Optimisation of the Autothermal NH3 Production Process for Power-to-Ammonia
Abstract
1. Introduction
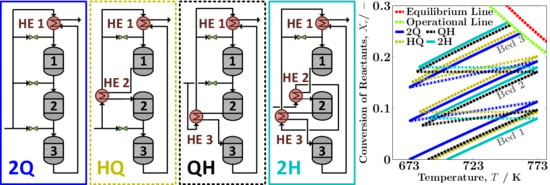
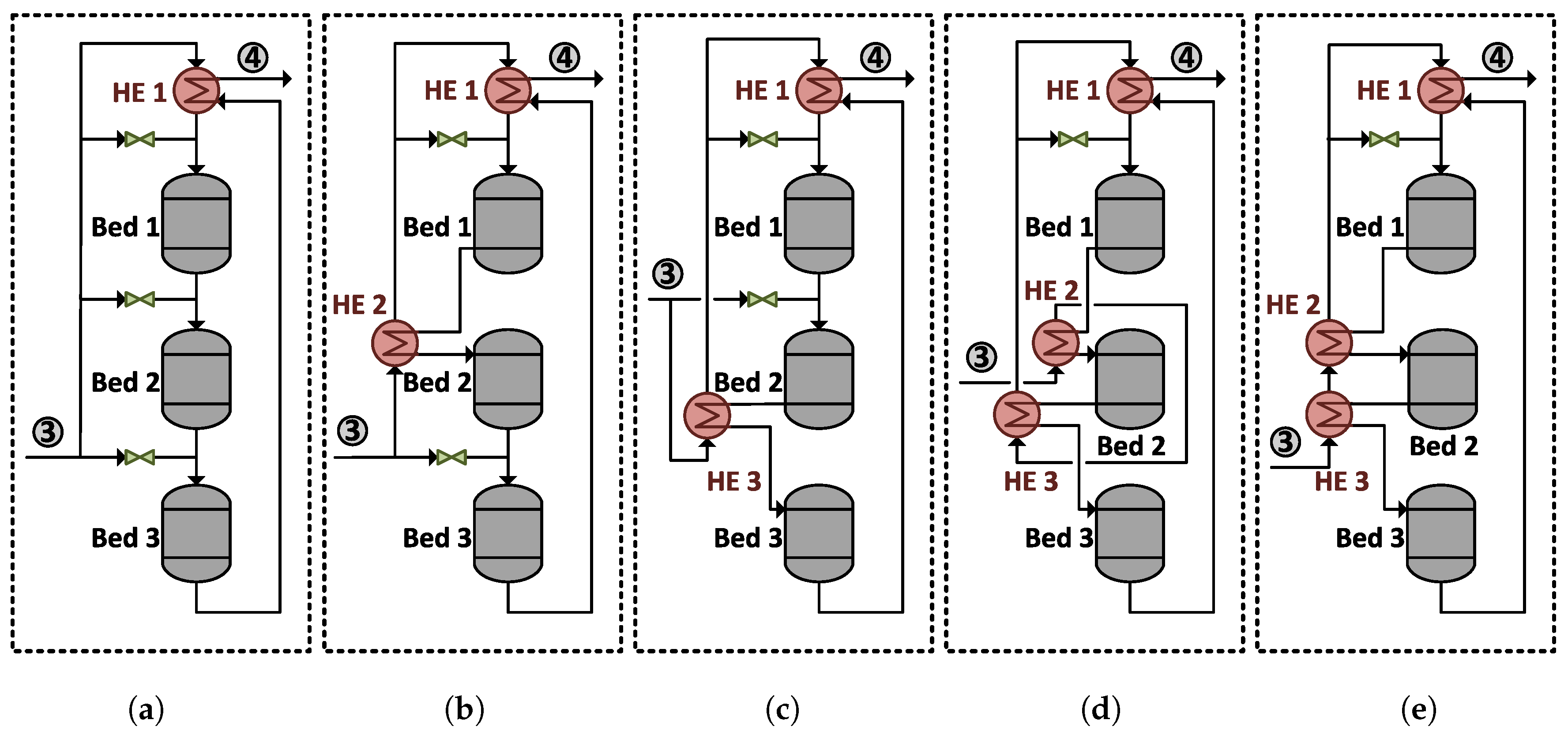
Ammonia Synthesis Reactor Systems
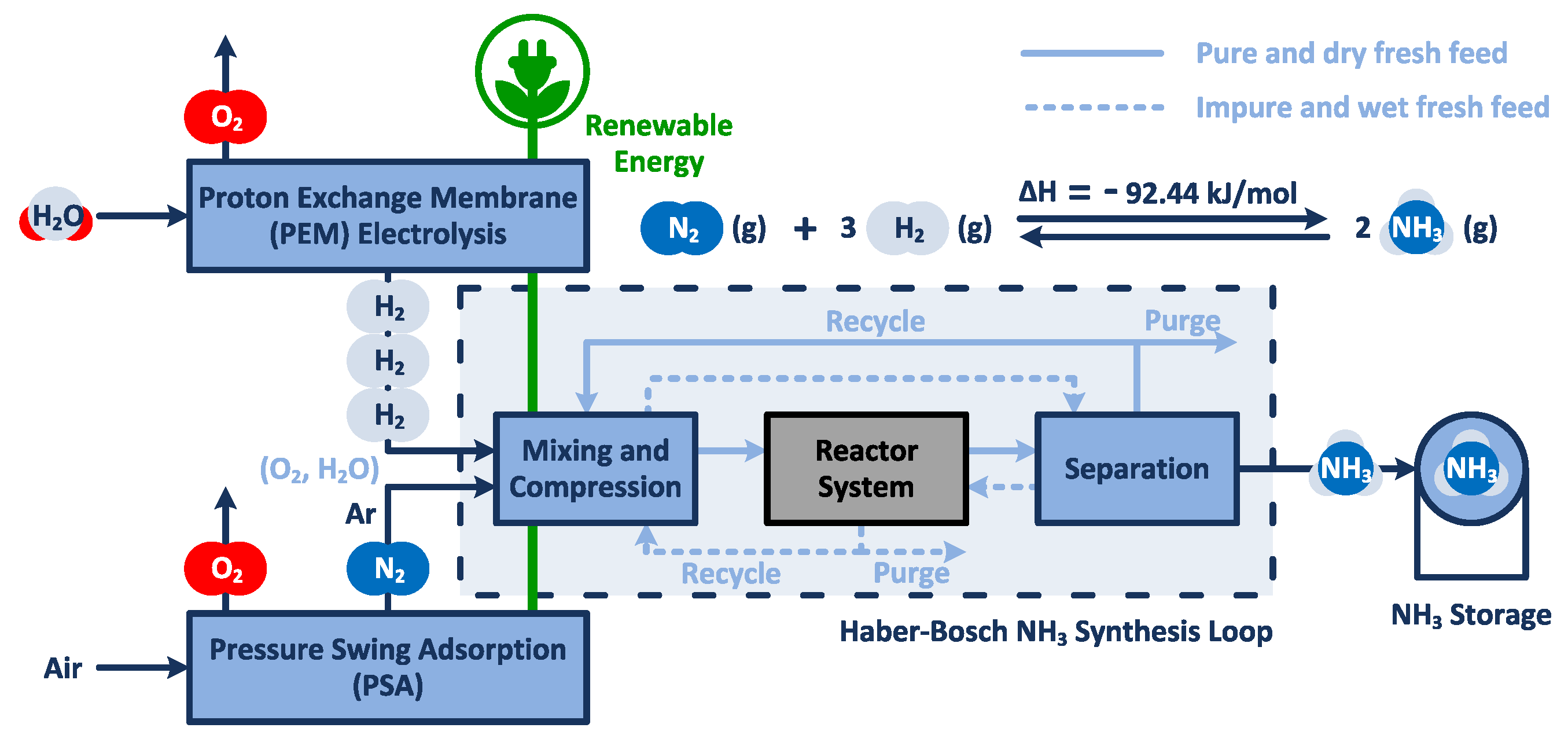
2. Methodology
2.1. Mathematical Model
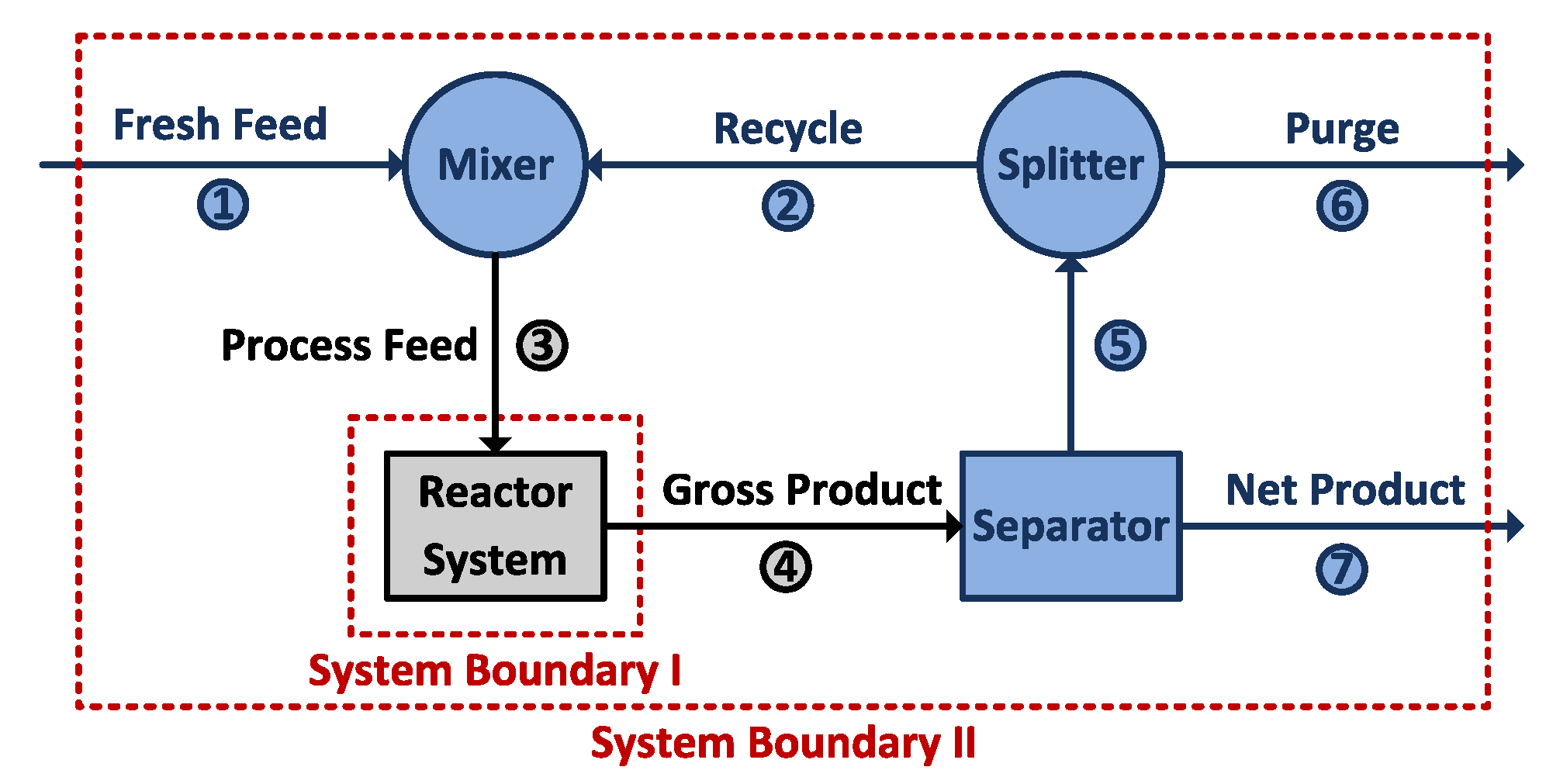
2.1.1. System Boundary I
Catalyst Bed
Mixer
Heat Exchanger
2.1.2. System Boundary II
2.2. Problem Formulation
2.2.1. Design Performance
System Boundary I
System Boundary II
2.2.2. Off-Design Performance
System Boundary I
System Boundary II
2.3. Problem Implementation
3. Results and Discussion
3.1. Design Performance
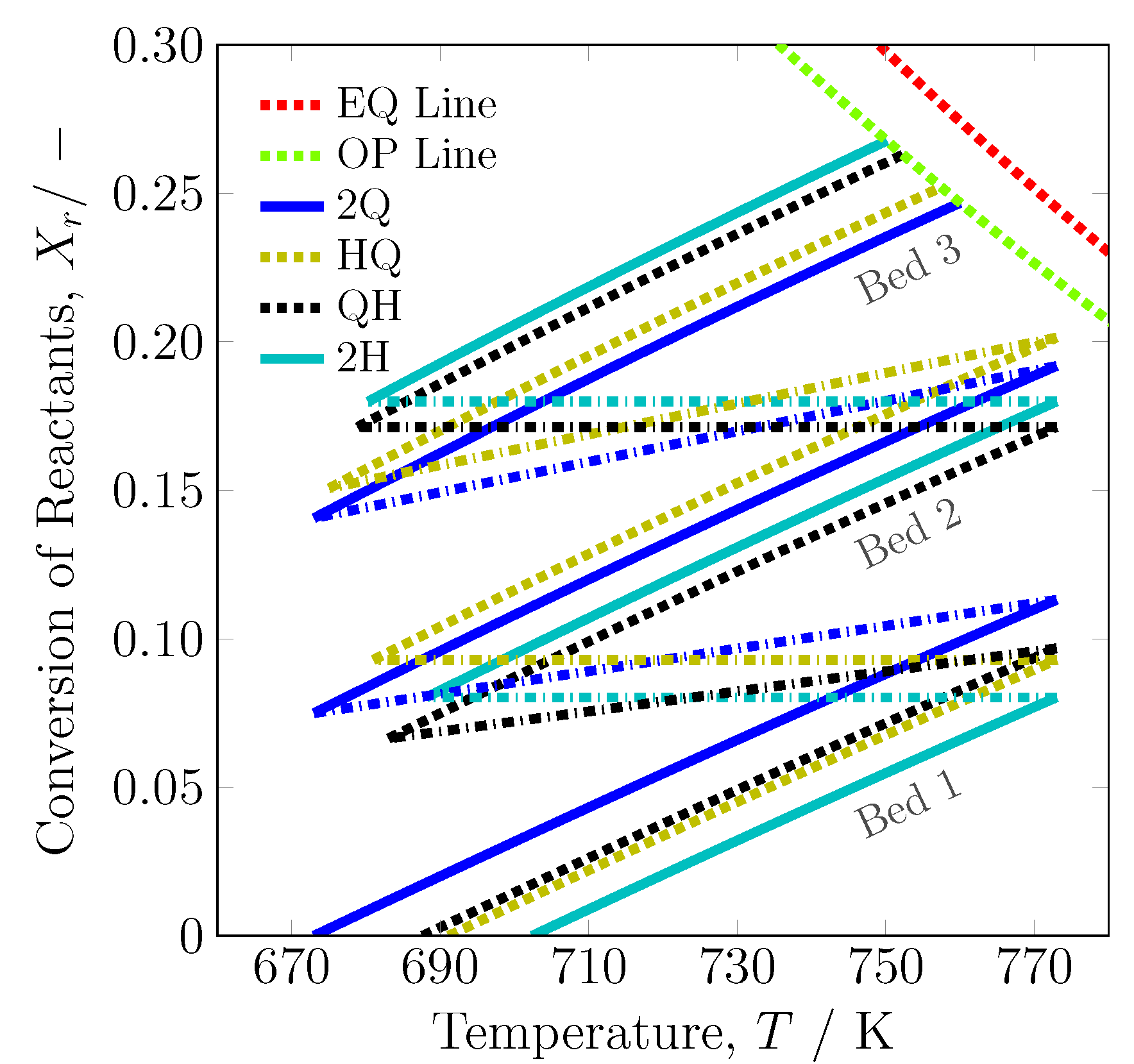
3.1.1. System Boundary I
3.1.2. System Boundary II
3.2. Off-Design Performance
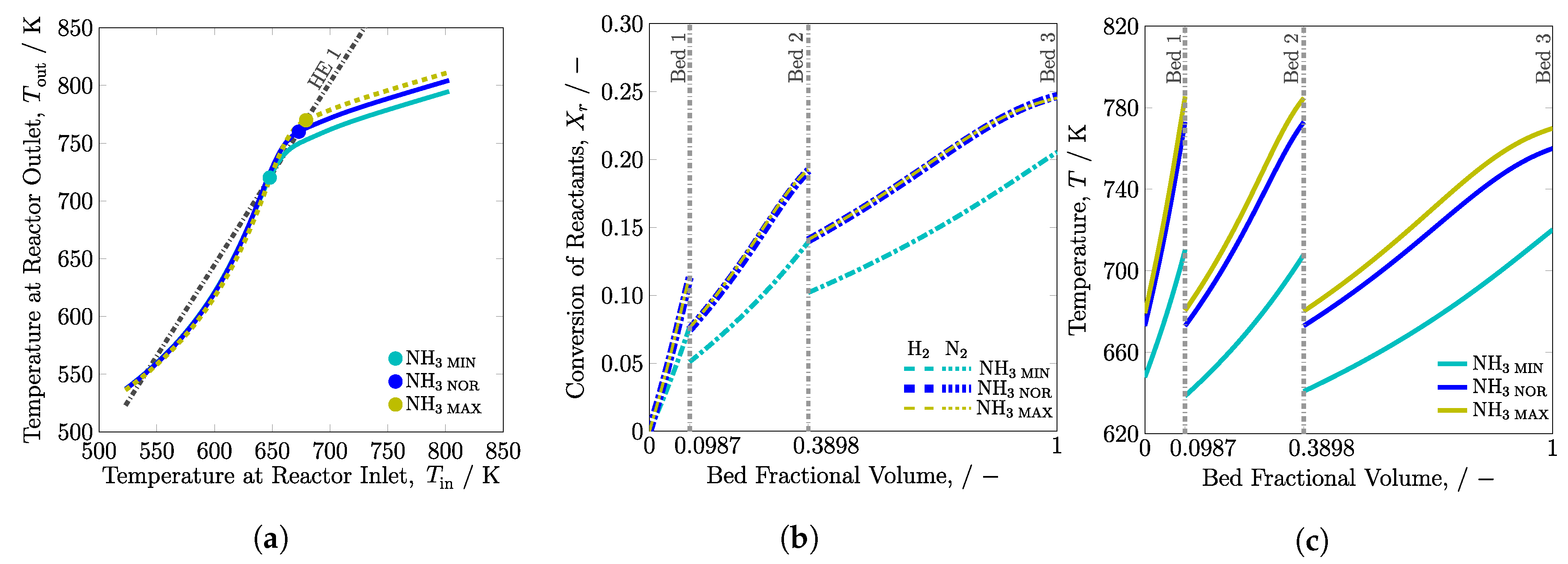
3.2.1. System Boundary I
3.2.2. System Boundary II
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| List of Symbols | |
| Specific heat capacity / kJ kg−1 K−1 | |
| Heat of reaction / kJ kmol−1 | |
| Mass flow rate / kg h−1 | |
| Molecular flow rate / kmol h−1 | |
| P | Pressure / bar |
| p | Purge fraction |
| Rate of reaction / kmol m−3 h−1 | |
| T | Temperature / K |
| Gross NH3 production / kg h−1 | |
| V | Volume of catalyst bed / m3 |
| X | Conversion of reactant / - |
| x | Mass fraction / - |
