Mathematical Modeling and Simulation on the Stimulation Interactions in Coalbed Methane Thermal Recovery
Abstract
1. Introduction
2. Experimental Observation of Methane Sorption Under Variable Temperature
2.1. Experimental Program
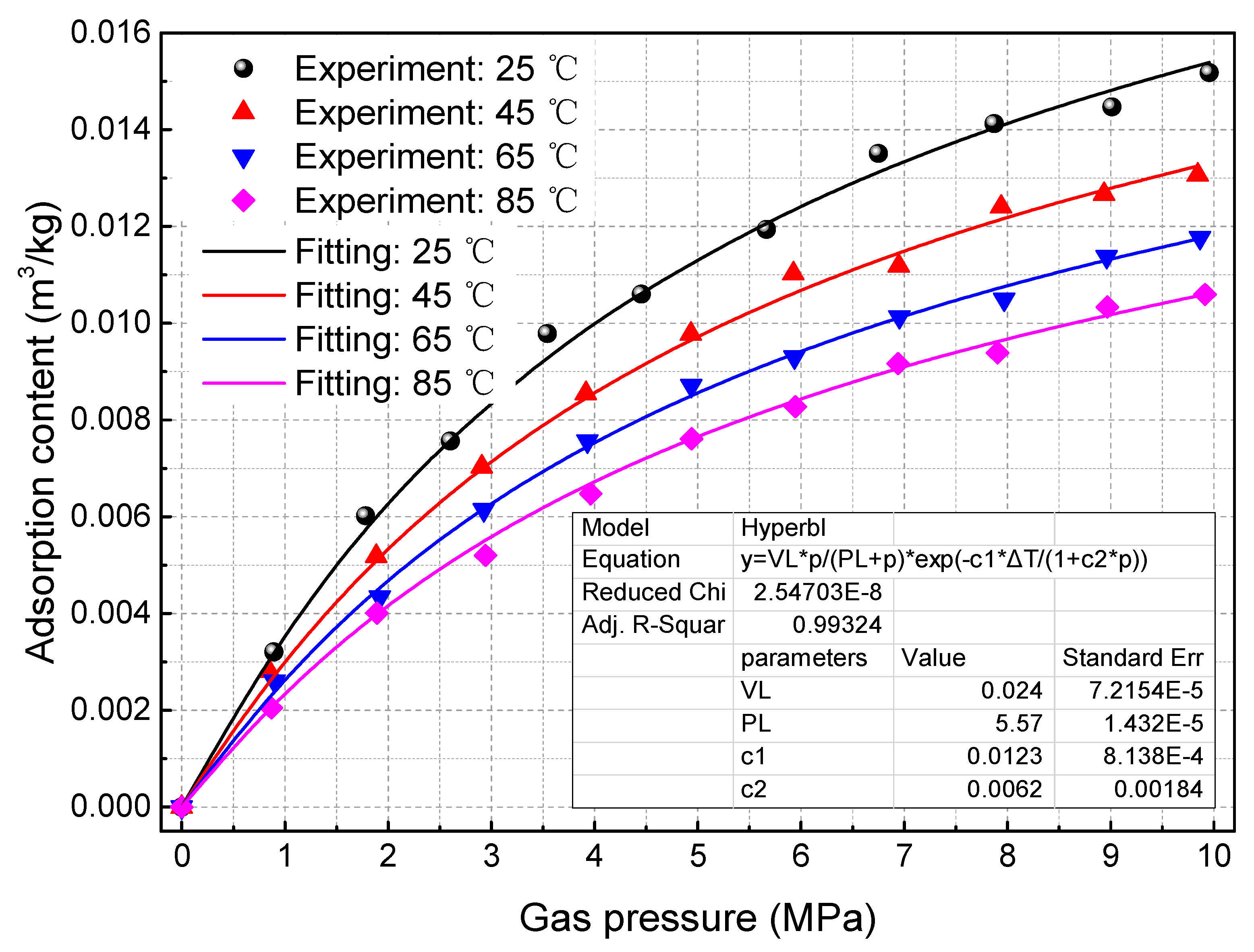
2.2. Effects of Temperature on Methane Adsorption Content
2.3. Volumetric Stain Induced by Methane Desorption
3. Mathematical Model
3.1. Coalbed Deformation Equation
3.2. Methane Flow Equation
3.3. Heat Transport Equation
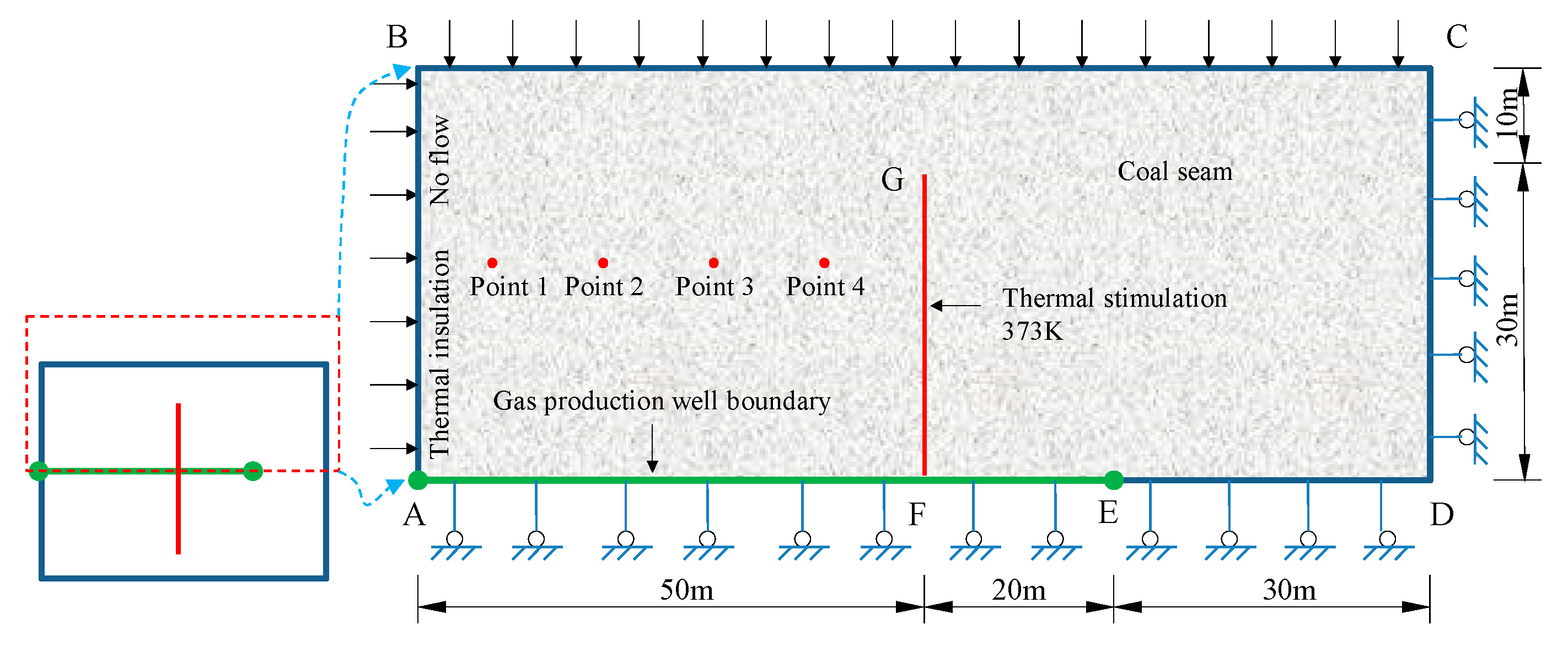
4. Modeling on CBM thermal Recovery
5. Modeling Results
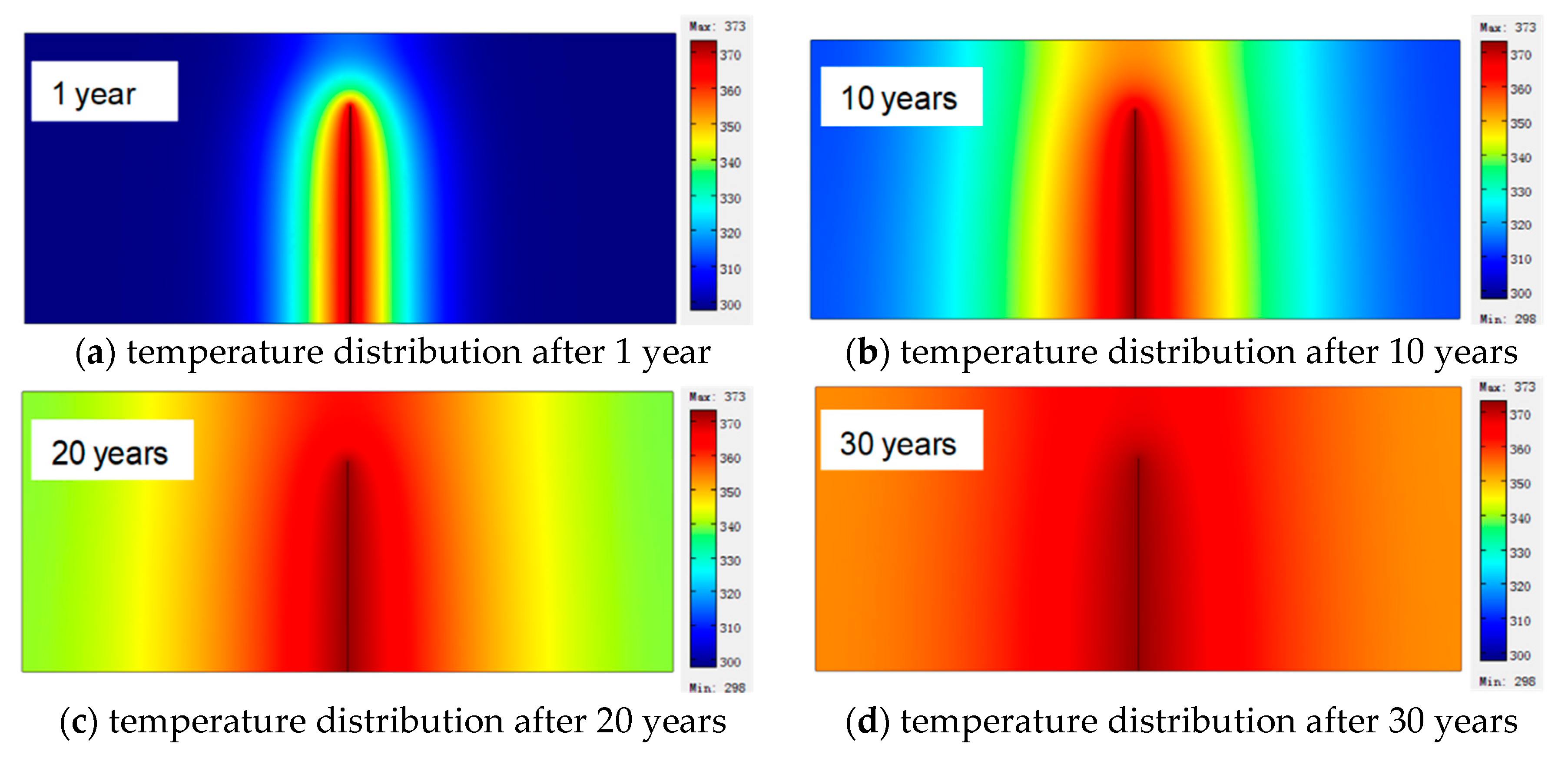
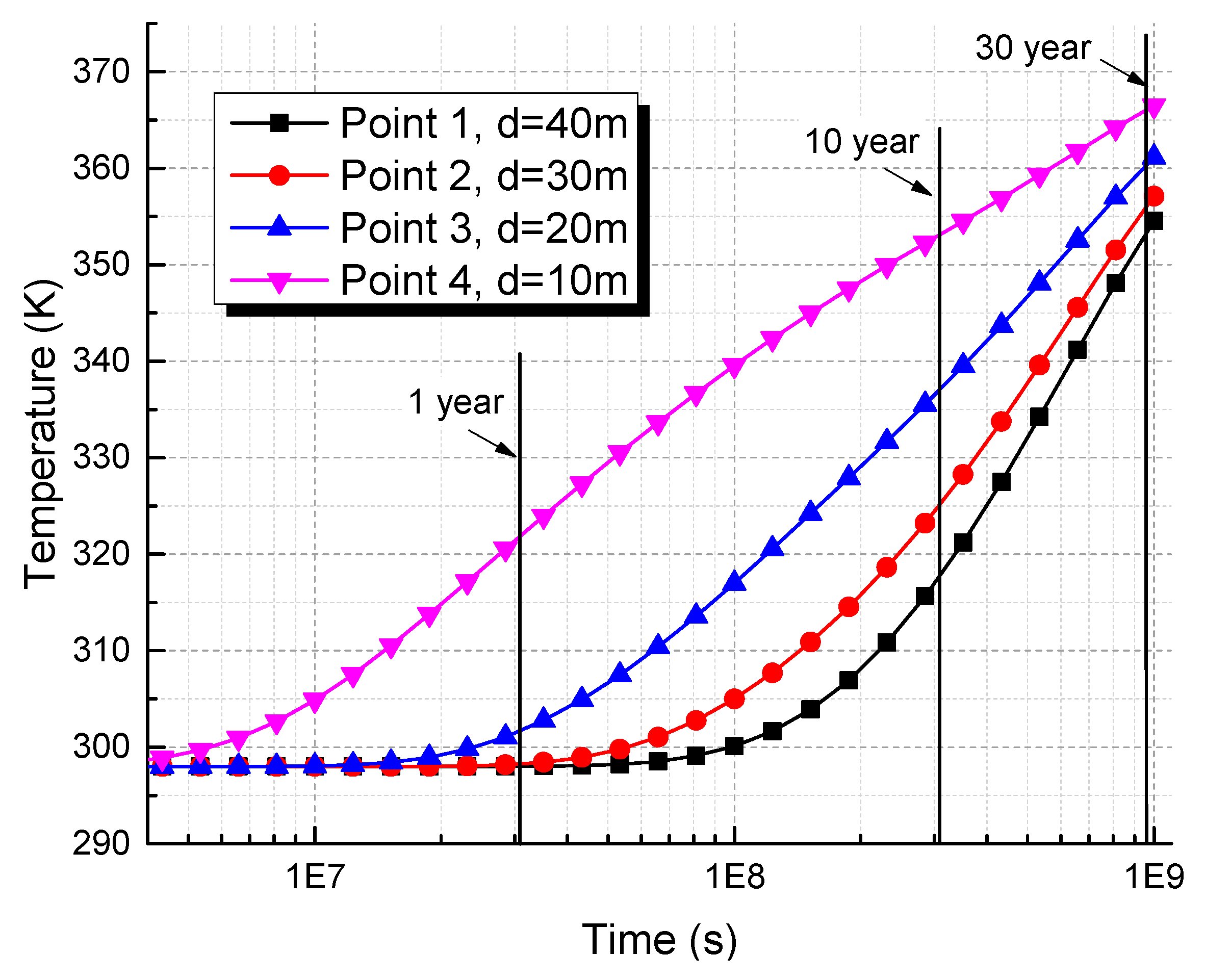
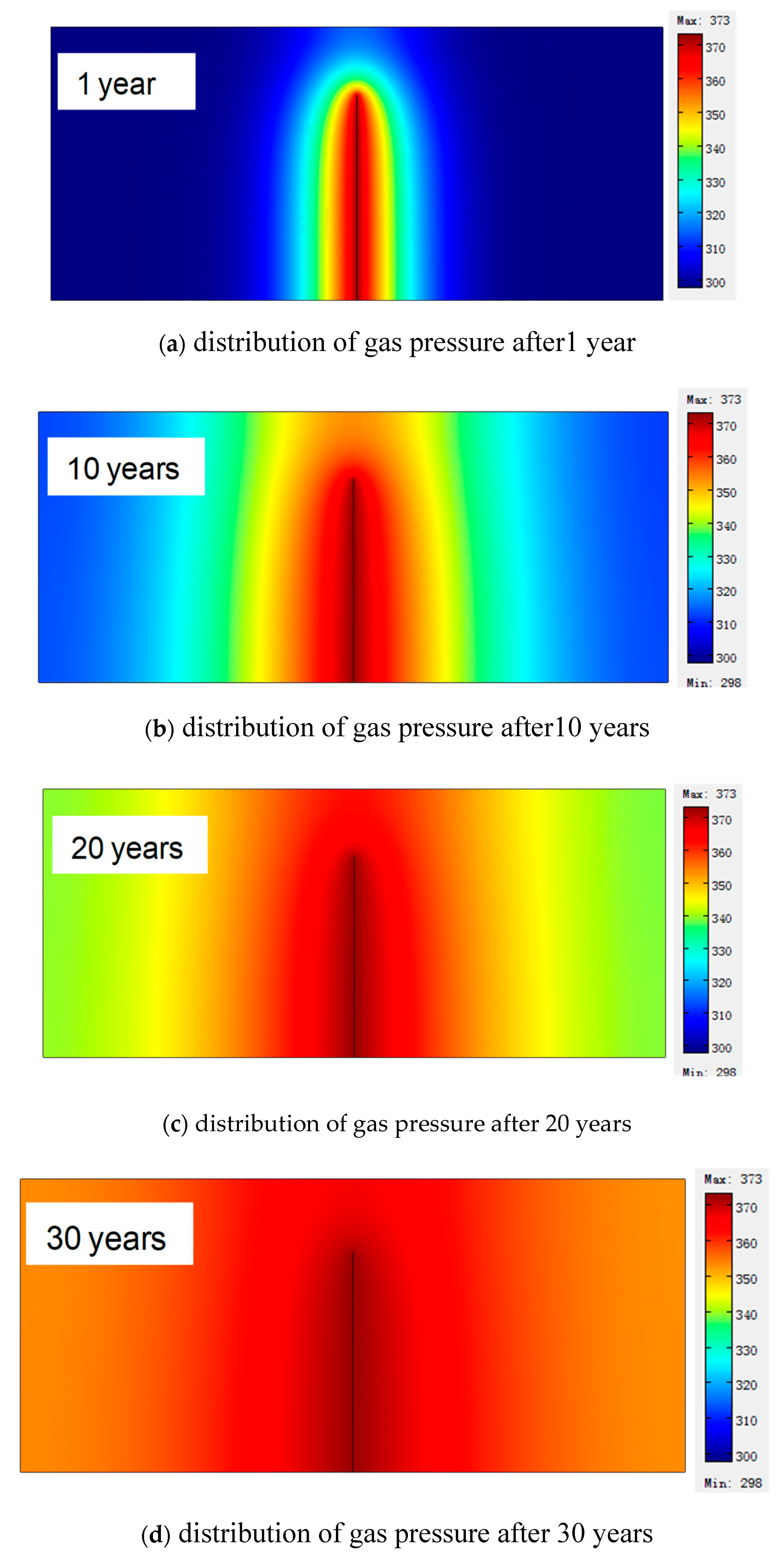
5.1. Distribution of Coalbed Temperature
5.2. Evolution of Methane Pressure
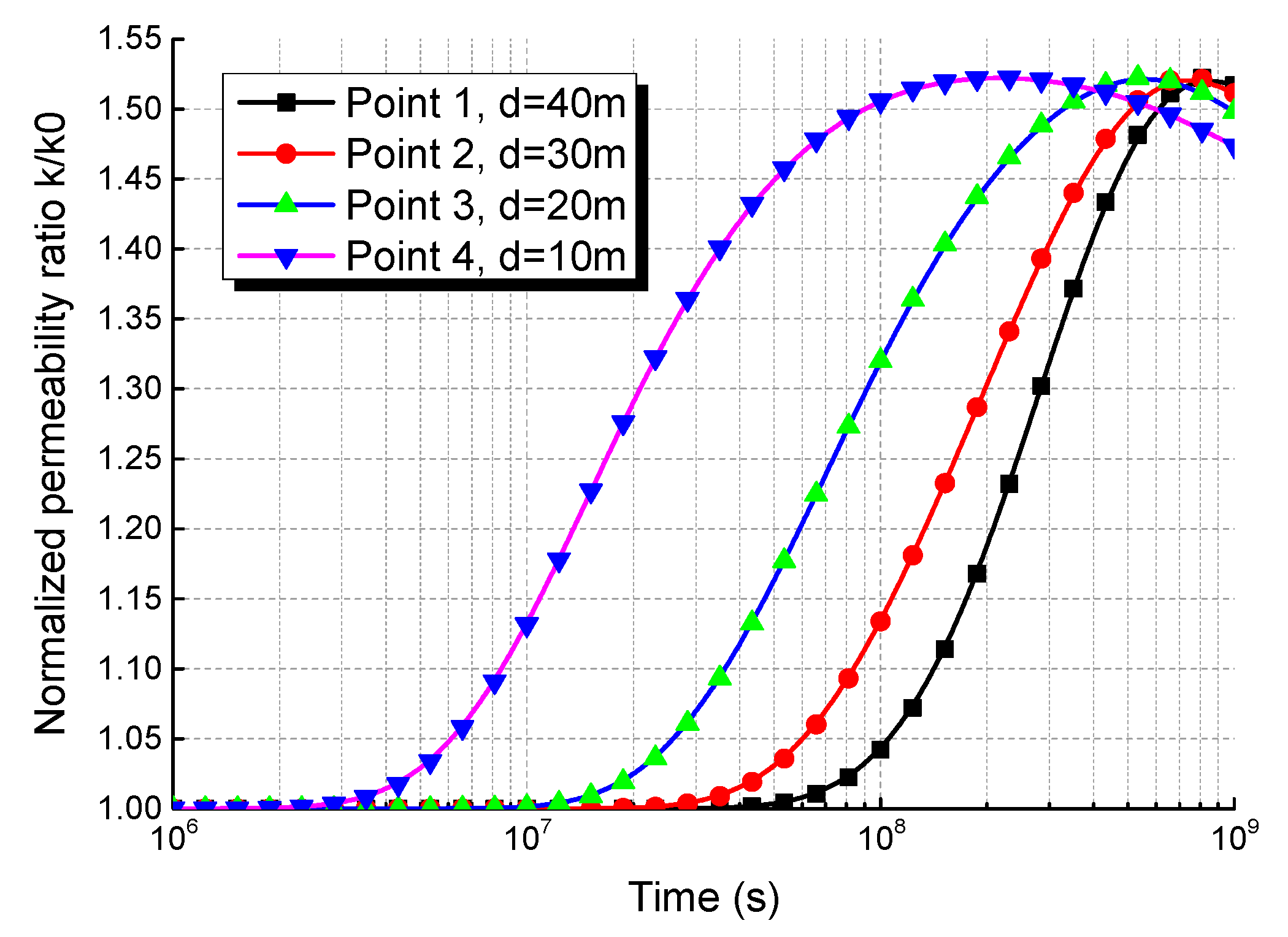
5.3. Evolution of Coalbed Permeability
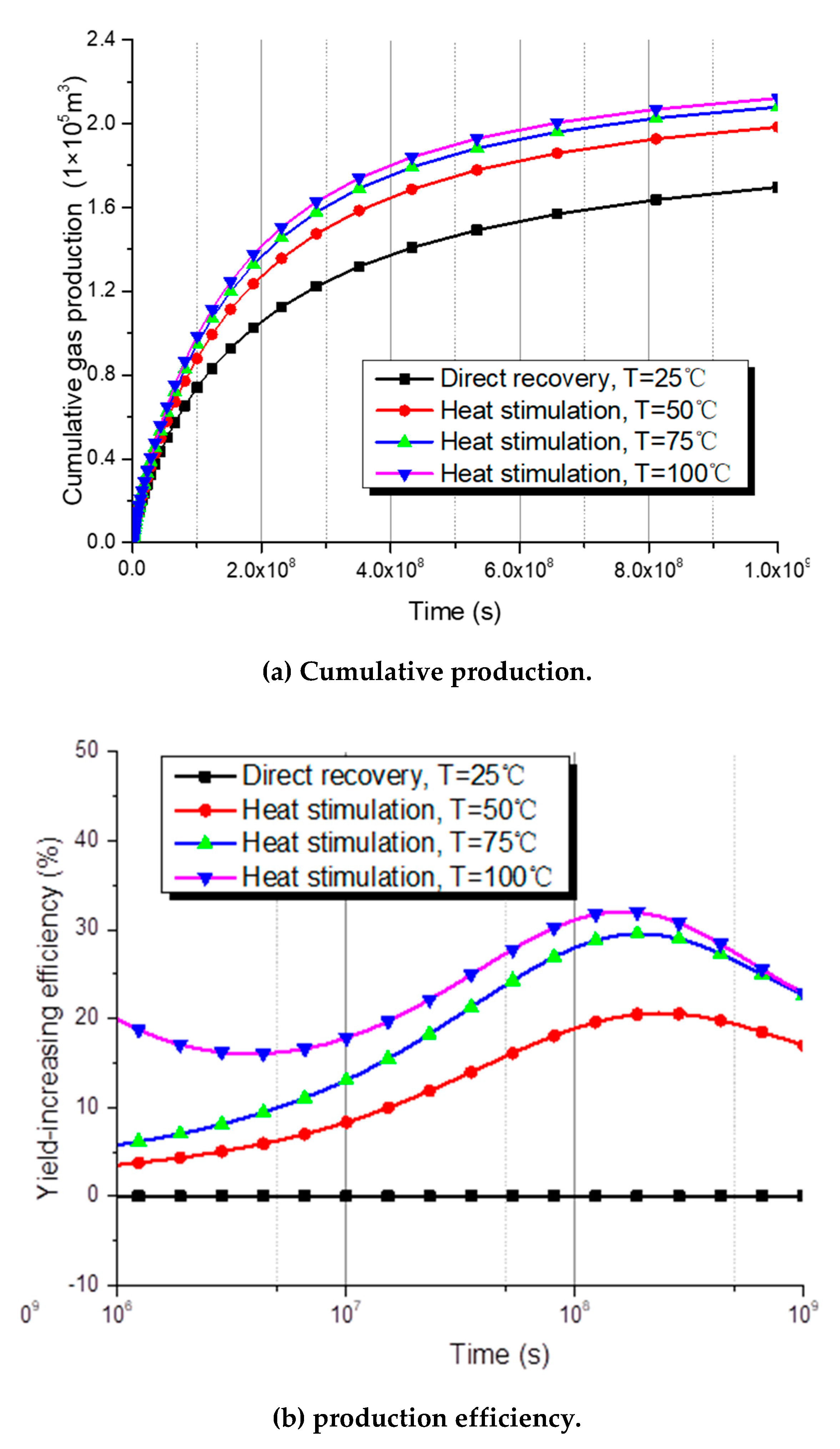
5.4. Methane Production with Different Thermal Stimulation Temperature
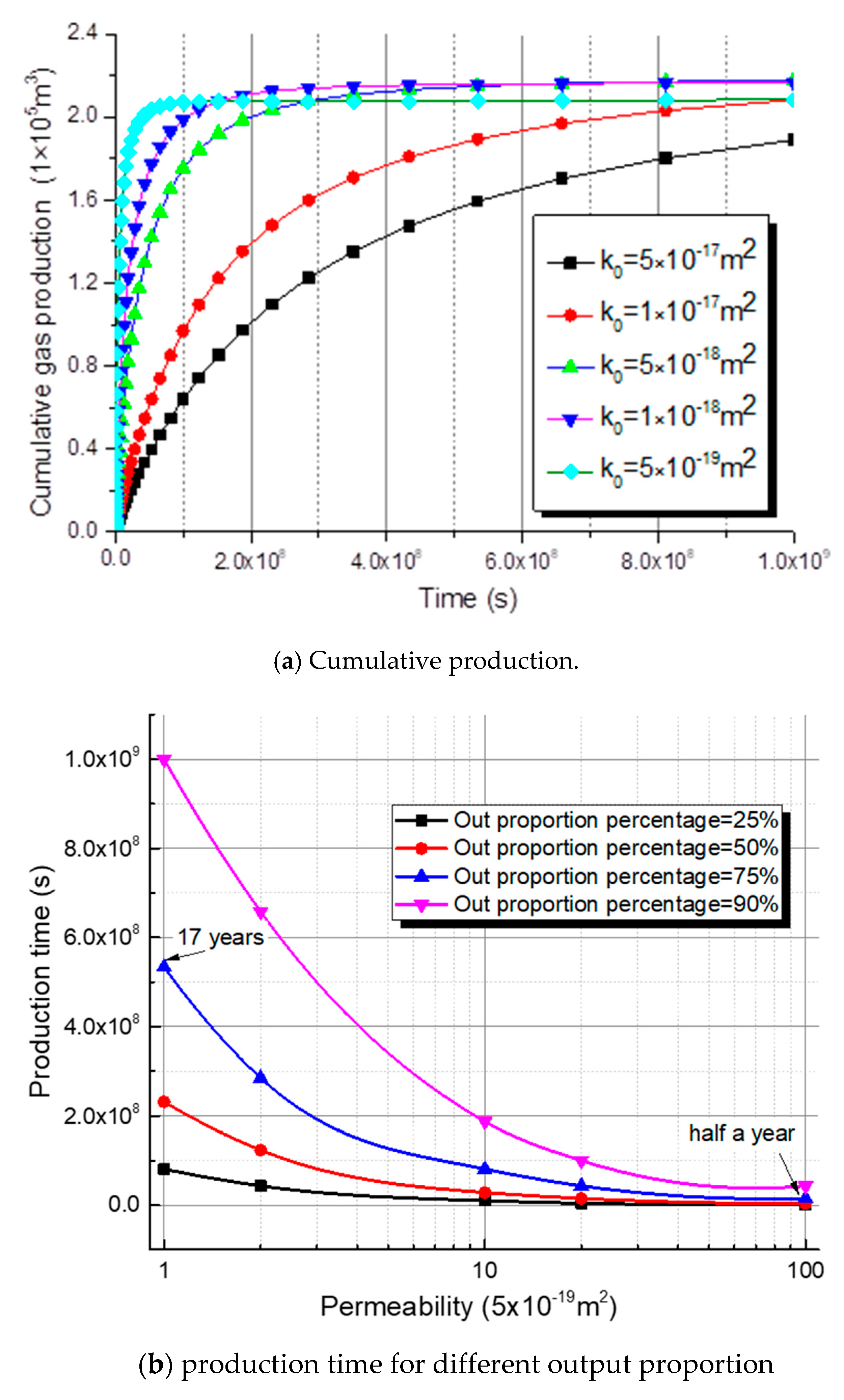
5.5. Methane Production with Different Initial Permeability
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Al-Jubori, A.; Johnston, S.; Boyer, C.; Lambert, S.W.; Bustos, O.A.; Pashin, J.C.; Wray, A. Coalbed methane: Clean energy for the world. Oilfield Rev. 2009, 21, 4–13. [Google Scholar]
