300 rpm. Sun et al. [115] replaced the solvent by 40% ethanol for extracting puerarin from this plant material. The usage of an ethanol–water mixture for UAE also led to the successful quantification of puerarin, daidzin, daidzein, and genistein in the roots of Pueraria lobata and P. thomsonii [116]. Another study compared UAE to the classical techniques in the extraction of isoflavones from Iris tectorum [117]. The authors confirmed that UAE (in optimal condition: 70% methanol in water, 45 °C, 150 W, and 45 min) produced the highest extraction yields for the target isoflavones. Song et al. [118] isolated eleven major isoflavonoids from the xylem and bark of Radix Astragali by UAE using methanol. Rostagno et al. [170] compared UAE to conventional maceration in extraction of isoflavones from soya beans. The yield of the target compounds was found to be higher in the UAE extracts. The optimal UAE parameters were 333.15 K, 200 W sonification power, 20 min single run at 24 kHz frequency. Three different aqueous mixtures with ethanol, methanol, and acetonitrile (in the concentration interval from 30% to 70% v/v), and two temperature levels (10 and 60 °C) were tested. UAE was determined as a rapid and reliable technique. In this study, the ultrasonic bath was substituted by a probe horn and it yielded similar results. Pananun et al. conducted a very interesting experiment [126]. The researchers used high-power ultrasonication to extract isoflavones from defatted soybean with aqueous, acidified acetonitrile. The chosen parameters were 20 kHz ultrasound power, and changing amplitudes (18–54 μm) for 1 and 3 min. This process has not been studied in detail, but the extraction efficiency has been increasingly compared to conventional techniques.
Higher extraction efficiency compared to the simpler technique can be accomplished by a combination of various energy sources. A study conducted by Hu et al. introduced a new method for the rapid extraction of isoflavones from Pueraria lobata Ohwi [136]. They combined microwave and ultrasound energy sources. Isolation of isoflavones from plant material by ultrasound, combined microwave- and vacuum-drying technologies, proved to be a good alternative: The time required was 20-fold shorter than by reflux-extraction. The microwave-vacuum method additionally reduced the processing time 10-fold. Moreover, this combined extraction technique showed no negative impact on the isoflavonoid content.
In conclusion, compared to the maceration and Soxhlet extraction, UAE has better merits such as time- and solvent-saving, requires lower temperature levels, and gives larger extract yields. However, the selectivity of this technique is comparable with that of the conventional extraction methods. The extract obtained contains a mixture of phenolic compounds and must be purified before the next step of identification or practical use [116,118], especially when fruits were subjected to extraction [85]. High-power sonication of soya beans reduced the total phenolic content, but the concentration of the major isoflavones (genistein, daidzein, and glycitein) increased 10-fold [126], so UAE can be a promising selective extracting technique with the proper extractant.
4.2.2. Microwave-Assisted Extraction (MAE)
MAE is an extraction technique that uses microwave power to promote the pouring of analytes from the sample matrix into the solvent. Microwave radiation works on the dipoles of polar and polarizable materials and causes heating near the surface of the materials. The heat is transferred by conduction. MAE can be considered as a selective method in the case of polar molecules and solvents with high dielectric constant [11]. Compared to conventional extraction methods, MAE offers a combination of advantages: The possibility to use fewer toxic solvents; reduced extraction time, solvent amount, energy, and processing costs; and increased recovery. Nevertheless, this technique is limited to small molecules stable under microwave heating conditions. Further added cycles of MAE led to lower yield of phenolics including flavanones, as a result of their oxidation [171]. Anthocyanins may not be suitable for MAE because of their potential thermal instability [171]. Table 4 lists the developed methods for MAE of flavonoids from several plant matrices. Laghari et al. [93] also investigated the extraction, identification, and antioxidative properties of flavonoids from Cassia angustifolia leaves and flowers and compared the efficiency of MAE and UAE to conventional extraction methods. The results obtained showed that the microwave extraction method is the better option for the total flavonoids extraction (Table 4). There is another example for MAE as a better extraction option, but not as a selective option: MAE conditions (solvent concentration, extraction time, and microwave power) for flavonoids and phenols extraction from Chinese quince (Chaenomeles sinensis) were optimized using designed experiments to maximize recovery by the method [172]. Different solvents can be used to extract flavonoids by this technique (Table 4): Water [134]; alcohol [127,130,131,132]; alcohol/water mixture [129,135]; or ionic liquids [133].
The extracts obtained by MAE are rich in some flavonoid fractions because of the plant material. For example, Rostagno et al. [129] obtained an extract from soya bean rich in isoflavones by MAE using methanol/water (1:1) as the solvent. However, MAE is not highly selective technique. Scientific literature reports studies on the extraction of flavonoids by MAE: Saponins [173], phenols [121,132,133,171], and coumarins [130]. Increased efficiency is associated with an increase in the amount of the flavonoid fraction and of the selectivity, respectively. Liu et al. [135] modified the MAE method as a first step for HPLC-fluorescence determination of isoflavones from P. thomsonii roots. The optimized extraction step was based on a 30-min sample soaking in 70% methanol, and a subsequent microwave exposure to 600 W for 11 min.
Two ecofriendly and cost-efficient variants of MAE were developed: Genistein and biochanin A were isolated from Dalbergia odorifera T. Chen leaves by microwave-assisted aqueous two-phase extraction (MA-ATPE) [137]. Ethanol, K2HPO4/(NH4)SO4/citrate, and deionized water built an aqueous two-phase system. First, a salt was dissolved in the water, then ethanol was added, and the solution was mixed until two phases were formed. The building of an aqueous two-phase system was attributed to the reciprocal exclusion of ions and ethanol on one hand, and their high affinity for the water on other hand. From the investigated salts, K2HPO4 was picked up, and it could be reused. Compared to conventional MAE and the under-reflux-extraction, the amount of target compounds in the extracts was higher by applying the new procedure. Another combined green technique for isoflavone extraction from the roots of pigeon pea was investigated, which was named deep eutectic solvent-based MAE (DES-MAE) [138]. The optimal conditions for DES-MAE were designed by the single factor and Box–Behnken tests: 30% water in 1,6-hexanediol/choline chloride (7:1, n/n), 80 °C, microwave power of 600 W, and 11-min run cycle. The advantage of DES-MAE compared to the other extraction methods was the higher extraction efficiency. MA-ATPE and DES-MAE showed an enormous potential as environmentally friendly methods for the rapid and efficient extraction of isoflavonoids from plant samples.
4.2.3. Pressurized Liquid Extraction (PLE)
PLE has also been described as accelerated solvent extraction by different research groups. PLE applies high pressure, whereby the solvents remain liquid above their boiling point. The obtained effects are high solubility and high diffusion rate of hydrophobic compounds in the solvent, and high penetration of the solvent into the matrix [15]. Compared to other methods described above in this review, PLE requires less extraction time and solvent consumption and has better repeatability. Moreover, this technique can be automated which allows extraction in a shorter time with a minimum amount of solvent. For the first time, the Dionex Corporation introduced it in 1995 [174]. Contrary to the claim of some researchers that high temperature and pressure destroy the thermolabile compounds, Gizir et al. [144] successfully applied PLE to obtain an anthocyanin-rich extract from black carrots using acidified water as the solvent. The authors shared the opinion that the degradation rate of anthocyanins is time dependent. They fixed temperatures up to 100 °C, under 50 bar pressure, and a short processing time of a few minutes to overcome the possibility of molecular destruction. For this technique the choice of solvents is critical. The extraction must be as selective as possible, but solvents must be harmless, of lower toxicity, and easy to remove [13]. Solvent mixtures (e.g., methanol/ethanol–water) were found to be more effective and environment-friendly than pure organic solvents (Table 4). A successful optimal extraction of anthocyanins from lyophilized red grape skins was carried out by PLE using acidified water as a solvent. The acidified methanol was picked up from six solvents due to the reached maximum recovery rates of anthocyanins and total phenolics in the extracts tested. The optimal PLE conditions were 333.15 K and 10.1 MPa. This led to the decreasing of the solvent consumption and a total extraction time of 3 min [145]. After optimization, Santos et al. reported improvements in the recovery of anthocyanins (by 13%) and other phenolic compounds (by 8%) compared to the method of percolation under low pressure [123]. The optimized PLE process had the following parameters: 5 MPa, 553 K, 9 min, and static mode. Similarly, in another study [140], PLE was used for optimized extraction of flavones from parsley flakes. The researcher team used two-binary solvent systems: 50:50 v/v (ethanol/water and acetone/water). The flush volume significantly reduced the extractant consumption and the waste amounts obtained were lower. High recoveries (~94%) of flavonols and falvan-3-ols from Rheum palmatun were observed using 80% aqueous methanol by PLE [146]. Because of that, the method was suggested for quality control application. Chang et al. [148] investigated the influence of the ethanol/water ratio and the effects of the physiochemical parameters, including solvent flow rate and feed loading, on the efficiency of PLE of isoflavones from soybean flakes. Using 80%, aqueous ethanol achieved 95% recovery of the extraction method. Another study described the use of PLE in the micropreparative isolation of isoflavones from plants of the genus Trifolium L. [147]. Different extraction parameters (e.g., type of solvents, temperature, and the number of extraction cycles) were studied. The optimal extraction efficiency was achieved using methanol–water (75:25, v/v) at 125 °C. In this study, PLE showed significant advantages over conventional solvent extraction and UAE: Lower cost, high yield of isoflavone aglycones, relatively high precision and accuracy. Many other research teams have reported successful applications of PLE in flavonoid extraction from different plant matrices (Table 4).