| Y | Mole percentage / mol % |
| z | Adjustable parameters |
| Greek Symbols | |
| Efficiency / % | |
| Stoichiometric coefficient / - | |
| Heat exchanger effectiveness / - | |
| Subscripts | |
| b | Catalyst bed |
| Heat exchanger | |
| m | Mixer |
| q | Quench stream |
| r | Reactant |
| Reactor system | |
| EQ | Equilibrium |
| in | Inlet |
| MAX | Maximum |
| MIN | Minimum |
| NOR | Normal |
| out | Outlet |
References
- Morgan, E.; Manwell, J.; McGowan, J. Wind-powered ammonia fuel production for remote islands: A case study. Renew. Energy 2014, 72, 51–61. [Google Scholar] [CrossRef]
- Fuhrmann, J.; Hülsebrock, M.; Krewer, U. Energy storage based on electrochemical conversion of ammonia. In Transition to Renewable Energy Systems; Stolten, D., Scherer, V., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; Chapter 33; pp. 691–706. [Google Scholar] [CrossRef]
- Patil, A.; Laumans, L.; Vrijenhoef, H. Solar to ammonia—via Proton’s NFuel units. Procedia Eng. 2014, 83, 322–327. [Google Scholar] [CrossRef]
- Reese, M.; Marquart, C.; Malmali, M.; Wagner, K.; Buchanan, E.; McCormick, A.; Cussler, E.L. Performance of a small-scale Haber process. Ind. Eng. Chem. Res. 2016, 55, 3742–3750. [Google Scholar] [CrossRef]