- Yuan, L. Theory and practice of integrated coal production and gas extraction. Int. J. Coal Sci. Technol. 2015, 2, 3–11. [Google Scholar] [CrossRef]
- Towler, B.; Firouzi, M.; Underschultz, J.; Rifkin, W.; Garnett, A.; Schultz, H.; Witt, K. An overview of the coal seam gas developments in Queensland. J. Nat. Gas Sci. Eng. 2016, 31, 249–271. [Google Scholar] [CrossRef]
- Zhou, F.; Xia, T.; Wang, X.; Zhang, Y.; Sun, Y.; Liu, J. Recent developments of coal mine methane extraction and utilization in china: A review. J. Nat. Gas Sci. Eng. 2016, 31, 437–458. [Google Scholar] [CrossRef]
- Noack, K. Control of gas emissions in underground coal mines. Int. J. Coal Geolo. 1998, 35, 57–82. [Google Scholar] [CrossRef]
- Slastunov, S.V.; Prezent, G.M.; Kolikov, K.S. Experimental work on methane recovery at the Lenin Mine of the Karaganda Basin. J. Min. Sci. 1999, 35, 531–535. [Google Scholar] [CrossRef]
- Mutyala, S.; Fairbridge, C.; Paré, J.J.; Bélanger, J.M.; Ng, S.; Hawkins, R. Microwave applications to oil sands and petroleum: A review. Fuel Process. Technol. 2010, 91, 127–135. [Google Scholar] [CrossRef]
- Singh, A.K.; Baumann, G.; Henninges, J.; Görke, U.J.; Kolditz, O. Numerical analysis of thermal effects during carbon dioxide injection with enhanced gas recovery: A theoretical case study for the Altmark gas field. Environ. Earth Sci. 2012, 67, 497–509. [Google Scholar] [CrossRef]