Pressurized hot water extraction (PHWE) is a variation of PLE. This technique is also known as pressurized low-polarity water extraction and supercritical water extraction: The operating characteristics of the pressurized water used are temperature range above the boiling point and below the critical point of water, which are 273 K at 0.1 MPa and 647 K at 22.1 MPa, respectively [13]. Under subcritical conditions, the intermolecular hydrogen bonds of water break down, and its dielectric constant decreases. At a temperature level of 250 °C, this constant is reduced to 27 [175], which is similar to the dielectric constant of ethanol (Ɛ = 24) and methanol (Ɛ = 33). The replacement of ethanol or methanol with water significantly reduces costs and makes this technique more environmentally friendly. The selectivity of this method relies on the solubility of the flavonoids which depends on the temperature conditions. The aqueous solubility of a variety of hydrophobic compounds increases at temperatures above 373.15 K and the water remains incompressible with further pressure rising under the operating conditions. High temperature has some unacceptable consequences, including degradation of thermolabile phenolic compounds and the necessity of further purification. In summary, the careful optimization of extraction temperature is important for PHWE. For example, PHWE resulted in enhanced extraction of flavonols from Seabuckthorn at 150 °C [150] and anthocyanins from dried red grape skin at 100–110 °C [145]. Rodriguez–Meizoso et al., [149] in an extensive research, concluded that more polar flavonoids, such as flavonols, flavones, flavonones, and anthocyanins, can be selectively extracted at a temperature of 100–120 °C. Above 120 °C and at long-term exposure, the flavonoids are destroyed. PHWE has industrial applications: Ko et al. [176] conducted a pilot-scale commercialization of the process. The effects of extraction parameters (temperature, pressure, time, material type, and sample/solvent ratio) on PHWE of flavonoids from dried satsuma mandarin peel were studied. The research team found the following optimal conditions: 130 °C, 15-min extraction time, and solute/solvent ratio of 1/34. The yield of flavonoids obtained under laboratory and pilot conditions was similar: 117.8 and 113.4 mg/g, respectively. The flavonoids recovery of PHWE in the pilot plant was 96.3%, which demonstrated the potential of this method for industrial application.
PHWE is not required for quantitative extraction of isoflavones [177]. Even at elevated temperatures and moderate pressure, water is not as effective as methanol, ethanol, and their mixtures with water for the isolation of isoflavones [87]. Moreover, the degradation of glucoside and malonyl forms was detected at higher temperature levels during PHWE [178,179].
4.2.4. Supercritical Fluid Extraction (SFE)
Supercritical fluid can be defined as a fluid above its specific critical temperature and pressure. Under these conditions, it exists in a phase, which has the properties of both liquids and gases at its critical point. Critical pressure is the minimum pressure required to convert a gas into a liquid state at its own critical temperature. The critical temperature of a gas is the temperature at which it does not become liquid under the application of extra pressure [13]. Temperature and pressure are the factors that determine the bringing of a substance into its critical region. The supercritical fluid behaves more like a gas, but has the solvating properties of a liquid. For example, CO2 has this characterisic at above 31.1 °C and 7380 kPa. Supercritical-CO2 (SC-CO2) extraction has several very important advantages: Good solvating of non-polar analytes, low costs, and low toxicity of CO2, and obtaining of a concentrated dry matter (CO2 can be easily evaporated). To achieve greater solubility for polar compounds, a small amount of ethanol and methanol (defined as modifiers) were added. The huge disadvantage of this extracting technique was the use of an expensive installation.
Different bioactive flavonoid compounds, including catechin, epicatechin, rutin, myricetin, luteolin, apigenin, and naringenin, were obtained from spearmint (Mentha spicata L.) leaves using conventional Soxhlet extraction and SC-CO2 extraction at different extraction schemes and parameters [103]. The effect of different parameters such as temperature (40, 50, and 60 °C), pressure (100, 200, and 300 bar), and dynamic extraction time (30, 60, and 90 min) on the SC-CO2 extraction of spearmint flavonoids was investigated. The extracts obtained by Soxhlet and and the optimized SC-CO2 extraction were further analyzed by HPLC to determine their chemical profile. Comparable results were obtained with the optimal SC-CO2 extraction condition (60 °C, 200 bar, 60 min) and 70% ethanol as a solvent. Soxhlet extraction showed a higher crude extract yield (257.67 mg/g) compared to the SC-CO2 extraction (60.57 mg/g). SC-CO2 extract contained more main flavonoid compounds (seven bioactive flavonoids) in a higher concentration compared to 70% ethanol Soxhlet extract (five bioactive flavonoids). Therefore, SC-CO2 extraction is considered as an alternative selective process regarding the bioactive flavonoid compounds of spearmint leaves.
The efficiency of SC-CO2 regarding polar analytes may be increased by adding a modifier [180]. The solvating properties of the fluid could be adjusted by optimization of pressure and/or temperature, which led to a relatively high selectivity [177]. The most important factor for the efficiency of the extraction method was the high solubility of the target substances in the supercritical extractant. Therefore, many parameters that affect the solubility and the resulting yield had to be taken into account. The optimal operating conditions for the phenolic compound extraction from grape marc and elderberry were investigated [156]. The researchers obtained extracts with high anthocyanin content. They used ethanol, ethyl-acetate, and acetone in different ratios with water as the modifier in a single-step mode. Temperatures of 20, 40, and 60 °C were applied. The influence of medium pH on the yield and degradation of anthocyanins was investigated, and two-step extractions, combining SFE and conventional batch extractions, were applied, too. Mixtures of organic solvent and water at 60 °C were shown to be the most effecive conventional solvents in single-step extractions. Pre-treatment of the natural material with SC-CO2 (with or without ethanol as co-solvent) improved the extraction of polyphenols from the grape marc. This method provided a promising environmentally friendly alternative to the pre-treatment of the plant materials. Acidification of the medium resulted in a higher anthocyanin content in the extracts. However, the degradation of the anthocyanins during storage was higher, which led to the loss of the intensive color. Ghafoor et al. [158] used SC-CO2 modified by 7% ethanol for the extraction of total phenolic compounds. The anthocyanin fraction was dominant. In a similar study of Murga et al. [156], a grape seed extract rich in phenols, including (+) catechin and (−) epicatechin, was obtained using the SC-CO2 technique with ethanol as a modifier. In a study of Rostagno et al., an enhanced extraction efficiency of soy isoflavones (daidzein, genistein, daidzin and genistin) was obtained using higher modifier concentrations (80% methanol in water) [154]. The authors explained these results with an increase of CO2 polarity and nearly constant density. The highest isoflavone yields were achieved by increasing the pressure (higher density of SC-CO2) and CO2 flow rates (higher mass of SC-CO2). Raising the temperature (from 40 to 70 °C) can enhance the solubility of other matrix components and decrease the yield of isoflavones. Araújo et al. [155] found the optimal conditions of SFE for soybean isoflavones. An earlier step of the plant material preparation was thermohydration at 50 °C and pH 5.0. The researchers investigated the impact of various parameters (temperature, pressure, and added co-solvent) and compared the results obtained to the traditional solid–liquid extraction with 80% methanol in water. The SC-CO2 results showed the highest yield of the target isoflavones at the following parameters: 60 °C, 380 bar, extraction time 15 min, and 10% acetonitrile. To extract isoflavonoid glycosides, a modifier addition was not enough. HPLC analysis quantification of daidzein and genistein showed that through solid–liquid extraction a higher quantity of daidzein and genistein was obtained than the amount acquired by SFE. For this reason, SFE cannot be recommended for quantitative isoflavone extraction.