- Science & Technology Facilities Council. Available online: https://stfc.ukri.org/news/uk-team-develop-worlds-first-green-energy-storage-demonstrator/ (accessed on 28 May 2019).
- Appl, M. Ammonia. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar] [CrossRef]
- Cinti, G.; Frattini, D.; Jannelli, E.; Desideri, U.; Bidini, G. Coupling Solid Oxide Electrolyser (SOE) and ammonia production plant. Appl. Energy 2017, 192, 466–476. [Google Scholar] [CrossRef]
- Sánchez, A.; Martín, M. Optimal renewable production of ammonia from water and air. J. Clean. Prod. 2018, 178, 325–342. [Google Scholar] [CrossRef]
- Wang, G.; Mitsos, A.; Marquardt, W. Conceptual design of ammonia-based energy storage system: System design and time-invariant performance. AIChE J. 2017, 63, 1620–1637. [Google Scholar] [CrossRef]
- Malmali, M.; Reese, M.; McCormick, A.V.; Cussler, E.L. Converting Wind Energy to Ammonia at Lower Pressure. ACS Sustain. Chem. Eng. 2018, 6, 827–834. [Google Scholar] [CrossRef]
- Strelzoff, S. Technology and Manufacture of Ammonia; John Wiley & Sons Inc: New York, NY, USA, 1981. [Google Scholar]
- Khademi, M.H.; Sabbaghi, R.S. Comparison between three types of ammonia synthesis reactor configurations in terms of cooling methods. Chem. Eng. Res. Des. 2017, 128, 306–317. [Google Scholar] [CrossRef]