- Wang, H.; Ajao, O.; Economides, M.J. Conceptual study of thermal stimulation in shale gas formations. J. Nat. Gas Sci. Eng. 2014, 21, 874–885. [Google Scholar] [CrossRef]
- Xue, L.; Dai, C.; Wang, L.; Chen, X. Analysis of Thermal Stimulation to Enhance Shale Gas Recovery through a Novel Conceptual Model. Geofluids 2019, 2019. [Google Scholar] [CrossRef]
- Salmachi, A.; Haghighi, M. Feasibility study of thermally enhanced gas recovery of coal seam gas reservoirs using geothermal resources. Energy Fuels 2012, 26, 5048–5059. [Google Scholar] [CrossRef]
- Li, B.; Yang, K.; Xu, P.; Xu, J.; Yuan, M.; Zhang, M. An experimental study on permeability characteristics of coal with slippage and temperature effects. J. Pet. Sci. Eng. 2019, 175, 294–302. [Google Scholar] [CrossRef]
- Khoshnevis, N.; Khosrokhavar, R.; Nick, H.; Bruhn, D.F.; Bruining, H. Injection of disposal water from a geothermal reservoir into a gas reservoir. J. Pet. Sci. Eng. 2019, 178, 616–628. [Google Scholar] [CrossRef]
- Lu, B.X. Numerical simulations of temperature field of coalbed methane with heat injection based on ANSYS. In Proceedings of the 2012 International Conference on Computer Science and Electronics Engineering, Hangzhou, China, 23–25 March 2012; Volume 2. [Google Scholar]
- Shahtalebi, A.; Khan, C.; Dmyterko, A.; Shukla, P.; Rudolph, V. Investigation of thermal stimulation of coal seam gas fields for accelerated gas recovery. Fuel 2016, 180, 301–313. [Google Scholar] [CrossRef]