4.2.5. Enzyme-Assisted Extraction (EAE)
EAE is an enzymatic pre-treatment which is carried out by the addition of specific hydrolyzing enzymes (e.g., cellulase, α-amylase, and pectinase) during the extraction step [77]. By using enzymes, the cell wall is broken and the structural polysaccharides and lipid bodies are hydrolyzed under moderate conditions, which is reflected in high yields. This approach is considered a novel and effective technique for the extraction of a large group of secondary plant metabolites with antioxidant properties, including flavonoids [14]. In this regard, several parameters must be taken into account in order for the extraction process to be effective: Reaction temperature, extraction time, pH of the system, enzyme concentration, and the particle size of the substrate. Maier et al. [159] used a mixture of pectinolytic and cellulolytic enzymes (2:1 ratio, 2-h treatment, at 40 °C, and pH 4.0) to extract bioactive compounds including anthocyanins and non-anthocyanin flavonoidsfrom grape pomace. The yield obtained was higher compared to sulfite-assisted extraction. The methodology led to isolation of phenol-rich fractions. Wallace and Burong [86] developed a selective EAE-procedure for isoflavone extraction from soya beans, chickpeas, Trifolium subterraneum L. leaves, Lupinus albus seeds, and Mentha piperita leaves using the hydrolyzing enzymes of the plants treated: The first step was the suspension of the ground material in water, followed by the warming (up to 62 °C at the treatment of Trifolium subterraneum L leaves) and waiting for the hydrolysis to finish (from 10 min to a few hours or overnight). After that, the pH value had to be adjusted up to 9,6–12 to obtain a soluble isoflavone fraction, and, finally, the precipitation of isoflavones from the filtrate was carried out by pH adjustment up to 3.5–5.6 and the precipitated isoflavone fraction was filtrated. The precipitate could be dissolved in ethanol with subsequent modification by the addition of acetone, whereby all dissolved sugars, saponins, and proteins were expected to be more or less precipitated.
The EAE is suitable for the extraction of various bioactive substances from plant matrices, but the fraction obtained after filtration is rich in water-soluble small molecule compounds, including flavonoids. After this procedure, it is possible to isolate the flavonoids step by step through changing the pH and adding different solvents [86]. However, this makes EAE a non-selective method regarding flavonoid extraction from plant materials.
4.2.6. Matrix Solid-Phase Dispersion (MSPD)
A popular alternative to the solid–liquid extraction method is MSPD. By means of this technique, the target compounds are extracted, fractionated, and prepared [177]. The process involves simultaneous homogenization, extraction, and purification, during which most of the problems associated with the classical methods are eliminated. Visnevschi–Necrasov et al. [160] developed a MSPD method for extraction and determination of 12 isoflavones in Trifolium pratense L.: Dried leaf samples were blended with hydrophobic C18-resin, placed in small columns, and isoflavones were extracted with a mixture of dichloromethane and methanol. Contrary to the standard extraction techniques, MSPD needs no specific labor equipment, less solvent, and it is fast, inexpensive, and more environmentally friendly. The selectivity depends on the eluent choice. Hong et al. [161] developed a MSPD method for the simultaneous extraction and isolation of kaempferol and other polyphenols such as hydroxysafflor yellow A from Carthamus tinctorius L., using silica gel as a dispersing sorbent (sorbent/sample ratio 3:1), and methanol:water (1:3, v:v). Graphene-encapsulated silica was used as an adsorbent in MSPD of polymethoxylated flavonoids from dried Murraya paniculata (L.) Jack leaves with recovery above 92.61% [162]. Compared to another five sorbents (graphene, silica gel, C18-resins, diatomaceous earth, and neutral alumina), graphene-encapsulated silica demonstrated better extraction efficiency regarding the target analytes.
4.2.7. Negative Pressure Cavitation Extraction (NPCE)
NPCE is a new type of cavitation, suitable for the isolation of thermo-unstable plant byproducts [177]. The main point of the process is a continuous introduction of nitrogen stream into the extraction system. Because of the negative pressure, N2-bubbles arise in the liquid–solid system and a highly unstable gas–liquid–solid phase is built. These processes promote the turbulence, collision, and mass transfer between the extractant and the plant matrix, which leads to an effective extraction of the target analytes. During the process, a low temperature is kept, which makes it effective, simple, low-cost, and eco-friendly. NPCE shows potential for industrial application [181].
There is another advantage of this method: At room temperature processing, thermosensitive compounds such as isoflavonoids can be quantitatively extracted [163,166].
Dong et al. [165] isolated flavones from Radix Scutellariae by NPCE at the following optimal technological parameters: 40 mL/g solid, 75% ethanol in water, 60 min extraction time, and −0.07 MPa vacuum degree. The researchers concluded that NPCE has a good extraction efficiency compared to the other conventional extraction methods. The efficiency of NPCE increased significantly in combination with ultra-sonication. Wang et al. [167] developed a UAE method for the extraction of flavonols from Flos Sophorae immaturus combined with negative pressure cavitation (NPC-UAE). The authors reported that the NPC-UAE extraction yield for six compounds was 1.27–1.62 and 1.17–1.40 folds higher than the output of NPCE and UAE, respectively. The optimal extraction conditions were: 72% ethanol, 16-min treatment time, liquid-to-solid ratio 25:1 mL/g, ultrasonic intensity 0.347 W/cm2, negative pressure −0.07 MPa, and temperature 60 °C.
4.3. Other
Zhang et al. [74] developed a new method of flavonoid extraction based on mechanochemical-extraction technology. Physico-chemical transformations, generated by mechanical force, were used to obtain a solid fraction rich in flavonones and flavanonols: Pulverized S. flavescens roots and solid reagents (basic salts) were co-ground in a stainless-steel ball planetary mill for 10 min. After extraction with water for 20 min, the solution pH was adjusted to 4–5 by citric acid. Next followed a condensation and precipitant analysis. The highest yield of 35.17 mg/g was achieved by grinding the roots with Na2CO3 (15%) at 440 rpm/min for 17.0 min and using 25 mL solvent (water) per gram of solid material. The authors concluded that the extracts obtained by MPET, including kurarinol, kushenol I/N, and kurarinone determined by HPLC-MS/MS, showed good selective extraction.
Kurepa et al. [168] reported a preparation of nanoparticles, which deliver in vivo bioactive agents to their target cells: The anatase TiO2 nanoparticles smaller than 20 nm enter plant cells, readily conjugate flavonoids rich in enediol and catechol groups in situ, and exit plant cells as nanoparticle-flavonoid conjugates. Nano-harvesting has a huge advantage: It eliminates the use of organic solvents. This approach opens new options for the use of the nanomaterials for simultaneous isolation and testing of bioactive properties of plant-synthesized compounds. The described technique for flavonol and anthocyanins extraction has even more advantages: Selectivity, no sample preparation, and preparation of materials for direct use. Futhermore, flavonoid nanoparticles can be easily prepared from limited amounts of material from different plant organs. Flavonoid/nanoparticle complexes obtained under sterile conditions in physiologically compatible buffers may be used for the treatment of mammalian cells immediately after the plant cell-based isolation step. Furthermore, flavonoid nanoconjugates isolated from various sources may also serve as a selection platform for screens aimed at the identification of flavonoid-binding biomolecules.
5. Conclusions
Plants are an inexhaustible source of flavonoids. The most important step in the plant raw material processing is the extraction and isolation of target compounds. A lot of research teams compared classical and modern methods for the extraction of flavonoids from plant materials and discussed their pluses and minuses. The focus of our study was the selectivity of the reviewed methods. Moreso, selectivity is equal to less time consuming, cost-effective, and environmentally friendly. The sample preparation and the extraction steps are equally important in the development of a selective extraction method of flavonoids.
Usually flavonoids and polyphenols are co-extracted by the application of classical techniques. When plants are a rich source of flavonoids, the extracts obtained are rich in flavonoids as well. The efficiency of extraction is higher by grinding the plant material and using alcohols as extractants. The extraction of thermo unstable flavonoids is dependent on the temperature and the treatment time. By applying green extraction techniques, such as MAE, UAE, and MSPD, the resulting yields are large, but this concerns both flavonoids and polyphenols. These techniques can be promising methods for selective flavonoids extraction with the proper extractant. PLE was more effective than MAE and UAE by using a methanol–water or ethanol–water medium. More polar flavonoids, such as flavonols, flavones, flavonones and anthocyanins, could be selectively extracted at temperature 100–120 °C by PHWE. Furthermore, PHWE had industrial applications, but was not required for the extraction of isoflavones. The interest in SC-CO2 extraction was due to its high efficiency for non-polar analytes and the low cost and low toxicity of CO2. A relatively high selectivity of anthocyanins, flavonols, and flavonones extraction could be achieved by pressure and temperature modifying. However, SFE was not recommended for quantitative isoflavone extraction. EAE was a pre-extracting step rather than selective extraction procedure, because it was always followed by liquid–liquid extraction or another enrichment step. At room temperature processing, thermosensitive compounds such as isoflavonoids could be extracted quantitatively by NPCE. The application of NPCE in combination with UAE for the extraction of flavonols and flavones had a better extraction efficiency compared to the other conventional extraction methods, NPCE and UAE. Two separate methods developed—using anatase TiO2 nanoparticles, and a method based on mechanochemical-extraction technology—are promising techniques for the selective extraction of flavonoids from plant matrices.