- Luyben, W.L. Design and Control of a Cooled Ammonia Reactor. In Plantwide Control; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2012; pp. 273–292. [Google Scholar] [CrossRef]
- Gaines, L.D. Optimal Temperatures for Ammonia Synthesis Converters. Ind. Eng. Chem. Process Des. Dev. 1977, 16, 381–389. [Google Scholar] [CrossRef]
- Akpa, J.G.; Raphael, N.R. Optimization of an Ammonia Synthesis Converter. World J. Eng. Technol. 2014, 2, 305–313. [Google Scholar] [CrossRef]
- Elnashaie, S.S.; Abashar, M.E.; Al-Ubaid, A.S. Simulation and optimization of an industrial ammonia reactor. Ind. Eng. Chem. Res. 1988, 27, 2015–2022. [Google Scholar] [CrossRef]
- Elnashaie, S.; Alhabdan, F. A computer software package for the simulation and optimization of an industrial ammonia converter based on a rigorous heterogeneous model. Math. Comput. Model. 1989, 12, 1589–1600. [Google Scholar] [CrossRef]
- Azarhoosh, M.J.; Farivar, F.; Ale Ebrahim, H. Simulation and optimization of a horizontal ammonia synthesis reactor using genetic algorithm. RSC Adv. 2014, 4, 13419–13429. [Google Scholar] [CrossRef]
- Farivar, F.; Ebrahim, A. Modeling, Simulation, and Configuration Improvement of Horizontal Ammonia Synthesis Reactor. Chem. Prod. Process Model. 2014, 9, 89–95. [Google Scholar] [CrossRef]
- Cheema, I.I.; Krewer, U. Operating Envelope of Haber-Bosch Process Design for Power-to-Ammonia. RSC Adv. 2018, 8, 34926–34936. [Google Scholar] [CrossRef]