- Li, H.; Kang, J.; Zhou, F.; Qiang, Z.; Li, G. Adsorption heat features of coalbed methane based on microcalorimeter. J. Loss Prev. Process Ind. 2018, 55, 437–449. [Google Scholar] [CrossRef]
- Miklová, B.; Staf, M.; Kyselová, V. Influence of ash composition on high temperature CO2 sorption. J. Environ. Chem. Eng. 2019, 7, 103017. [Google Scholar] [CrossRef]
- Okolo, G.N.; Everson, R.C.; Neomagus, H.W.; Sakurovs, R.; Grigore, M.; Bunt, J.R. The carbon dioxide, methane and nitrogen high-pressure sorption properties of South African bituminous coals. Int. J. Coal Geol. 2019, 209, 40–53. [Google Scholar] [CrossRef]
- Sakurovs, R.; Day, S.; Weir, S.; Duffy, G. Temperature dependence of sorption of gases by coals and charcoals. Int. J. Coal Geol. 2008, 73, 250–258. [Google Scholar] [CrossRef]
- Charrière, D.; Pokryszka, Z.; Behra, P. Effect of pressure and temperature on diffusion of CO2 and CH4 into coal from the Lorraine basin (France). Int. J. Coal Geol. 2010, 81, 373–380. [Google Scholar] [CrossRef]
- Guan, C.; Liu, S.; Li, C.; Wang, Y.; Zhao, Y. The temperature effect on the methane and CO2 adsorption capacities of Illinois coal. Fuel 2018, 211, 241–250. [Google Scholar] [CrossRef]