In conclusion, there is no universal extraction method and each extraction procedure is unique to certain plants. The design of sample preparation and extraction methods must be consistent with the study objectives, samples, and target compounds. For the extraction technique to be selective, it must incorporate an optimal solvent or mixture of solvents and a suitable technique. Last but not least, its optimization is important for a variety of applications, e.g., preparation of extracts for pharmaceutical usage, with a minimum level of impurities. In addition, when the method selected as the most appropriate one needs to be standardized, it must achieve an acceptable degree of repeatability and reproducibility.
Author Contributions
M.T., V.A., Z.Y., D.I., and T.D. contributed equally to the manuscript compilation and revisions. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Bulgarian Ministry of Education and Science under the National Research Programme “Healthy Foods for a Strong Bio-Economy and Quality of Life” approved by DCM # 577/17.08.2018.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| UAE | Ultra sonic-assisted extraction |
| MAE | Microwave-assisted extraction |
| MA-ATPE | Microwave-assisted aqueous two-phase extraction |
| DES-MAE | Deep eutectic solvent-based microwave-assisted extraction |
| PLE | Pressurized liquid extraction |
| PHWE | Pressurized hot water extraction |
| SFE | Supercritical fluid extraction |
| SC-CO2 | Supercritical-CO2 |
| EAE | Enzyme-assisted extraction |
| NPCE | Negative pressure cavitation extraction |
| LDL | Low-density lipoprotein |
| SPE | Solid phase extraction |
| HPLC | High performance liquid extraction |
References
- Doughari, J.H. Phytochemicals: Extraction Methods, Basic Structures and Mode of Action as Potential Chemotherapeutic Agents. In Phytochemical—A Global Perspective of Their Role in Nutrition and Health; Rao, V., Ed.; InTechOpen: Rijeka, Croatia, 2012; pp. 1–33. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.C.; Gulliya, B. Chemistry and Pharmacology of Flavonoids—A Review. Indian J. Pharm. Educ. Res. 2019, 53, 8–20. [Google Scholar] [CrossRef]
- Hayashi, T.; Sawa, K.; Kawasaki, M.; Arisawa, M.; Shimizu, M.; Morita, N. Inhibition of cow’s milk xanthine oxidase by flavonoids. J. Nat. Prod. 1988, 51, 345–348. [Google Scholar] [CrossRef]
- Walker, E.; Pacold, M.; Perisic, O.; Stephens, L.; Hawkins, P.T.; Wymann, M.P.; Williams, R.L. Structural determinations of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell 2000, 6, 909–919. [Google Scholar] [CrossRef]
- Korkina, L.; Afanasev, I. Antioxidant and chelating properties of flavonoids. Adv. Pharmacol. 1997, 38, 151–163. [Google Scholar]
- Kerry, N.; Abbey, M. Red wine and fractionated phenolic compounds prepared from red wine inhibit low density lipoprotein oxidation in vitro. Atherosclerosis 1997, 135, 93–102. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron chelation by the powerful antioxidant flavonoid quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 196. [Google Scholar]
- Stobiecki, M.; Kachlicki, P. Isolation and Identification of Flavonoids. In The Science of flavonoids; Grotewold, E., Ed.; Springer Science & Business Media, Inc.: New York, NY, USA, 2006; pp. 47–71. [Google Scholar]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Selvamuthukumaran, M.; Shi, J. Recent advances in extraction of antioxidants from plant by-products processing industries. Food Qual. Saf. 2017, 1, 61–81. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Raks, V.; Al-Suod, H.; Buszewski, B. Isolation, Separation, and Preconcentration of Biologically Active Compounds from Plant Matrices by Extraction Techniques. Chromatographia 2018, 81, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Smith, R. Before the inject ion-modern methods of sample preparation for separation techniques. J. Chromatogr. A 2003, 1000, 3–27. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Havsteen, B. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Alzand, K.I.; Mohamed, M.A. Flavonoids: Chemistry, Biochemistry and Antioxidant activity. J. Pharm. Res. 2012, 5, 4013–4020. [Google Scholar]
- Ollanketo, M.; Riekkola, M.L. Column-switching technique for selective determination of flavonoids in Finnish berry wines by high-performance liquid chromatography with diode array detection. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 1339–1351. [Google Scholar] [CrossRef]
- Arts, I.C.W.; van de Putte, B.; Hollman, P.C.H. Catechin Contents of Foods Commonly Consumed in The Netherlands. 1. Fruits, Vegetables, Staple Foods, and Processed Foods. J. Agric. Food Chem. 2000, 48, 1746–1751. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J. Agric. Food Chem. 2000, 48, 5331–5337. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Lean, M.E.J.; McDonald, M.S.; Black, C. Quantitative analysis of the flavonoids content of commercial tomatoes, onions, lettuce and celery. J. Agric. Food Chem. 1997, 45, 590–595. [Google Scholar] [CrossRef]
- Raffo, A.; la Malfa, G.; Fogliano, V.; Maiani, G.; Quaglia, G. Seasonal variations in antioxidant components of cherry tomatoes (Lycopersicon esculentum cv. Naomi F1). J. Food Compost. Anal. 2006, 19, 11–19. [Google Scholar] [CrossRef]
- Hara, Y.; Luo, S.J.; Wickremasinghe, R.L.; Yamanishi, T. Special issue on tea. Food Rev. Int. 1995, 11, 371–542. [Google Scholar]
- Xu, J.Z.; Leung, L.K.; Huang, Y.; Chen, Z.Y. Epimerisation of tea polyphenols in tea drinks. J. Sci. Food Agric. 2003, 83, 1617–1621. [Google Scholar] [CrossRef]
- Price, K.R.; Rhodes, M.J.C.; Barnes, K.A. Flavonol glycoside content and composition of tea infusions made from commercially available teas and tea products. J. Agric. Food Chem. 1998, 46, 2517–2522. [Google Scholar] [CrossRef]
- Natsume, M.; Osakabe, N.; Yamagishi, M.; Takizawa, T.; Nakamura, T.; Miyatake, H.; Hatano, T.; Yoshida, T. Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal-phase and reversed-phase HPLC. Biosci. Biotechnol. Biochem. 2000, 64, 2581–2587. [Google Scholar] [CrossRef]
- Gu, L.; House, S.E.; Wu, X.; Ou, B.; Prior, R.L. Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products. J. Agric. Food Chem. 2006, 54, 4057–4061. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.A.; Cienfuegos-Jovellanos, E.; Marin, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerda, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; et al. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J. Agric. Food Chem. 2007, 55, 3926–3935. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, Y.J.; Kim, D.O.; Lee, H.J.; Lee, C.Y. Major phenolics in apple and their contribution to the total antioxidant capacity. J. Agric. Food Chem. 2003, 51, 6516–6520. [Google Scholar] [CrossRef] [PubMed]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.J.; Bozonnet, S.; Mullen, W.; Jenkins, G.I.; Lean, M.E.; Crozier, A. Occurrence of flavonols in tomatoes and tomato-based products. J. Agric. Food Chem. 2000, 48, 2663–2669. [Google Scholar] [CrossRef] [PubMed]
- Lugasi, A.; Hovari, J. Flavonoid aglycons in foods of plant origin. II. Fresh and dried fruits. Acta Aliment. 2002, 31, 63–71. [Google Scholar] [CrossRef]
- Mullen, W.; Stewart, A.J.; Lean, M.E.J.; Gardner, P.; Duthie, G.G.; Crozier, A. Effect of freezing and storage on the phenolics, ellagitannins, flavonoids, and antioxidant capacity of red raspberries. J. Agric. Food Chem. 2002, 50, 5197–5201. [Google Scholar] [CrossRef] [PubMed]
- Wada, L.; Boxin, O. Antioxidant activity and phenolic content of Oregon caneberries. J. Agric. Food Chem. 2002, 50, 3495–3500. [Google Scholar] [CrossRef]