- Bañares-Alcãntara, R.; Dericks, G., III; Fiaschetti, M.; Grünewald, P.; Lopez, J.M.; Tsang, E.; Yang, A.; Ye, L.; Zhao, S. Analysis of Islanded NH3-Based Energy Storage Systems; Technical Report; University of Oxford: Oxford, UK, 2015. [Google Scholar]
- Faraoni, V.; Mancusi, E.; Russo, L.; Continillo, G. Bifurcation analysis of periodically forced systems via continuation of a discrete map. In European Symposium on Computer Aided Process Engineering-11; Gani, R., Jørgensen, S.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 9, pp. 135–140. [Google Scholar] [CrossRef]
- Mancusi, E.; Maffettone, P.L.; Gioia, F.; Crescitelli, S. Nonlinear analysis of heterogeneous model for an industrial ammonia reactor. Chem. Prod. Process Model. 2009, 4, 1–23. [Google Scholar] [CrossRef]
- Rovaglio, M.; Manca, D.; Cortese, F.; Mussone, P. Multistability and robust control of the ammonia synthesis loop. In European Symposium on Computer Aided Process Engineering-11; Gani, R., Jørgensen, S.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 9, pp. 723–730. [Google Scholar] [CrossRef]
- Elnashaie, S.S.E.H.; Elshishini, S.S. Modelling, Simulation and Optimization of Industrial Fixed Bed Catalytic Reactors; Gordon and Breach Science Publishers: Yverdon, Switzerland, 1993; Volume 7. [Google Scholar]