- Crosdale, P.J.; Moore, T.A.; Mares, T.E. Influence of moisture content and temperature on methane adsorption isotherm analysis for coals from a low-rank, biogenically-sourced gas reservoir. Int. J. Coal Geol. 2008, 76, 166–174. [Google Scholar] [CrossRef]
- Zhang, L.; Aziz, N.; Ren, T.X.; Wang, Z. Influence of temperature on coal sorption characteristics and the theory of coal surface free energy. Procedia Eng. 2011, 26, 1430–1439. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Liu, S.; Li, W.; Tang, X. Temperature effect on gas adsorption capacity in different sized pores of coal: Experiment and numerical modeling. J. Pet. Sci. Eng. 2018, 165, 821–830. [Google Scholar] [CrossRef]
- Akbarzadeh, H.; Chalaturnyk, R.J. Structural changes in coal at elevated temperature pertinent to underground coal gasification: A review. Int. J. Coal Geol. 2014, 131, 126–146. [Google Scholar] [CrossRef]
- Niu, S.; Zhao, Y.; Hu, Y. Experimental ivestigation of the temperature and pore pressure effect on permeability of lignite under the in situ condition. Trans. Porous Media 2014, 101, 137–148. [Google Scholar] [CrossRef]
- Zhang, X.G.; Ranjith, P.G.; Perera, M.S.A.; Ranathunga, A.S.; Haque, A. Gas transportation and enhanced coalbed methane recovery processes in deep coal seams: A review. Energy Fuels 2016, 30, 8832–8849. [Google Scholar] [CrossRef]