- Maatta-Riihinen, K.R.; Kamal-Eldin, A.; Torronen, A.R. Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (family Rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187. [Google Scholar] [CrossRef]
- Romani, A.; Mulinacci, N.; Pinelli, P.; Vincieri, F.F.; Cimato, A. Polyphenolic content in five Tuscany cultivars of Olea europaea L. J. Agric. Food Chem. 1999, 47, 964–967. [Google Scholar] [CrossRef]
- Maeda, T.; Kakuta, H.; Sonoda, T.; Motoki, S.; Ueno, R.; Suzuki, T.; Oosawa, K. Antioxidation capacities of extracts from green, purple, and white asparagus spears related to polyphenol concentration. HortScience 2005, 40, 1221–1224. [Google Scholar] [CrossRef]
- Carmona, M.; Sanchez, A.M.; Ferreres, F.; Zalacain, A.; Tomas-Barberan, F.; Alonso, G.L. Identification of the flavonoid fraction in saffron spice by LC/DAD/MS/MS: Comparative study of samples from different geographical origins. Food Chem. 2007, 100, 445–450. [Google Scholar] [CrossRef]
- Chang, S.; Tan, C.; Frankel, E.N.; Barrett, D.M. Low-density lipoprotein antioxidant activity of phenolic compounds and polyphenol oxidase activity in selected clingstone peach cultivars. J. Agric. Food Chem. 2000, 48, 147–151. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Cienfuegos-Jovellanos, E.; Marin, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerda, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; et al. HPLC-DAD-ESIMS analysis of phenolic compounds in nectarines, peaches, and plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef]
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, S.; Gebhardt, S. Flavonoid content of U.S. fruits, vegetables, and nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar] [CrossRef]
- Milbury, P.E.; Chen, C.Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of flavonoids and phenolics and their distribution in almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef]
- Kreft, S.; Knapp, M.; Kreft, I. Extraction of rutin from buckwheat (Fagopyrum esculentum Moench) seeds and determination by capillary electrophoresis. J. Agric. Food Chem. 1999, 47, 4649–4652. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Vlahov, G. Flavonoids in three olive (Olea europaea) fruit varieties during maturation. J. Sci. Food Agric. 1992, 58, 157–159. [Google Scholar] [CrossRef]
- Miyake, Y.; Shimoi, K.; Kumazawa, S.; Yamamoto, K.; Kinae, N.; Osawa, T. Identification and antioxidant activity of flavonoid metabolites in plasma and urine of eriocitrin-treatedrats. J. Agric. Food Chem. 2000, 48, 3217–3224. [Google Scholar] [CrossRef]
- Rousseff, R.L.; Martin, S.F.; Youtsey, C.O. Quantitative survey of narirutin, naringin, hesperidin, and neohesperidin in citrus. J. Agric. Food Chem. 1987, 35, 1027–1030. [Google Scholar] [CrossRef]
- Justesen, U.; Knuthsen, P.; Leth, T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J. Chromatogr. A 1999, 799, 101–110. [Google Scholar] [CrossRef]
- Reinli, K.; Block, G. Phytoestrogen content of foods: A compendium of literature values. Nutr. Cancer 1996, 26, 123–148. [Google Scholar] [CrossRef]
- Wiseman, H.; Casey, K.; Clarke, D.B.; Barnes, K.A.; Bowey, E. Isoflavone aglycon and glucoconjugate content of high- and low-soy UK foods used in nutritional studies. J. Agric. Food Chem. 2002, 50, 1401–1410. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhao, J.; Meng, Q.; Li, S.P.; Wang, Y.T. Simultaneous determination of five flavonoids in licorice using pressurized liquid extraction and capillary electrochromatography coupled with peak suppression diode array detection. J. Chromatogr. A 2009, 1216, 7329–7335. [Google Scholar] [CrossRef]
- Leuner, O.; Havlik, J.; Hummelova, J.; Prokudina, E.; Novy, P.; Kokoska, L. Distribution of isoflavones and coumestrol in neglected tropical and subtropical legumes. J. Sci. Food Agric. 2013, 93, 575–579. [Google Scholar] [CrossRef]
- Chukwumah, Y.C.; Walker, L.T.; Verghese, M.; Bokanga, M.; Ogutu, S.; Alphonse, K. Comparison of extraction methods for the quantification of selected phytochemicals in peanuts (Arachis hypogaea). J. Agric. Food Chem. 2007, 55, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Shaari, A.R.; Azimi, A. Effect of Drying Methods on Metabolites Composition of Misai Kucing (Orthosiphon stamineus) Leaves. APCBEE Procedia 2012, 2, 178. [Google Scholar] [CrossRef]
- Gupta, K.K.; Taneja, S.C.; Dhar, K.L.; Atal, C.K. Flavonoids of Andrographis paniculata. Phytochemistry 1983, 22, 314–315. [Google Scholar] [CrossRef]
- L’azaro, M.L. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Bama, P.; Ramachandran, J.; Kalaichelvan, P.T.; Deccaraman, M.; Vijayalakshimi, M.; Dhamotharan, R.; Dananjeyan, B.; Bama, S.S. Ethnobotanical study of medicinal plants used by traditional users in Villupuram district of Tamil Nadu, India. J. Med. Plant Res. 2010, 4, 1089–1101. [Google Scholar]
- Lin, L.J.; Huang, X.B.; Lv, Z.C. Isolation and identification of flavonoids components from Pteris vittata L. SpringerPlus 2016, 5, 1649. [Google Scholar] [CrossRef]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.Y.; Kim, K.N.; Kim, H.S. Quantitative analysis of two major flavonoid aglycones in acid hydrolyzed samples of Angelica keiskei by HPLC. Food Sci. Biotechnol. 2003, 12, 415–418. [Google Scholar]
- Murlidhar, A.; Babu, K.S.; Sankar, T.R.; Redenna, P.; Reddy, G.V.; Latha, J. Antiinflammatory activity of flavonoid fraction isolated from stem bark of Butea monosperma (Lam): A mechanism based study. Int. J. Phytopharm. 2010, 1, 124–132. [Google Scholar]
- Sannomiya, M.; Fonseca, V.B.; Silva, M.A.D.; Rocha, L.R.M.; Dos Santos, L.C.; Hiruma-Lima, C.A.; Souza Brito, A.R.M.; Vilegas, W. Flavonoids and antiulcerogenic activity from Byrsonima crassa leaves extracts. J. Ethnopharm. 2005, 97, 1–6. [Google Scholar] [CrossRef]
- Agarwal, M.; Kamal, R. Studies on flavonoid production using in-vitro cultures of Momordica charantia. Indian J. Biotechnol. 2007, 6, 277–279. [Google Scholar]
- Arora, S.; Itankar, P. Extraction, isolation and identification of flavonoid from Chenopodium album aerial parts. J. Tradit. Complement. Med. 2018, 8, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Janmeda, P. Extraction, isolation and identification of flavonoid from Euphorbia neriifolia leaves. Arab. J. Chem. 2017, 10, 509–514. [Google Scholar] [CrossRef]
- Ghoulami, S.; Idrissi, A.I.; Fkih-Tetouani, S. Phytochemical study of Mentha longifolia of Morocco. Fitoterapia 2001, 72, 596–598. [Google Scholar] [CrossRef]
- Kogawa, K.; Kazuma, K.; Kato, N.; Noda, N.; Suzuki, M. Biosynthesis of malonylated flavonoid glycosides on basis of malonyl transferase activity in the petals of Clitoria ternatea. J. Plant Physiol. 2007, 164, 886–894. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Luis, J.C.; Johnson, C.B. Seasonal variations of rosmarinic and carnosic acids in rosemary extracts. Analysis of their in vitro antiradical activity. Span. J. Agric. Res. 2005, 3, 106–112. [Google Scholar] [CrossRef]
- Mueller, A.; Ganzera, M.; Stuppner, H. Analysis of the aerial parts of Verbena officinalis L. by micellar electrokinetic capillary chromatography. Chromatographia 2004, 60, 193–197. [Google Scholar]
- Zhang, Q.; Yu, J.; Wang, Y.; Su, W. Selective Extraction of Flavonoids from Sophora flavescens Ait by Mechanochemistry. Molecules 2016, 21, 989. [Google Scholar] [CrossRef] [PubMed]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [PubMed]