- Dyson, D.C.; Simon, J.M. Kinetic expression with diffusion correction for ammonia synthesis on industrial catalyst. Ind. Eng. Chem. Fundam. 1968, 7, 605–610. [Google Scholar] [CrossRef]
- Skogestad, S. Chemical and Energy Process Engineering; Taylor & Francis: Boca Raton, FL, USA, 2008. [Google Scholar]
- Sinnott, R.; Towler, G. Chemical Engineering Design: SI Edition; Chemical Engineering Series; Elsevier Science: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Kakaç, S.; Liu, H.; Pramuanjaroenkij, A. Heat Exchangers: Selection, Rating, and Thermal Design, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Morud, J.C.; Skogestad, S. Analysis of instability in an industrial ammonia reactor. AIChE J. 1998, 44, 888–895. [Google Scholar] [CrossRef]
- Thirumaleshwar, M. Fundamentals of Heat and Mass Transfer; Always Learning; Pearson Education: London, UK, 2009. [Google Scholar]
- Nicol, W.; Hildebrandt, D.; Glasser, D. Crossing reaction equilibrium in an adiabatic reactor system. Dev. Chem. Eng. Miner. Process. 1998, 6, 41–54. [Google Scholar] [CrossRef]
- Van Heerden, C. Autothermic processes. Ind. Eng. Chem. 1953, 45, 1242–1247. [Google Scholar] [CrossRef]