- Wang, K.; Du, F.; Wang, G. Investigation of gas pressure and temperature effects on the permeability and steady-state time of Chinese anthracite coal: An experimental study. J. Nat. Gas Sci. Eng. 2017, 40, 179–188. [Google Scholar] [CrossRef]
- Yin, G.; Jiang, C.; Wang, J.G.; Xu, J. Combined effect of stress, pore pressure and temperature on methane permeability in anthracite coal: An experimental study. Trans. Porous Media 2013, 100, 1–16. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranjith, P.G.; Choi, S.K.; Airey, D. Investigation of temperature effect on permeability of naturally fractured black coal for carbon dioxide movement: An experimental and numerical study. Fuel 2012, 94, 596–605. [Google Scholar] [CrossRef]
- Teng, T.; Wang, J.G.; Gao, F.; Ju, Y.; Jiang, C. A thermally sensitive permeability model for coal-gas interactions including thermal fracturing and volatilization. J. Nat. Gas Sci. Eng. 2016, 32, 319–333. [Google Scholar] [CrossRef]
- Pandey, S.N.; Chaudhuri, A.; Kelkar, S. A coupled thermo-hydro-mechanical modeling of fracture aperture alteration and reservoir deformation during heat extraction from a geothermal reservoir. Geothermics 2017, 65, 17–31. [Google Scholar] [CrossRef]
- Tong, F.; Jing, L.; Zimmerman, R.W. A fully coupled thermo-hydro-mechanical model for simulating multiphase flow, deformation and heat transfer in buffer material and rock masses. Int. J. Rock Mech. Min. Sci. 2010, 47, 205–217. [Google Scholar] [CrossRef]