- Marston, A.; Hostettmann, K. Separation and quantification of flavonoids. In Flavonoids: Chemistry, Biochemistry and Applications; Andersen, O.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–32. [Google Scholar]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind. Crops Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Tzanova, M.T.; Grozeva, N.H.; Gerdzhikova, M.A.; Argirova, M.D.; Pavlov, D.H.; Terzieva, S.R. Flavonoid content and antioxidant activity of Betonica bulgarica Degen et Neič. Bulg. Chem. Commun. 2018, 50, 90–97. [Google Scholar]
- Temidayo, A.R. Extraction and Isolation of Flavonoids Present in the Methanolic Extract of Leaves of Acanthospermum Hispidium Dc. Glob. J. Med. Plant Res. 2013, 1, 111–123. [Google Scholar]
- Mediani, F.A.; Khatib, A.; Tan, C.P. Cosmos Caudatus as a potential source of polyphenolic compounds: Optimisation of oven drying conditions and characterisation of its functional properties. Molecules 2013, 18, 10452–10464. [Google Scholar] [CrossRef]
- Borhan, M.Z.; Ahmad, R.; Rusop, M.M.; Abdullah, S. Impact of Nano powders on Extraction Yield of Centella asiatica. Adv. Mater. Res. 2013, 667, 246–250. [Google Scholar] [CrossRef]
- Hossain, M.A.; Rahman, S.M.M. Isolation and characterisation of flavonoids from the leaves of medicinal plant Orthosiphon stamineus. Arab. J. Chem. 2015, 8, 218–221. [Google Scholar] [CrossRef]
- Pk, M.U.; Talukder, R.I.; Sarkar, M.K.I.; Rahman, T.; Pervin, R.; Rahman, M.; Zenat, E.A.; Akther, L. Effect of Solvents on Phytochemicals Content and Antioxidant Activity of Ganoderma lucidum. Open Microbiol. J. 2019, 13, 10–15. [Google Scholar]
- Baber, R. Phytoestrogens and post reproductive health. Maturitas 2010, 66, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Damián-Reyna, A.A.; González-Hernández, J.C.; Chávez-Parga, M.C. Current procedures for extraction and purification of citrus flavonoids. Rev. Colomb. Biotecnol. 2016, 18, 135–147. [Google Scholar]
- Wallace, R.G.W.; Burong, G.W.B. Extraction of Flavonoids. DE Patent DE60114151T2, 20 July 2006. [Google Scholar]
- Li-Hsun, C.; Ya-Chuan, C.; Chieh-Ming, C. Extracting and purifying isoflavones from defatted soybean flakes using superheated water at elevated pressures. Food Chem. 2004, 84, 279–285. [Google Scholar] [CrossRef]
- Cosa, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Musa, Y.M. Isolation and Purification of Flavonoids from the Leaves of Mitracarpushirtus Plant. IOSR J. Appl. Chem. 2015, 8, 1–3. [Google Scholar]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Lu, H.; Yang, K.; Zhan, L.; Lu, T.; Chen, X.; Cai, X.; Zhou, C.; Li, H.; Qian, L.; Lv, G.; et al. Optimization of Flavonoid Extraction in Dendrobium officinale Leaves and Their Inhibitory Effects on Tyrosinase Activity. Int. J. Anal. Chem. 2019. [Google Scholar] [CrossRef]
- Sulaiman, S.F.; Sajak, A.A.B.; Ooi, K.L.; Seow, E.M.; Sulaiman, S.; Sajak, A.; Ooi, K.; Supriatno, K.; Seow, E. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compos. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- Laghari, A.Q.; Memon, S.; Nelofar, A.; Laghari, A.H. Extraction, identification and antioxidative properties of the flavonoid-rich fractions from leaves and flowers of Cassia angustifolia. Am. J. Anal. Chem. 2011, 2, 871–878. [Google Scholar] [CrossRef]
- Megías, C.; Cortés-Giraldo, I.; Alaiz, M.; Vioque, J.; Girón-Calle, J. Isoflavones in chickpea (Cicer arietinum) protein concentrates. J. Funct. Foods 2016, 21, 186–192. [Google Scholar] [CrossRef]
- Fotso, G.W.; Maher, F.A.; Ngnintedo, D.; Ango, P.Y.; Kapche, D.G.F.W.; Ngameni, B.; Ngwenya, B.; Yeboah, S.O.; Ngadjui, B.T.; Andrae-Marobela, K. Three new isoflavonoids with antioxidant properties from Ptycholobium contortum (N.E.Br.) Brummitt (Leguminosae). Phytochem. Lett. 2015, 14, 254–259. [Google Scholar] [CrossRef]
- Ma, F.-Y.; Luo, M.; Zhao, C.-J.; Li, C.-Y.; Wang, W.; Gu, C.-B.; Wei, Z.-F.; Zu, Y.-G.; Fu, Y.-J. Simple and efficient preparation of biochanin A and genistein from Dalbergia odorifera T. Chen leaves using macroporous resin followed by flash chromatography. Sep. Purif. Technol. 2013, 120, 310–318. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ryu, Y.B.; Curtis-Long, M.J.; Yuk, H.J.; Cho, J.K.; Kim, J.Y.; Kim, K.D.; Lee, W.S.; Park, K.H. Flavanones and carotenoids from the roots of Amorpha fruticosa L. that inhibit bacterial neuraminidase. Food Chem. Toxicol. 2011, 49, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, X.; He, C. An isoflavonoid-enriched extract from Pueraria lobata (kudzu) root protects human umbilical vein endothelial cells against oxidative stress induced apoptosis. J. Ethnopharm. 2016, 193, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, H.; Yang, H.; Nie, L.; Zang, H. Rapid determination of major bioactive isoflavonoid compounds during the extraction process of kudzu (Pueraria lobata) by near-infrared transmission spectroscopy. Spectrochim. Acta A 2015, 137, 1403–1408. [Google Scholar] [CrossRef]
- Xiao, B.-X.; Feng, L.; Cao, F.-R.; Pan, R.-L.; Liao, Y.-H.; Liu, X.-M.; Chang, Q. Pharmacokinetic profiles of the five isoflavonoids from Pueraria lobata roots in the CSF and plasma of rats. J. Ethnopharmacol. 2016, 184, 22–29. [Google Scholar] [CrossRef]
- Lutz, M.; Martínez, A.; Martínez, E.A. Daidzein and genistein contents in seeds of quinoa (Chenopodium quinoa Willd.) from local ecotypes grown in arid Chile. Ind. Crops Prod. 2013, 49, 117–121. [Google Scholar] [CrossRef]
- Mun, S.-C.; Mun, G.-S. Dynamics of phytoestrogen, isoflavonoids, and its isolation from stems of Pueraria lobata (Willd.) Ohwi growing in Democratic People’s Republic of Korea. J. Food Drug Anal. 2015, 23, 538–544. [Google Scholar] [CrossRef]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hamid, A.; Zaidul, I.S.M. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process. 2011, 89, 67–72. [Google Scholar] [CrossRef]
- Pluempanupat, S.; Kumrungsee, N.; Pluempanupat, W.; Ngamkitpinyo, K.; Chavasiri, W.; Bullangpoti, V.; Koul, O. Laboratory evaluation of Dalbergia oliveri (Fabaceae: Fabales) extracts and isolated isoflavonoids on Aedes aegypti (Diptera: Culicidae) mosquitoes. Ind. Crops Prod. 2013, 44, 653–658. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Vankar, P.S.; Srivastava, J. Evaluation of Anthocyanin Content in Red and Blue Flowers. Int. J. Food Eng. 2010, 6, 4. [Google Scholar] [CrossRef]
- Oancea, S.; Stoia, M.; Coman, D. Effects of extraction conditions on bioactive anthocyanin content of Vaccinium corymbosum in the perspective of food applications. Procedia Eng. 2012, 42, 489–495. [Google Scholar] [CrossRef]
- Amid, A.; Salim, R.J.; Adenan, M. The Factors Affecting the Extraction Conditions for Newroprotective Activity of Centella asiatica evaluated by Metal Chelating Activity Assay. J. Appl. Sci. 2010, 10, 837–842. [Google Scholar]
- Anuradha, M.; Pragyandip, P.D.; Richa, K.; Murthy, P.N. Evaluation of Neuropharmacological Effects of Ethanolic Extract of Clitorea Ternatea Flowers. Pharmacol. Online 2010, 1, 284–292. [Google Scholar]
- Kwon, M.C.; Choi, W.Y.; Seo, Y.C.; Kim, J.S.; Yoon, C.S.; Lim, H.W.; Kim, H.S.; Ahn, J.H.; Lee, H.Y. Enhancement of the Skin-Protective Activities of Centella asiatica L. Urban by a Nanoencapsulation Process. J. Biotechnol. 2012, 157, 100–106. [Google Scholar] [CrossRef]
- Huie, C.W. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem. 2002, 373, 23–30. [Google Scholar] [CrossRef]
- Ebrahim, N.; Kershi, M.; Butnariub, M. Antioxidant Activity and Anthocyanin Content in Flower of Mirabilis jalab L. Collected from Yemen. World Appl. Sci. J. 2014, 29, 247–251. [Google Scholar]
- Lu, J.; Xu, Y.; Yang, M.; Fu, X.; Luo, F.; Li, Z. Optimization of ultrasound-assisted extraction of flavonoids from Cryptotaenia japonica Hassk, evaluation of antioxidant activity. J. Agric. Sci. 2015, 7, 138–146. [Google Scholar] [CrossRef][Green Version]
