1. Introduction
Raphanus sativus L., commonly referred to as radish or daikon, is a cruciferous vegetable that belongs to the Brassicaceae family and finds a myriad of applications in the daily diet and traditional medicine of Eastern societies. The radish is one of the most consumed vegetables in Asian countries, including Vietnam, China and Japan, and is regarded as an important ingredient in the treatment of liver and respiratory diseases [
1,
2]. Biological activities of radish are diverse and have been ascertained in many investigations, which were well summarized in a past review [
3]. In brief, the radish extract was shown to exhibit inhibitory effect on growth of G+, G’’ bacteria, antifungal, immunological, antioxidant, anticancer and antiviral properties. The antibacterial activity is mostly due to the presence of cysteine-rich peptides (Rs-AFP1 and Rs-AFP2), RAP-1 and RAP-2 protein, cafeic, ferulic and phydroxybenzoic acid. In addition, it was found that four types of the glucosinolate compound, including glucoraphenin, dehydroerucin, glucobrassicin, and glucoerucin are responsible for the pungency of
Raphanus sativus, predominate in roots of radish [
4]. Glucosinolate has been shown to take part in promoting enzymatic activities involving removal of carcinogens and preventing aging in the human body.
Different parts of the radish plant show varying degrees of biological activities [
5,
6]. While seeds and aerial parts of
Raphanus sativus have been long known for strong antifungal and antibacterial activity due to the presence of the raphanin compound, to date, current evidence has only documented moderate inhibitory effects of the
Raphanus sativus root against various bacteria species only and thus, making data with regard to antifungal activities of