- Abed, A.A.; Sołowski, W.T. A study on how to couple thermo-hydro-mechanical behaviour of unsaturated soils: Physical equations, numerical implementation and examples. Comput. Geotech. 2017, 92, 132–155. [Google Scholar] [CrossRef]
- Gao, F.; Xue, Y.; Gao, Y.; Zhang, Z.; Teng, T.; Liang, X. Fully coupled thermo-hydro-mechanical model for extraction of coal seam gas with slotted boreholes. J. Nat. Gas Sci. Eng. 2016, 31, 226–235. [Google Scholar] [CrossRef]
- Fan, C.; Elsworth, D.; Li, S.; Zhou, L.; Yang, Z.; Song, Y. Thermo-hydro-mechanical-chemical couplings controlling CH4 production and CO2 sequestration in enhanced coalbed methane recovery. Energy 2019, 173, 1054–1077. [Google Scholar] [CrossRef]
- Xia, T.; Zhou, F.; Liu, J.; Kang, J.; Gao, F. A fully coupled hydro-thermo-mechanical model for the spontaneous combustion of underground coal seams. Fuel 2014, 125, 106–115. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, S. Experimental and theoretical characterization of methane and CO2 sorption hysteresis in coals based on Langmuir desorption. Int. J. Coal Geol. 2017, 171, 49–60. [Google Scholar] [CrossRef]
- Rani, S.; Padmanabhan, E.; Prusty, B.K. Review of gas adsorption in shales for enhanced methane recovery and CO2 storage. J. Pet. Sci. Eng. 2018, 175, 634–643. [Google Scholar] [CrossRef]