- Huaneng, X.U.; Zhang, Y.; Chaohong, H. Ultrasonically assisted extraction of isoflavones from stem of Pueraria lobata (Willd.) Ohwi and its mathematical model. Chin. J. Chem. Eng. 2007, 15, 861–867. [Google Scholar]
- Sun, B.-L.; Yang, Y.-F.; Hu, X.; Xie, X.-X.; Meucci, J.; Fang, M.; Zhao, L.-J. Purification of puerarin from Pueraria lobata by FCPC versus HSCCC using small-volume columns. Chin. Herb. Med. 2014, 6, 140–144. [Google Scholar] [CrossRef]
- Zeng, A.; Xing, J.; Wang, C.; Song, J.; Li, C.; Yang, X.; Yang, G. Simultaneous analysis and retention behavior of major isoflavonoids in Radix Puerariae lobatae and Radix Puerariae thomsonii by high performance liquid chromatography with cyclodextrins as a mobile phase modifier. Anal. Chim. Acta. 2012, 712, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Z.; Wang, J. Ultrasound-assisted extraction of five isoflavones from Iris tectorum Maxim. Sep. Purif. Technol. 2011, 78, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-Z.; Yiu, H.H.W.; Qiao, C.-F.; Han, Q.-B.; Xu, H.X. Chemical comparison and classification of Radix Astragali by determination of isoflavonoids and astragalosides. J. Pharm. Biomed. Anal. 2008, 47, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Londono-Londono, J.; de Lima, V.R.; Lara, O.; Gil, A.; Pasa, T.B.; Arango, G.J.; Pineda, J.R. Clean recovery of antioxidant flavonoids from citrus peel: Optimizing an aqueous ultrasound-assisted extraction method. Food Chem. 2010, 119, 81–87. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodriguez, A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Plaza-Morales, M.; Giovagnoli-Vicuna, C.; Jamett, F. High hydrostatic pressure and ultrasound extractions of antioxidant compounds, sulforaphane and fatty acids from Chilean papaya (Vasconcellea pubescens) seeds: Effects of extraction conditions and methods. LWT Food Sci. Technol. 2015, 60, 525–534. [Google Scholar] [CrossRef]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. J. Food Eng. 2012, 108, 444–452. [Google Scholar] [CrossRef]
- Sahin, S.; Samli, R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochem. 2013, 20, 595–602. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Pananun, T.; Montalbo-Lomboy, M.; Noomhorm, A.; Grewell, D.; Lamsal, B. High-power ultrasonication-assisted extraction of soybean isoflavones and effect of toasting. Food Sci. Technol. 2012, 47, 199–207. [Google Scholar] [CrossRef]
- Xiao, W.; Han, L.; Shi, B. Optimization of microwave-assisted extraction of flavonoid from Radix astragali using response surface methodology. Sep. Sci. Technol. 2008, 43, 671–681. [Google Scholar] [CrossRef]
- Perino-Issartier, S.; Abert-Vian, M.; Chemat, F. Solvent free microwave-assisted extraction of antioxidants from sea buckthorn (Hippophae rhamnoides) food by-products. Food Bioprocess Technol. 2011, 4, 1020–1028. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Microwave-assisted extraction of soy isoflavones. Anal. Chim. Acta 2007, 588, 274–282. [Google Scholar] [CrossRef]
- Zhou, T.; Xiao, X.; Li, G.; Cai, Z.W. Study of polyethylene glycol as a green solvent in the microwave-assisted extraction of flavone and coumarin compounds from medicinal plants. J. Chromatogr. A 2011, 1218, 3608–3615. [Google Scholar] [CrossRef]
- Bai, X.L.; Yue, T.L.; Yuan, Y.H.; Zhang, H.W. Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J. Sep. Sci. 2010, 33, 3751–3758. [Google Scholar] [CrossRef]
- Svarc-Gajic, J.; Stojanovic, Z.; Carretero, A.S.; Roman, D.A.; Borras, I.; Vasiljevic, I. Development of a microwave-assisted extraction for the analysis of phenolic compounds from Rosmarinus officinalis. J. Food Eng. 2013, 119, 525–532. [Google Scholar] [CrossRef]
- Du, F.Y.; Xiao, X.H.; Luo, X.J.; Li, G.K. Application of ionic liquids in the microwave-assisted extraction of polyphenolic compounds from medicinal plants. Talanta 2009, 78, 1177–1184. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Rajan, K.S. Influence of microwave irradiation on kinetics and thermodynamics of extraction of flavonoids from Phyllanthus emblica. Braz. J. Chem. Eng. 2017, 34, 885–899. [Google Scholar]
- Liu, Y.-K.; Yan, E.; Zhan, H.-Y.; Zhang, Z.-Q. Response surface optimization of microwave-assisted extraction for HPLC-fluorescence determination of puerarin and daidzein in Radix Puerariae thomsonii. J. Pharm. Anal. 2011, 1, 13–19. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, T.; Wang, M.; Han, S.; Wan, P.; Fan, M. Extraction of isoflavonoids from Pueraria by combining ultrasound with microwave vacuum. Chem. Eng. Process. 2008, 47, 2256–2261. [Google Scholar] [CrossRef]
- Ma, F.-Y.; Gu, C.-B.; Li, C.-Y.; Luo, M.; Wang, W.; Zu, Y.-G.; Li, J.; Fu, Y.-J. Microwave-assisted aqueous two-phase extraction of isoflavonoids from Dalbergia odorifera T. Chen leaves. Sep. Purif. Technol. 2013, 115, 136–144. [Google Scholar] [CrossRef]
- Cui, Q.; Peng, X.; Yao, X.-H.; Wei, Z.-F.; Luo, M.; Wang, W.; Zhao, C.-J.; Fu, Y.-J.; Zu, Y.-G. Deep eutectic solvent-based microwave-assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Sep. Purif. Technol. 2015, 150, 63–72. [Google Scholar] [CrossRef]
- Alonso-Salces, R.M.; Korta, E.; Barranco, A.; Berrueta, L.A.; Gallo, B.; Vicente, F. Pressurized liquid extraction for the determination of polyphenols in apple. J. Chromatogr. A 2001, 933, 37–43. [Google Scholar] [CrossRef]
- Luthria, D.L. Influence of experimental conditions on the extraction of phenolic compounds from parsley (Petroselinum crispum) flakes using a pressurized liquid extractor. Food Chem. 2008, 107, 745–752. [Google Scholar] [CrossRef]
- Arapitsas, P.; Turner, C. Pressurized solvent extraction and monolithic column-HPLC/DAD analysis of anthocyanins in red cabbage. Talanta 2008, 74, 1218–1223. [Google Scholar] [CrossRef]
- Cheigh, C.I.; Chung, E.Y.; Chung, M.S. Enhanced extraction of flavanones hesperidin and narirutin from Citrus unshiu peel using subcritical water. J. Food Eng. 2012, 110, 472–477. [Google Scholar] [CrossRef]
- Pineiro, Z.; Palma, M.; Barross, C.G. Determination of catechins by means of extraction with pressurized liquids. J. Chromatogr. A 2004, 1026, 19–23. [Google Scholar] [CrossRef]
- Gizir, A.M.; Turker, N.; Artuvan, E. Pressurized acidified water extraction of black carrot (Daucus carota ssp. sativus var. atrorubens Alef.) anthocyanins. Eur. Food Res. Technol. 2008, 226, 363–370. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Howard, L.R. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Ji, T.; Jiang, G.; Hu, F. Simultaneous identification and quantification of five flavonoids in the seeds of Rheum palmatum L. by using accelerated solvent extraction and HPLC-PDA-ESI/MSn. Arab. J. Chem. 2019, 12, 1345–1352. [Google Scholar] [CrossRef]
- Zgórka, G. Pressurized liquid extraction versus other extraction techniques in micropreparative isolation of pharmacologically active isoflavones from Trifolium L. species. Talanta 2009, 79, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-C.; Chang, C.-M.J. Continuous hot pressurized fluids extraction of isoflavones and soyasaponins from defatted soybean flakes. J. Chin. Inst. Chem. Eng. 2007, 38, 313–319. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Marin, F.R.; Herrero, M.; Señorans, F.J.; Reglero, G.; Cifuentes, A.; Ibáñez, E. Subcritical Water Extraction of Nutraceuticals with Antioxidant Activity from Oregano. Chemical and Functional Characterization. J. Pharm. Biomed. Anal. 2006, 41, 1560–1565. [Google Scholar]