Raphanus sativus uncomprehensive [
7,
8,
9,
10]. For example, in a previous in vitro study involving 52 selected food-borne bacteria species, methanol extracts from white skin
Raphanus sativus roots were shown to inhibit the growth of 11 species (
Arthrobacter atrocyaneus,
Corynebacterium ammoniagenes,
Enterobacter hormaechei,
Kocuria rosea,
Neisseria subflava,
Pantoea agglomerans,
Proteus vulgaris,
Psychrobacter immobilis,
Shigella dysenteriae,
Bacillus sphaericus and
Corynebacterium flavescens) [
11]. This result was further extended in another similar study where growth-inhibitory effect of radish root juice was determined against five other microbial species including
Klebsiella pneumoniae,
Staphylococcus aureus,
Pseudomonas aeruginosa,
Enterococcus faecalis and
Escherichia coli with minimum inhibitory concentrations (MIC) ranging from 0.078 to 0.625 mg/mL [
12]. However, those reported antibacterial effects were not as high as those exhibited by standard drugs, which are respectively maxipime and ampicillin in the two studies.
Apart from the cysteine-rich peptides, the antibacterial activity of the taproot of
Raphanus sativus might be attributed specifically to its content of flavonoids, which figure for various health-beneficial effects and strong antimicrobial properties [
3]. The inhibition mechanism of flavonoids against microbial growth was implied in a review proposing that highly polar phenolic compounds, including flavonoids, tannins, and quinones, could bind to and thus interfering with the conformation of ABC transporter proteins in pathogenic fungi and microbes [
13,
14]. In Indian
Raphanus sativus roots, the total flavonoid content reached 43.5 mg CE/100 g fresh mass, which was comparable to those found in Indian apples and ladyfinger fruits, respectively at 48.5 and 48.2 mg CE/100 g fresh mass [