- Schulte-Schulze-Berndt, A.; Krabiell, K. Nitrogen generation by pressure swing adsorption based on carbon molecular sieves. Gas Sep. Purif. 1993, 7, 253–257. [Google Scholar] [CrossRef]



| Reactor Feed ③ Composition | |||
|---|---|---|---|
| YH2,③ /mol % | YN2,③ /mol % | YNH3,③ /mol % | YAr,③ /mol % |
| 68.12 | 22.71 | 4.17 | 5.00 |
| Reactor inlet ③ & normal operational conditions | |||
| ③/kg h−1 | P/bar | T③/K | /- |
| 662.54 | 200.00 | 523.00 | 0.00 |
| Volumes of catalyst beds | |||
| /m3 | /m3 | /m3 | /m3 |
| 0.0075 | 0.0221 | 0.0464 | 0.0760 |
| b1 | Tb1in | q2 | Tb2in | q3 | Tb3in | NH3 | |
|---|---|---|---|---|---|---|---|
| RS | /kg h−1 | /K | /kg h−1 | /K | /kg h−1 | /K | /kg h−1 |
| 2Q [20] | 321.70 | 673.00 | 163.83 | 673.00 | 177.01 | 673.00 | 121.11 |
| HQ | 495.80 | 691.14 | - | 681.01 | 166.74 | 675.07 | 123.70 |
| QH | 454.65 | 687.63 | 207.89 | 683.06 | - | 678.87 | 129.08 |
| 2H-2 | 662.54 | 702.39 | - | 688.16 | - | 680.30 | 131.38 |
| 2H-3 | 662.54 | 702.39 | - | 688.16 | - | 680.30 | 131.38 |
| Reactor | Fresh Feed | Purge Percentage | Purge 1 | Recycle 1 | Product 1 | Separator Efficiency |
|---|---|---|---|---|---|---|
| Systems | ① | ⑥ | ② | ⑦ | ||
| /kg h−1 | /% | /kg h−1 | /kg h−1 | /kg h−1 | /% | |
| 2Q [20] | 133.05 | 2.41 | 13.05 | 529.48 | 120.00 | 72.21 |
| HQ | 135.82 | 2.46 | 13.26 | 526.70 | 122.56 | 72.63 |
| QH | 141.58 | 2.56 | 13.69 | 520.95 | 127.90 | 73.44 |