- Kumar, M.S.Y.; Dutta, R.; Prasad, D.; Misra, K. Subcritical water extraction of antioxidant compounds from Seabuckthorn (Hippophae rhamnoides) leaves for the comparative evaluation of antioxidant activity. Food Chem. 2011, 127, 1309–1316. [Google Scholar] [CrossRef]
- Prado, J.M.; Dalmolin, I.; Carareto, N.D.; Basso, R.C.; Meirelles, A.J.; Oliveira, J.V.; Batista, E.A.; Meireles, M.A. Supercritical fluid extraction of grape seed: Process scale-up, extract chemical composition and economic evaluation. J. Food Eng. 2012, 109, 249–257. [Google Scholar] [CrossRef]
- Braga, M.E.; Santos, R.M.; Seabra, I.J.; Facanali, R.; Marques, M.O.; de Sousa, H.C. Fractioned SFE of antioxidants from maritime pine bark. J. Supercrit. Fluid. 2008, 47, 37–48. [Google Scholar] [CrossRef]
- Farias-Campomanes, A.M.; Rostagno, M.A.; Meireles, M.A. Production of polyphenol extracts from grape bagasse using supercritical fluids: Yield, extract composition and economic evaluation. J. Supercrit. Fluids 2013, 77, 70–78. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Villares, A.; Guillamón, E.; García-Lafuente, A.; Martínez, J.A. Sample preparation for the analysis of isoflavones from soybeans and soy foods. J. Chromatogr. A 2009, 1216, 2–29. [Google Scholar] [CrossRef]
- Araújo, J.M.A.; Silva, M.V.; Chaves, J.B.P. Supercritical fluid extraction of daidzein and genistein isoflavones from soybean hypocotyl after hydrolysis with endogenous β-glucosidases. Food Chem. 2007, 105, 266–272. [Google Scholar] [CrossRef]
- Murga, R.; Ruiz, R.; Beltrán, S.; Cabezas, J.L. Extraction of natural complex phenols and tannins from grape seeds by using supercritical mixtures of carbon dioxide and alcohol. J. Agric. Chem. 2000, 48, 3408–3412. [Google Scholar] [CrossRef] [PubMed]
- Vatai, T.; Škerget, M.; Knez, Ž. Extraction of phenolic compounds from elder berry and different grape marc varieties using organic solvents and/or supercritical carbon dioxide. J. Food Eng. 2009, 90, 246–254. [Google Scholar] [CrossRef]
- Ghafoor, K.; Park, J.; Choi, Y.H. Optimization of supercritical fluid extraction of bioactive compounds from grape (Vitis labrusca B.) peel by using response surface methodology. Innov. Food Sci. Emerg. Technol. 2010, 11, 485–490. [Google Scholar] [CrossRef]
- Maier, T.; Goppert, A.; Kammerer, D.R.; Schieber, A.; Carle, R. Optimization of a process for enzyme-assisted pigment extraction from grape (Vitis vinifera L.) pomace. Eur. Food Res. Technol. 2008, 227, 267–275. [Google Scholar] [CrossRef]
- Visnevschi-Necrasov, T.; Cunha, C.S.; Nunes, E.; Oliveira, M.B.P.P. Optimization of matrix solid-phase dispersion extraction method for the analysis of isoflavones in Trifolium pretense. J. Chromatogr. A 2009, 1216, 3720–3724. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Wang, Z.; Xu, T.; Li, C.; Li, W. Matrix solid-phase dispersion extraction followed by high performance liquid chromatography-diode array detection and ultra-performance liquid chromatography–quadrupole–time of flight–mass spectrometer method for the determination of the main compounds from Carthamus tinctorius L. (Hong-hua). J. Pharm. Biomed. Anal. 2015, 107, 464–472. [Google Scholar]
- Sun, T.; Li, X.; Yang, J.; Li, L.; Jin, Y.; Shi, X. Graphene-encapsulated silica as matrix solid-phase dispersion extraction sorbents for the analysis of poly-methoxylated flavonoids in the leaves of Murraya panaculata (L.) Jack. J. Sep. Sci. 2015, 38, 2132–2139. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Zu, Y.-G.; Fu, Y.-J.; Luo, M.; Gu, C.-B.; Wang, W.; Yao, X.-H. Negative pressure cavitation extraction and antioxidant activity of biochanin A and genistein from the leaves of Dalbergia odorifera T. Chen. Sep. Purif. Technol. 2011, 83, 91–99. [Google Scholar] [CrossRef]
- Liu, W.; Fu, Y.; Zu, Y.; Kong, Y.; Zhang, L.; Zu, B.; Efferth, T. Negative-pressure Cavitation Extraction for the Determination of Flavonoids in Pigeon Pea Leaves by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2009, 1216, 3841–3850. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.L.; Fu, J.Y.; Zu, Y.G.; Li, J.; Li, X.J.; Efferth, T. Negative pressure cavitation accelerated processing for extraction of main bioactive flavonoids from Radix Scutellariae. Chem. Eng. Process. 2011, 50, 780–789. [Google Scholar] [CrossRef]
- Zhao, B.-S.; Fu, Y.-J.; Wang, W.; Zu, Y.-G.; Gu, C.B.; Luo, M.; Efferth, T. Enhanced extraction of isoflavonoids from Radix Astragali by incubation pretreatment combined with negative pressure cavitation and its antioxidant activity. Innov. Food Sci. Emerg. Tech. 2011, 12, 577–585. [Google Scholar] [CrossRef]
- Wang, G.; Cui, Q.; Yin, L.J.; Li, Y.; Gao, M.Z.; Meng, Y.; Li, J.; Zhang, S.N.; Wang, W. Negative pressure cavitation based ultrasound-assisted extraction of main flavonoids from Flos Sophorae Immaturus and evaluation of its extraction kinetics. Sep. Purif. Technol. 2020, 244, 115805. [Google Scholar] [CrossRef]
- Kurepa, J.; Nakabayashi, R.; Paunesku, T.; Suzuki, M.; Saito, K.; Woloschak, G.E.; Smalle, J.A. Direct isolation of flavonoids from plants using ultra-small anatase TiO2 nanoparticles. Plant J. 2014, 77, 443–453. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trends Analyt. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of soy isoflavones. J. Chromatogr. A 2003, 1012, 119–128. [Google Scholar] [CrossRef]
- Trusheva, B.; Trunkova, D.; Bankova, V. Different extraction methods of biologically active components from propolis: A preliminary study. Chem. Cent. J. 2007, 1, 13. [Google Scholar] [CrossRef]
- Hui, T.; Ghafoor, K.; Choi, Y.H. Optimization of microwave-assisted extraction of active components from Chinese quince using response surface methodology. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 694–701. [Google Scholar]
- Song, J.Z.; Mo, S.F.; Yip, Y.K.; Qiao, C.F.; Han, Q.B.; Xu, H.X. Development of microwave assisted extraction for the simultaneous determination of isoflavonoids and saponins in radix astragali by high performance liquid chromatography. J. Sep. Sci. 2007, 30, 819–824. [Google Scholar] [CrossRef]
- Benthin, B.; Danz, H.; Hamburger, M. Pressurized liquid extraction of medicinal plants. J. Chromatogr. A 1999, 837, 211–219. [Google Scholar] [CrossRef]
- Vergara-Salinas, J.R.; Cuevas-Valenzuela, J.; Perez-Correa, J.R. Pressurized hot water extraction of polyphenols from plant material. In Biotechnology of Bioactive Compounds: Sources and Applications; Kumar, V.G., Tuohy, M.G., Lohani, M., O’Donovan, A., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; pp. 63–101. [Google Scholar]
- Ko, M.J.; Kwon, H.L.; Chung, M.S. Pilot-scale subcritical water extraction of flavonoids from satsuma mandarin (Citrus unshiu Markovich) peel. Innov. Food Sci. Emerg. Technol. 2016, 38, 175–181. [Google Scholar] [CrossRef]
- Blicharski, T.; Oniszczuk, A. Extraction Methods for the Isolation of Isoflavonoids from Plant Material. Open Chem. 2017, 15, 34–45. [Google Scholar] [CrossRef]
- Jung, S.; Murphy, P.A.; Sala, I. Isoflavone profiles of soymilk as affected by high-pressure treatments of soymilk and soybeans. Food Chem. 2008, 111, 592–598. [Google Scholar] [CrossRef]
- Moras, B.; Rey, S.; Vilarem, G.; Pontalier, P.-V. Pressurized water extraction of isoflavones by experimental design from soybean flour and Soybean Protein Isolate. Food Chem. 2017, 214, 9–15. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Roohinejad, S.; Kouba, M.; Barba, F.J.; Greiner, R.; Orlien, V.; Lebovka, N.I. Negative pressure cavitation extraction: A novel method for extraction of food bioactive compounds from plant materials. Trends Food Sci. Technol. 2016, 52, 98–108. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).