15]. This was supported by results of another study where flavonoids and alkaloids were consistently found in
Raphanus sativus root peel extracts regardless of employed solvent and extraction method [
16]. In terms of flavonoid composition in
Raphanus sativus root, it was revealed that radish root contains four major types of flavonoids including kaempferol, luteolin, quercetin and apigenin respectively at 32.3, 19.5, 5.2 and 2.2 mg/kg fresh weight [
17].
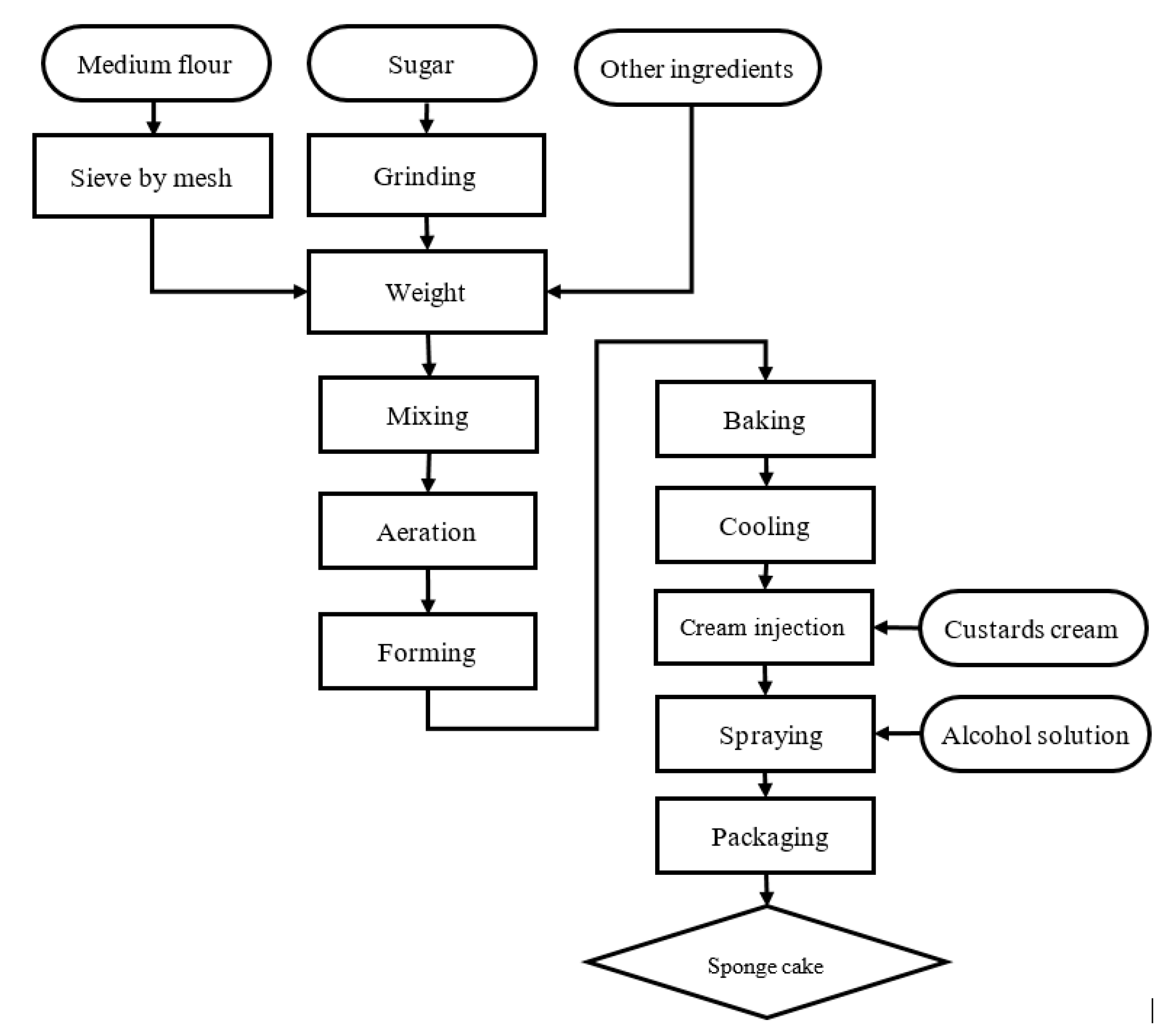
It is a common extrapolation in previous antimicrobial and antioxidant assays that Raphanus sativus taproots could act as a promising ingredient for manufacture of bio-preservatives used in foods. However, to our knowledge, it is difficult to comprehend this justification in absence of relevant results on antifungal activity. In addition, to permit feasible production