| 2H | 144.04 | 2.60 | 13.87 | 518.49 | 130.17 | 73.78 |
| Inert Gas Concentration | Flow Rate | Reactants Ratio | |||||
|---|---|---|---|---|---|---|---|
| RS | Design and | YAr,③ | NH3 | ③ | NH3 | H2,③:N2,③ | NH3 |
| Off-Design | /mol % | /kg h−1 | /kg h−1 | /kg h−1 | / | /kg h−1 | |
| 2Q | MIN | ||||||
| NOR [20] | |||||||
| MAX | |||||||
| HQ | MIN | ||||||
| NOR | |||||||
| MAX | |||||||
| QH | MIN | ||||||
| NOR | |||||||
| MAX | |||||||
| 2H-2 | MIN | ||||||
| NOR | |||||||
| MAX | |||||||
| 2H-3 | MIN | ||||||
| NOR | |||||||
| MAX | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheema, I.I.; Krewer, U. Optimisation of the Autothermal NH3 Production Process for Power-to-Ammonia. Processes 2020, 8, 38. https://doi.org/10.3390/pr8010038
Cheema II, Krewer U. Optimisation of the Autothermal NH3 Production Process for Power-to-Ammonia. Processes. 2020; 8(1):38. https://doi.org/10.3390/pr8010038
Chicago/Turabian StyleCheema, Izzat Iqbal, and Ulrike Krewer. 2020. "Optimisation of the Autothermal NH3 Production Process for Power-to-Ammonia" Processes 8, no. 1: 38. https://doi.org/10.3390/pr8010038
APA StyleCheema, I. I., & Krewer, U. (2020). Optimisation of the Autothermal NH3 Production Process for Power-to-Ammonia. Processes, 8(1), 38. https://doi.org/10.3390/pr8010038