of health-safe additives, optimization of flavonoid content accruing from the extraction process of the radish root as well as preservability and palatability evaluation of food supplemented with radish-derived preservatives should be carried out. Therefore, the objective of in this study is three-fold. First, we optimize the total flavonoid content in the extracts of the Raphanus sativus roots with respect to different experimental parameters. Second, the antifungal activity against Aspergillus flavus NBRC 33021, Aspergillus niger NBRC 4066, Aspergillus clavatus NBRC 33020, and Fusarium solani NBRC 31094 fungi of the obtained Raphanus sativus taproot extracts will be determined via well diffusion assays. Third, to assess the suitability of the extracts in food applications, we evaluate preservability, sensory characteristics and several important microbial indicators of a sponge cake formula incorporated with the dried extract of the Raphanus sativus root. The results are expected to aid further development of natural preservatives derived from abundant and inexpensive plant materials.
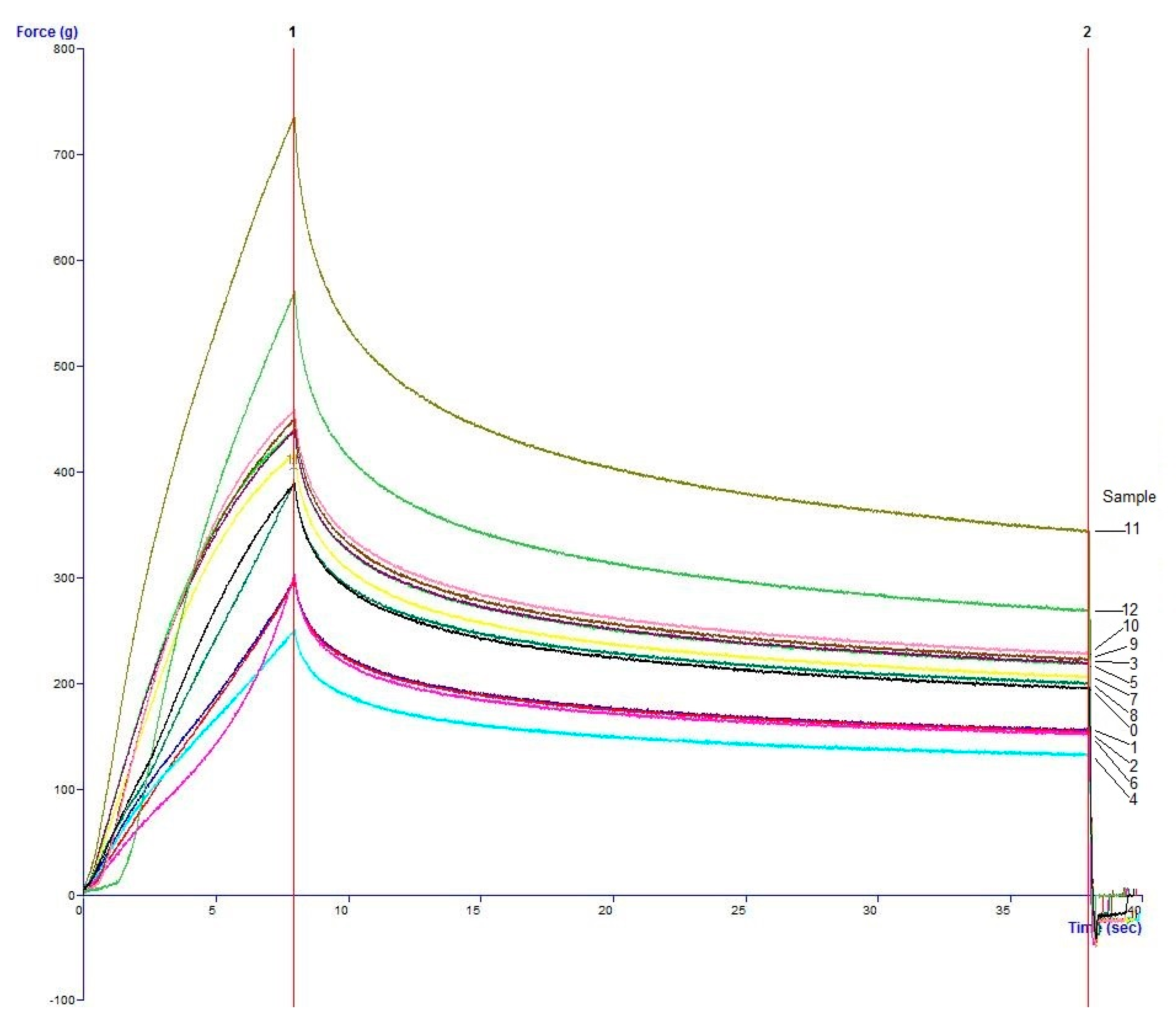
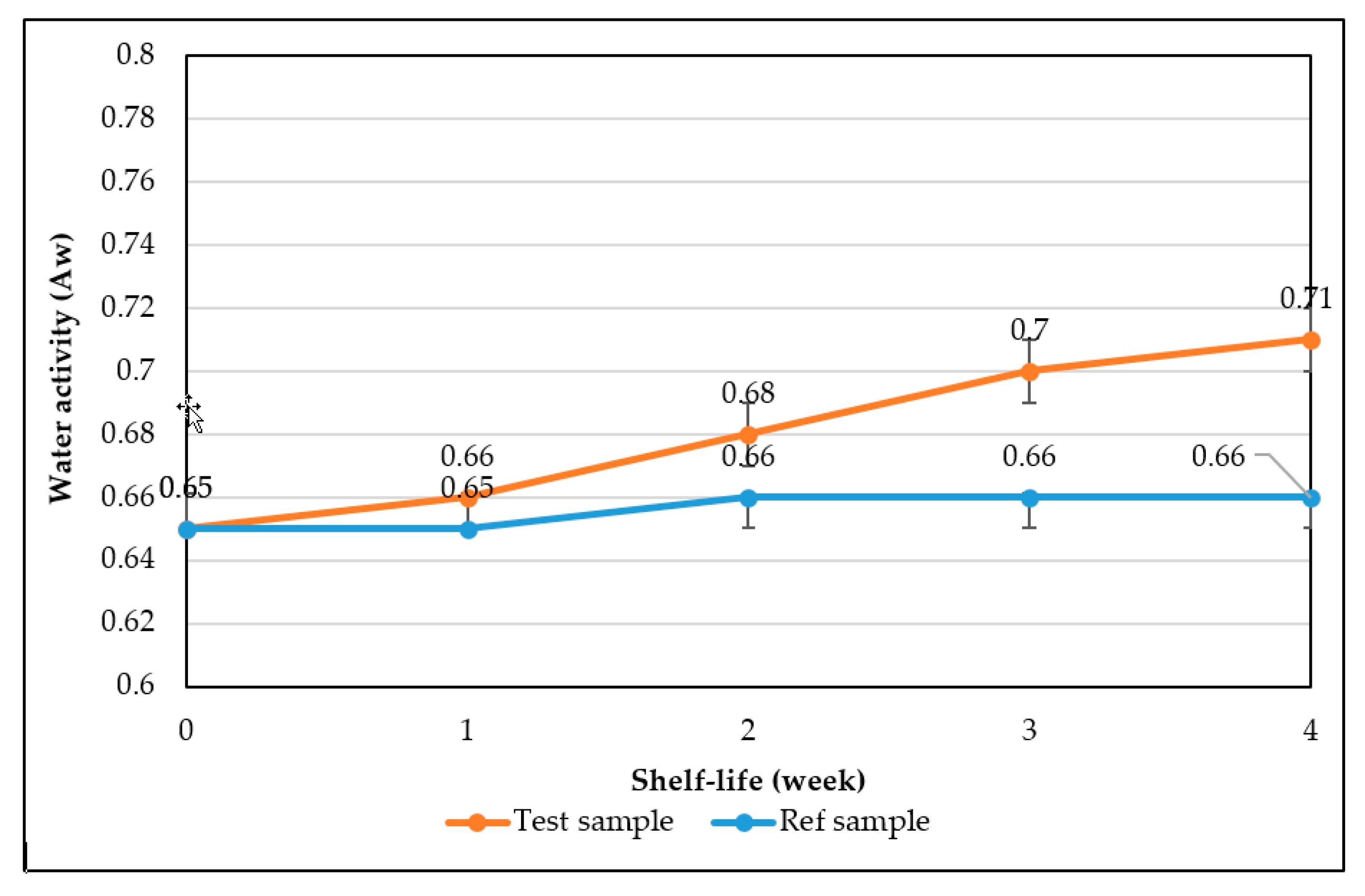
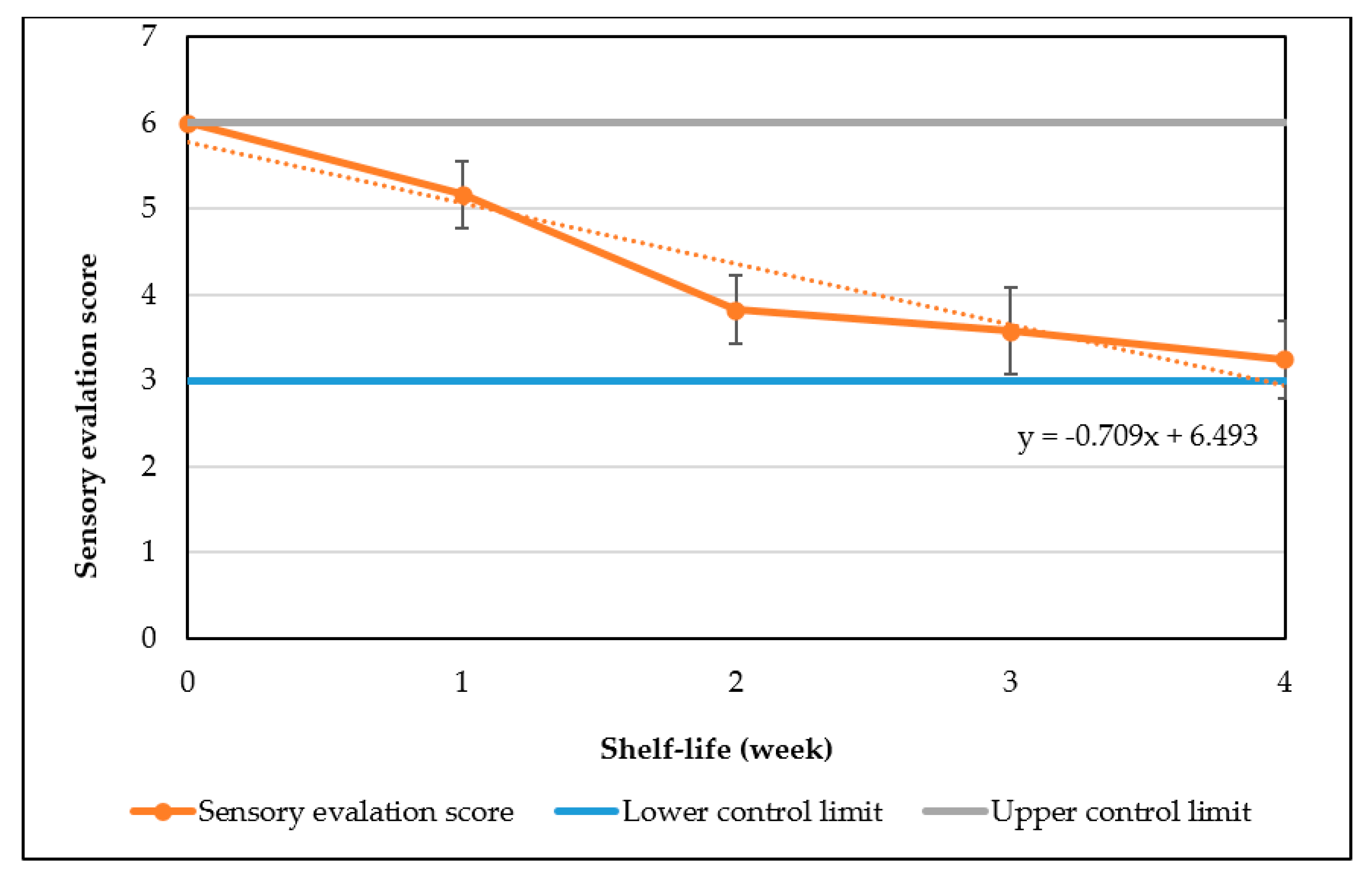
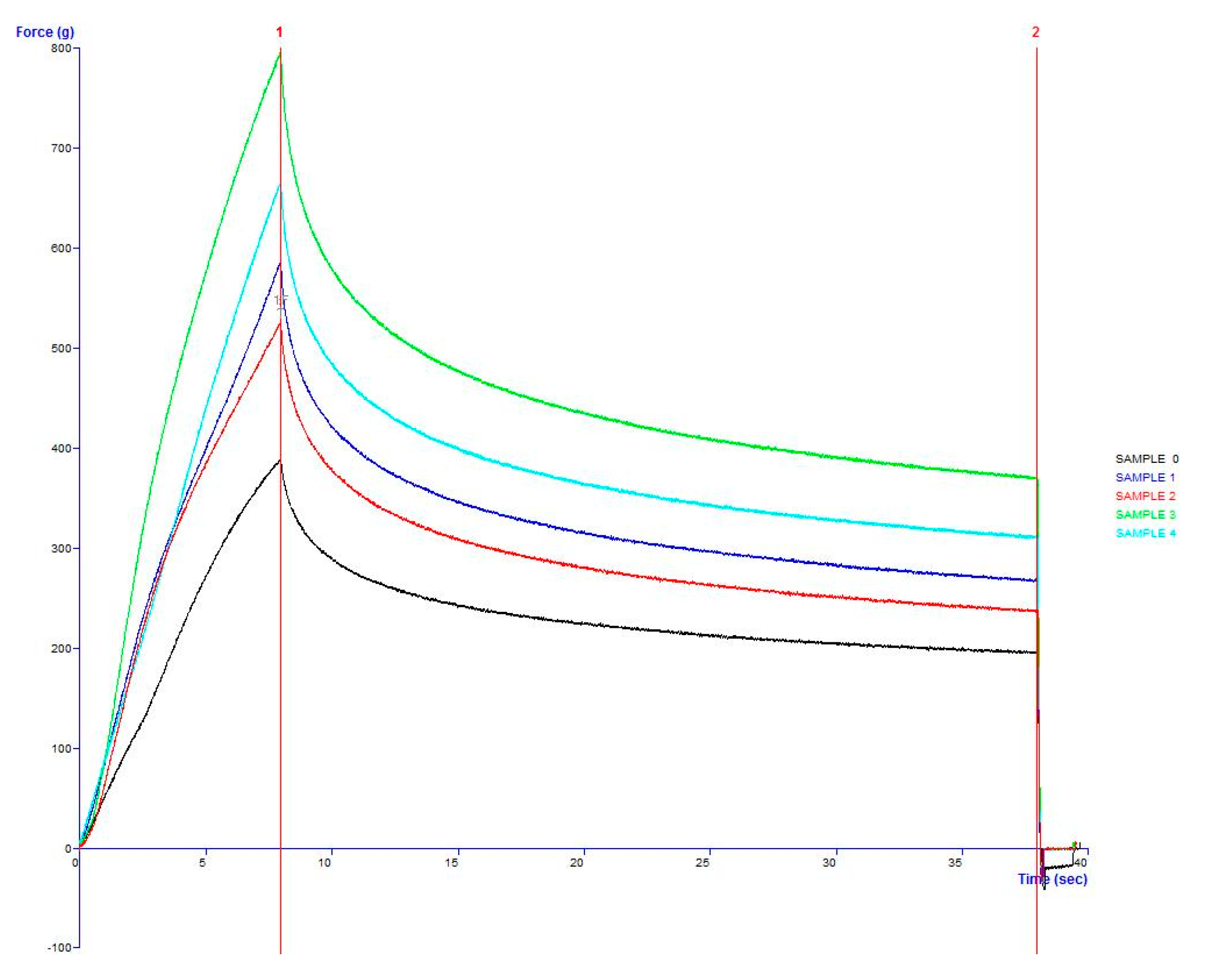
4. Conclusions
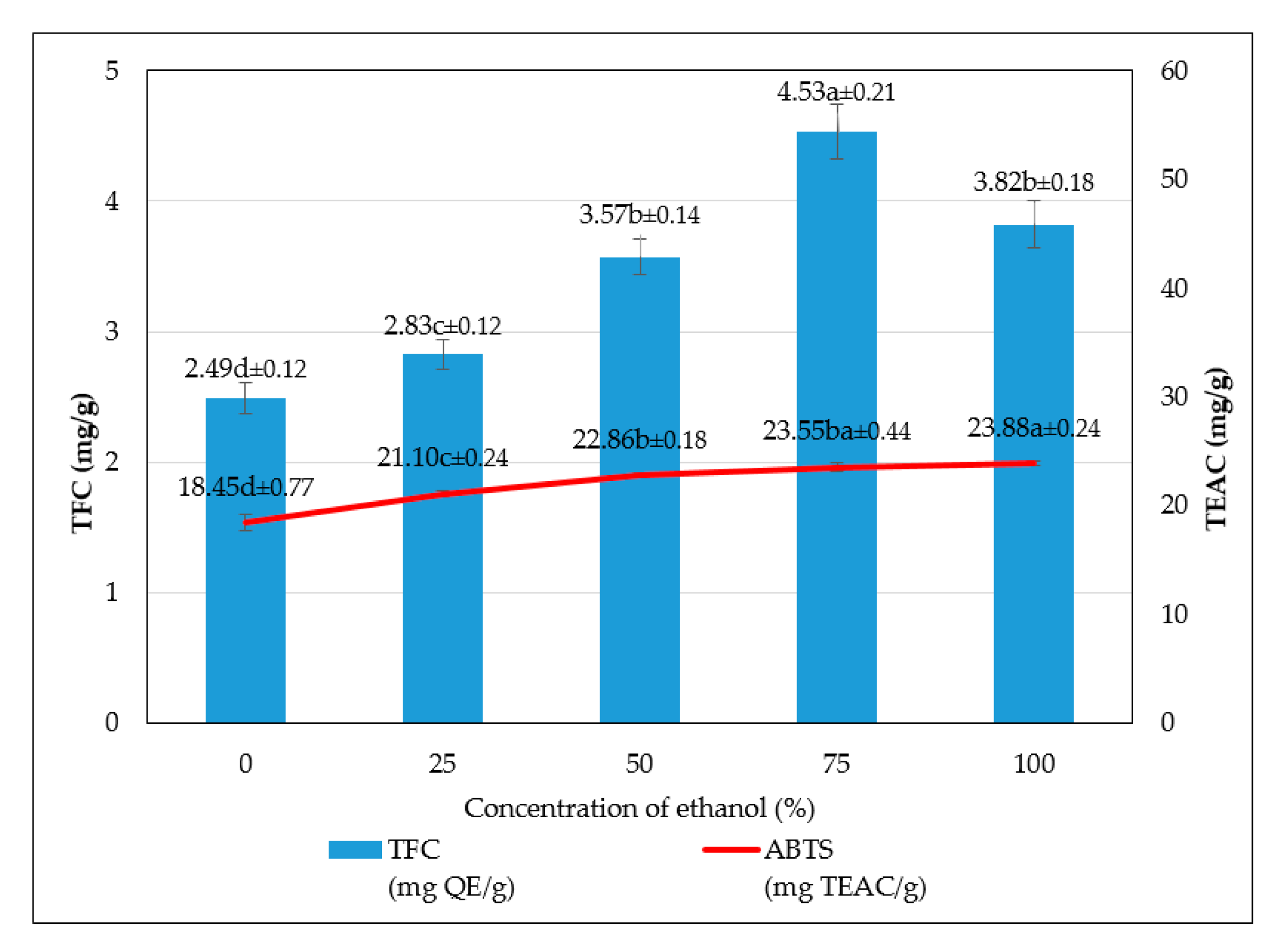
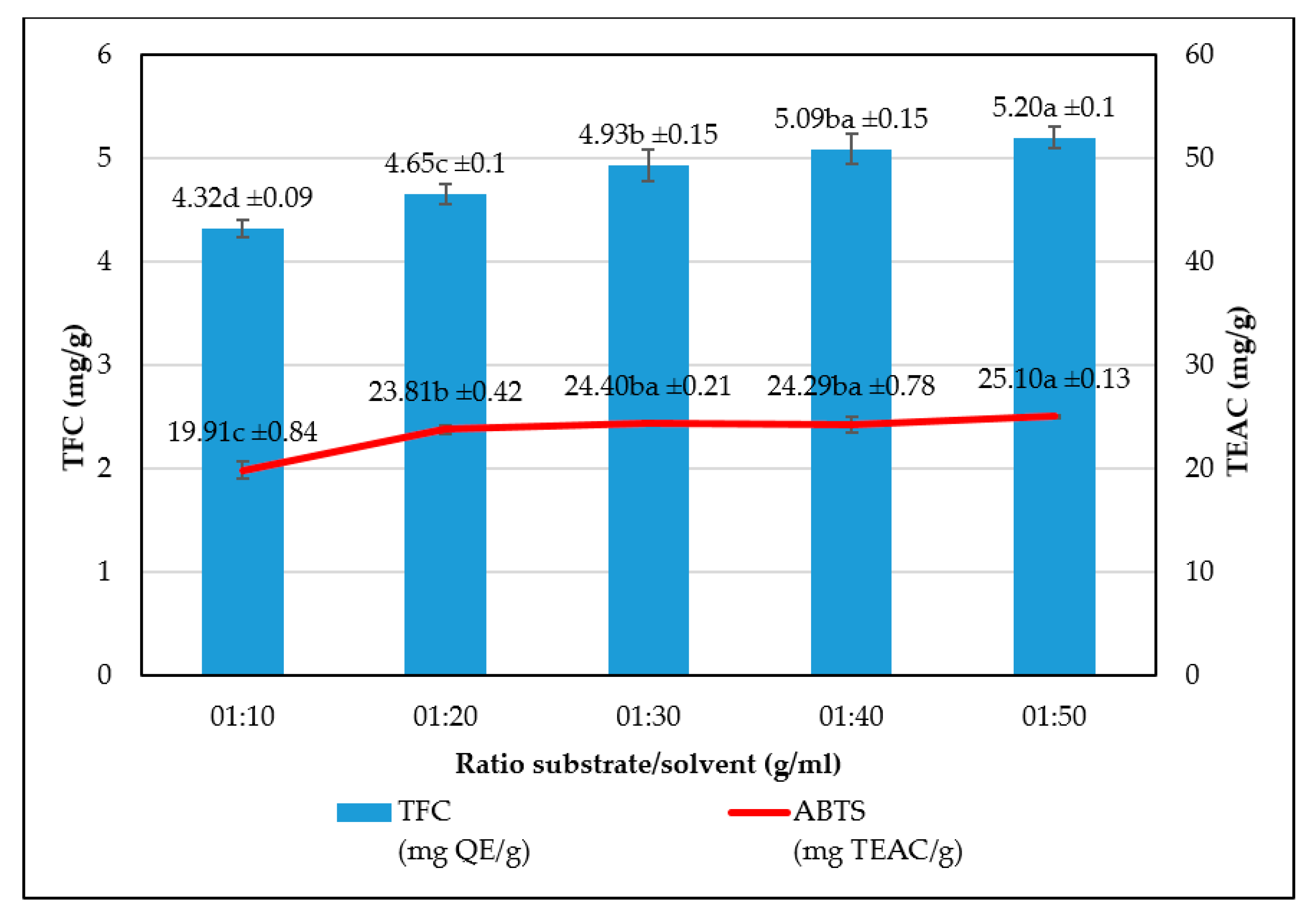
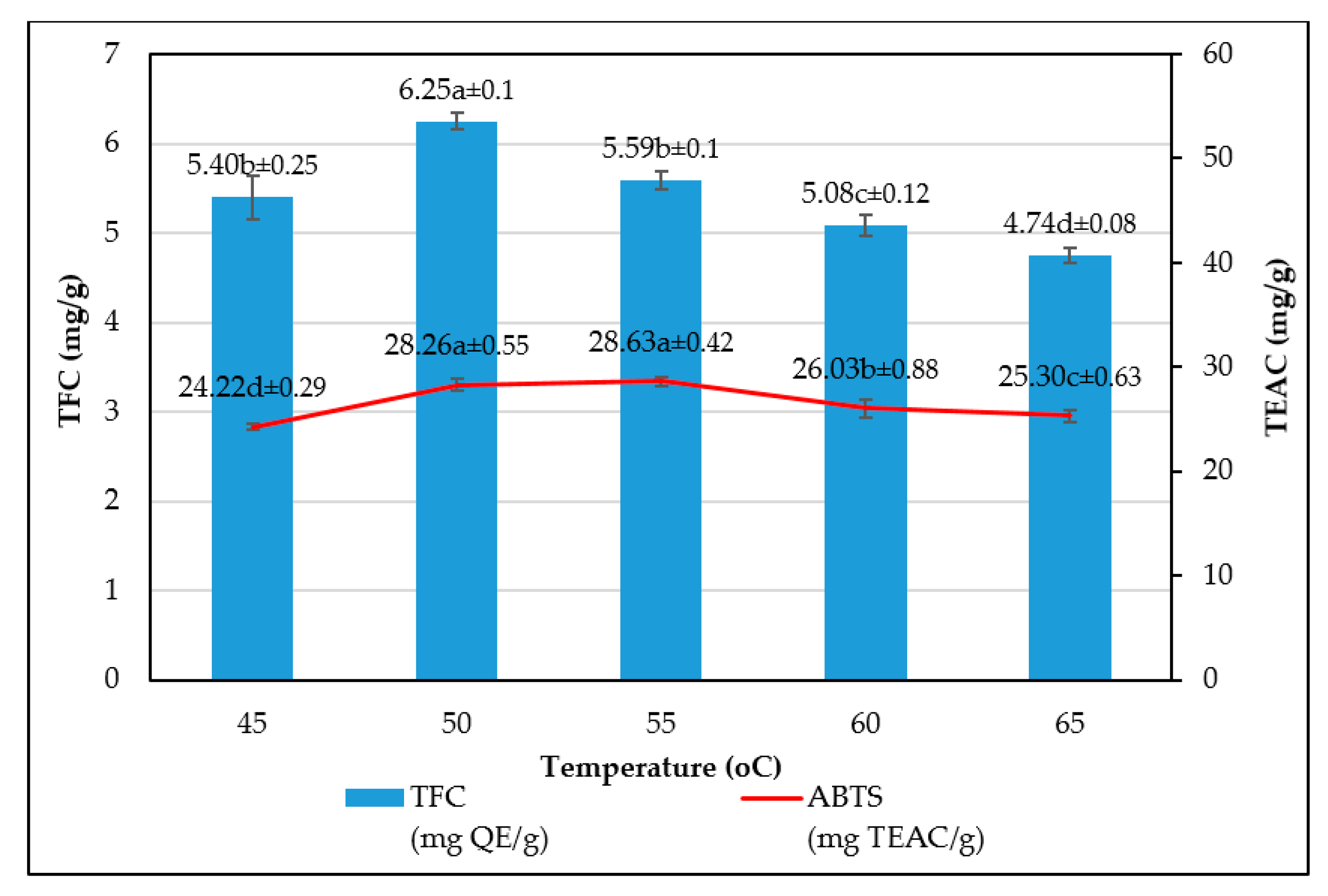
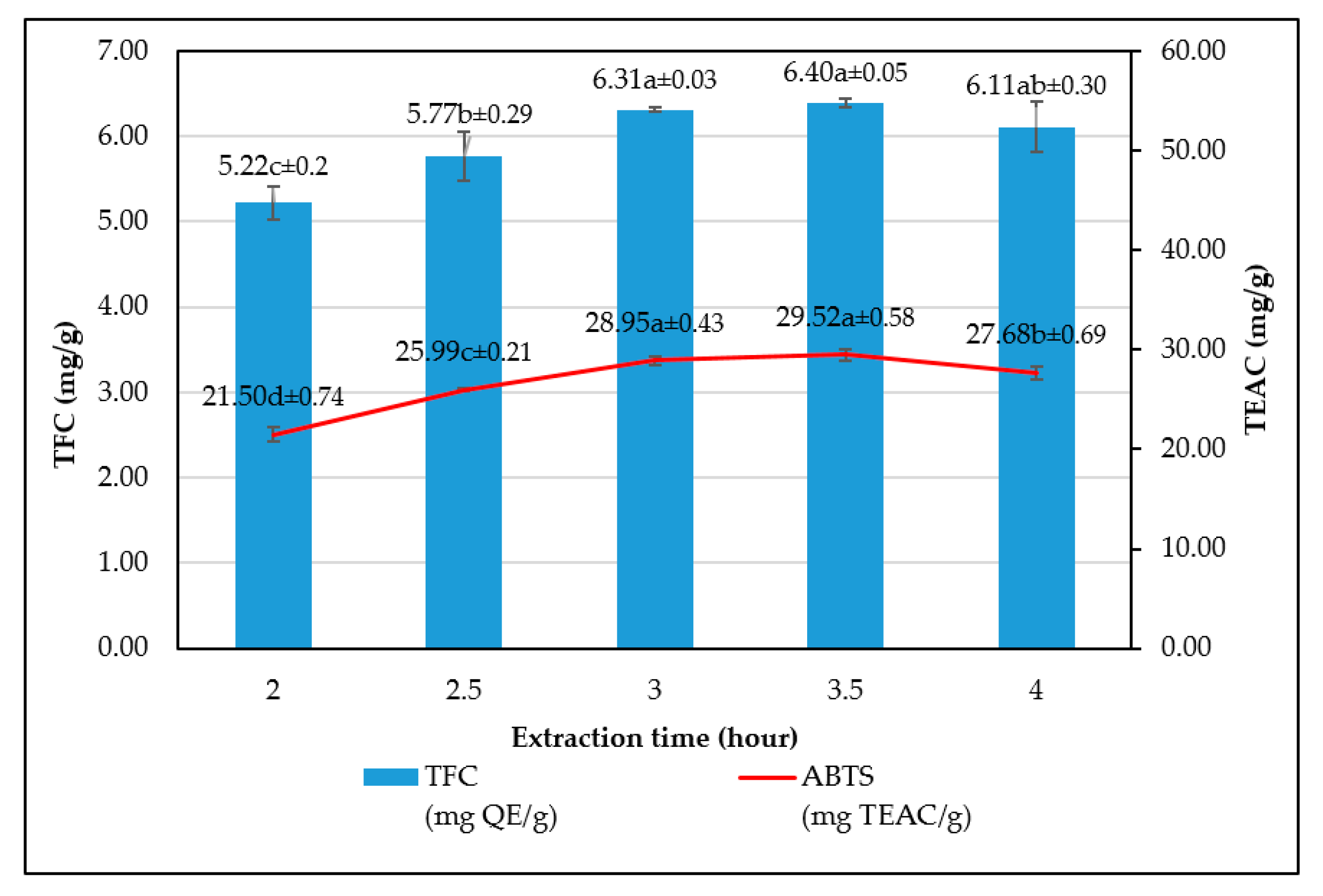
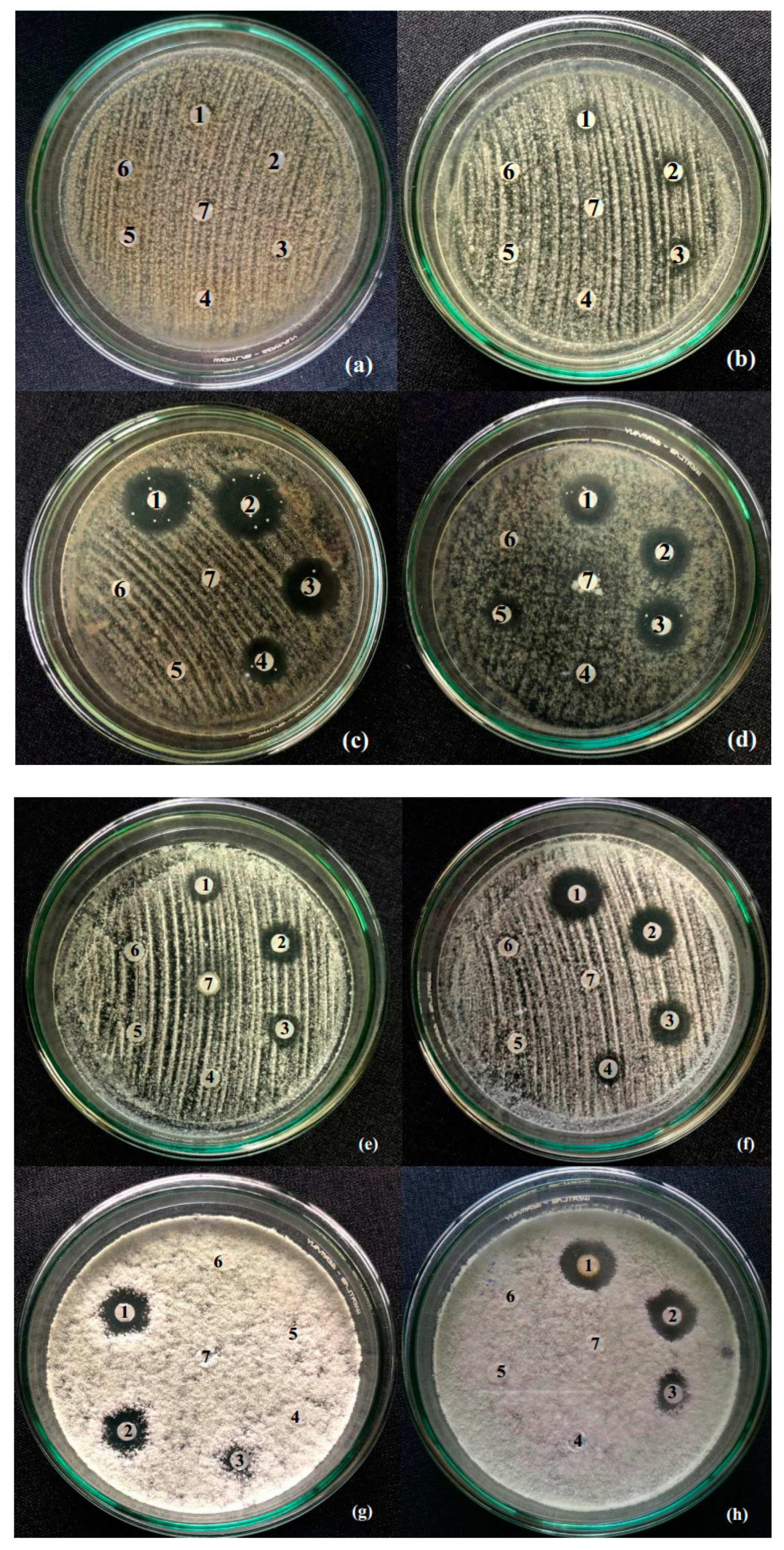
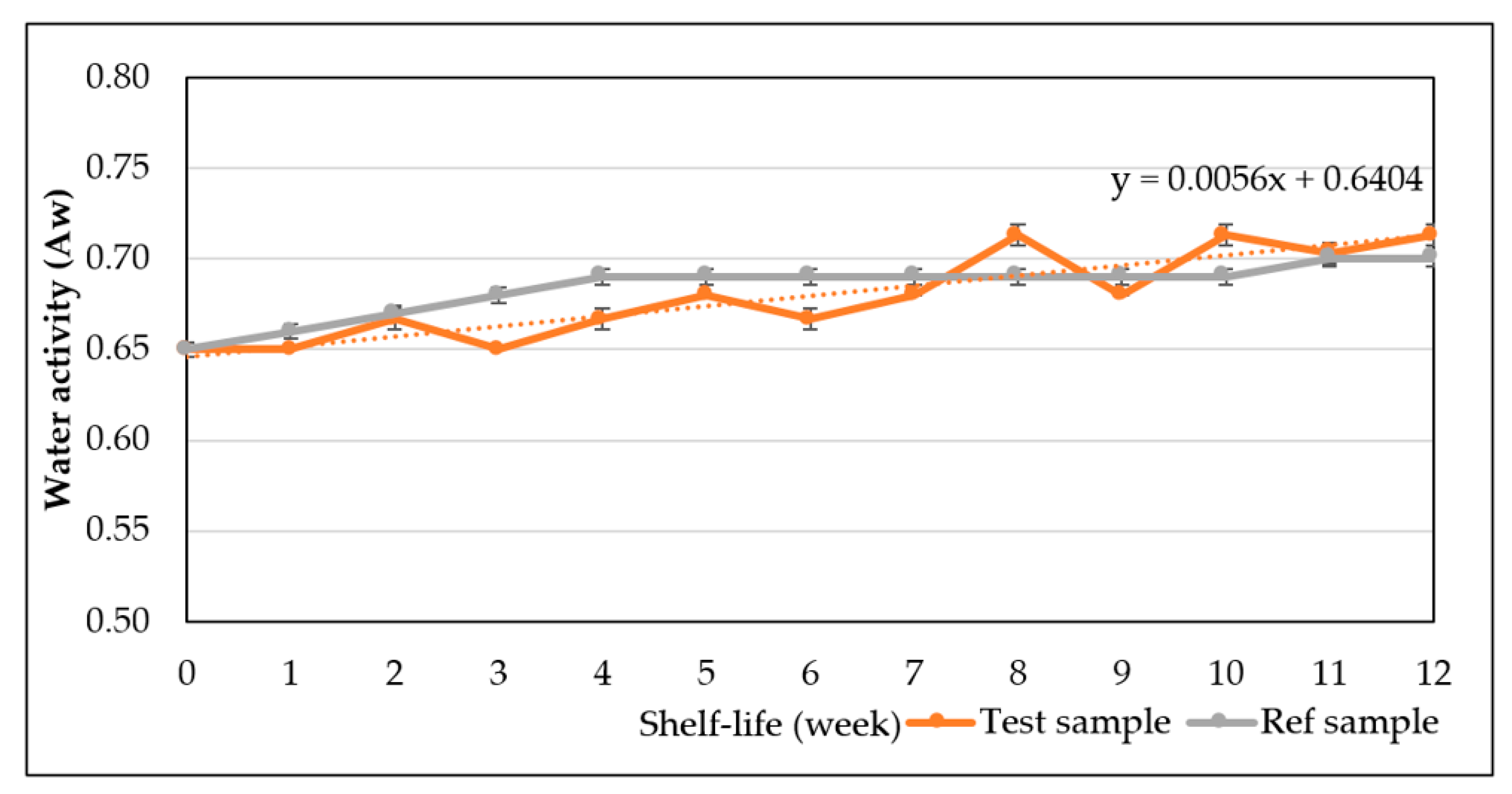
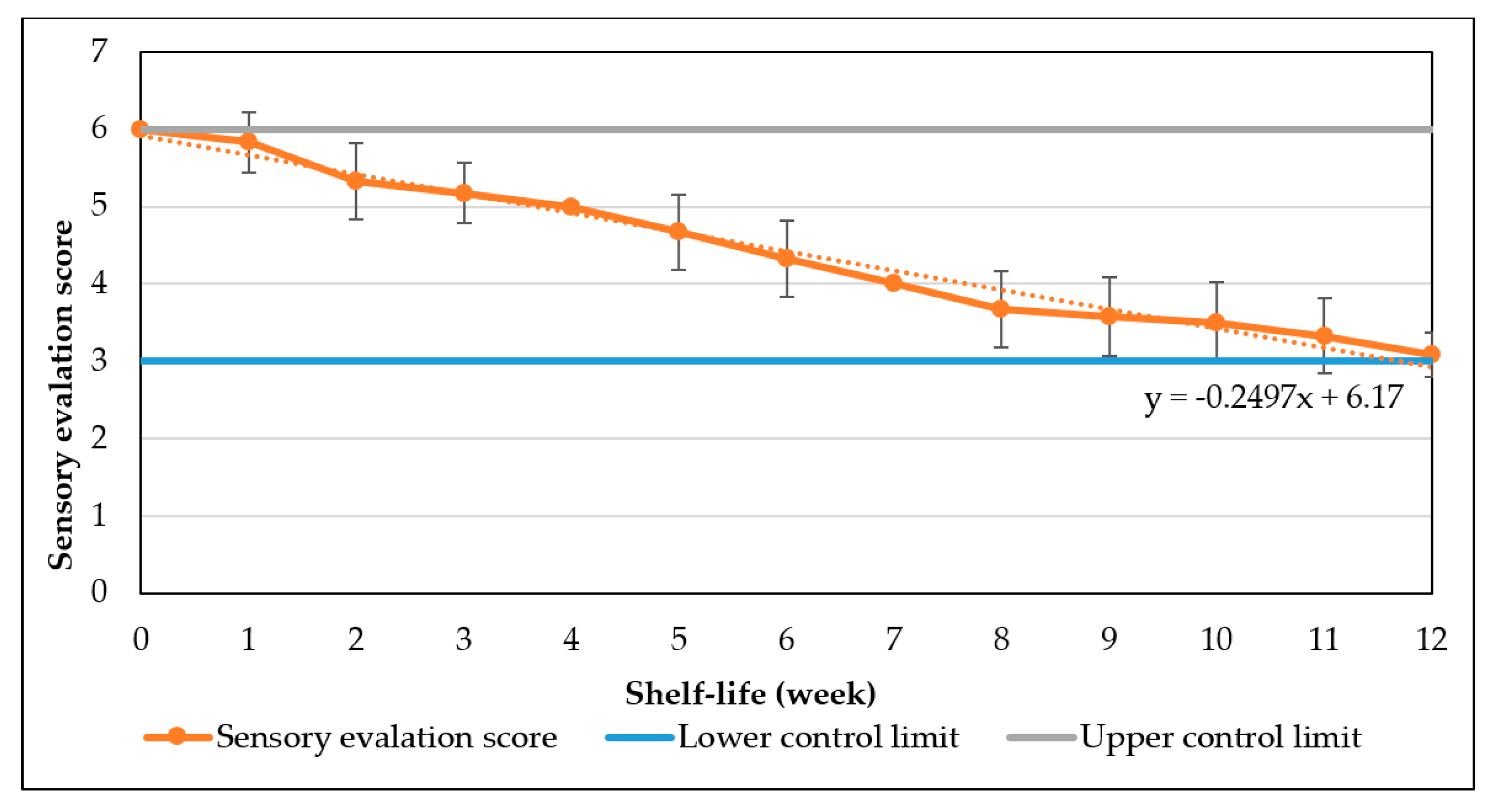
The study has optimized the total flavonoid content and antioxidant activity of the white radish root extract with regard to four extraction parameters including concentration of ethanol, ratio of solvent/material, temperature and time. Optimized parameters consisted of extraction temperature of 50 °C, the time of 3 h, ratio of 1:30 and ethanol concentration of 75%. These conditions correspond to the TFC content of 6.25 ± 0.1 mg/g and TEAC of 28.95 ± 0.43 mg/g. In vitro disk diffusion assay showed that WRE could inhibit four fungal species of Aspergillus flavus, Aspergillus niger, Aspergillus clavatus, and Fusarim solani with concentration of 75 mg/mL. For Aspergillus flavus and Fusarim solani, the MIC of WRE was 30 mg/mL. The figures were 20 and 10 mg/mL for Aspergillus clavatus and Aspergillus niger, respectively. To maintain acceptable physical, sensory and microbial parameters, sponge cakes added with WRE are suggested to have the shelf-life of 8 weeks at storage temperature of 20 °C or 2 weeks at 37 °C. These results suggest the use of white radish as a potential material for manufacture of natural preservatives.