- Kim, J.; Jang, Y.; Seomoon, H.; Lee, H.; Sung, W. A new sorption-corrected deconvolution method for production data analysis in a shale gas reservoir containing adsorbed gas. Int. J. Oil Gas Coal Technol. 2019, 20, 55–68. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Elsworth, D. How sorption-induced matrix deformation affects gas flow in coal seams: A new FE model. Int. J. Rock Mech. Min. Sci. 2008, 45, 1226–1236. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Chen, Z.; Elsworth, D.; Pone, D. A dual poroelastic model for CO2-enhanced coalbed methane recovery. Int. J. Coal Geol. 2011, 86, 177–189. [Google Scholar] [CrossRef]
- Liang, B. Study on temperature effects on the gas absorption performance. J. Heilongjiang Min. Inst. 2000, 10, 20–22. [Google Scholar]
- Zhu, W.C.; Wei, C.H.; Liu, J.; Qu, H.Y.; Elsworth, D. A model of coal-gas interaction under variable temperatures. Int. J. Coal Geol. 2011, 86, 213–221. [Google Scholar] [CrossRef]
- Qu, H.; Liu, J.; Chen, Z.; Wang, J.; Pan, Z.; Connell, L.; Elsworth, D. Complex evolution of coal permeability during CO2 injection under variable temperatures. Int. J. Greenh. Gas Control 2012, 9, 281–293. [Google Scholar] [CrossRef]
- Ibrahim, A.F.; Nasr-El-Din, H.A. A comprehensive model to history match and predict gas/water production from coal seams. Int. J. Coal Geol. 2015, 146, 79–90. [Google Scholar] [CrossRef]

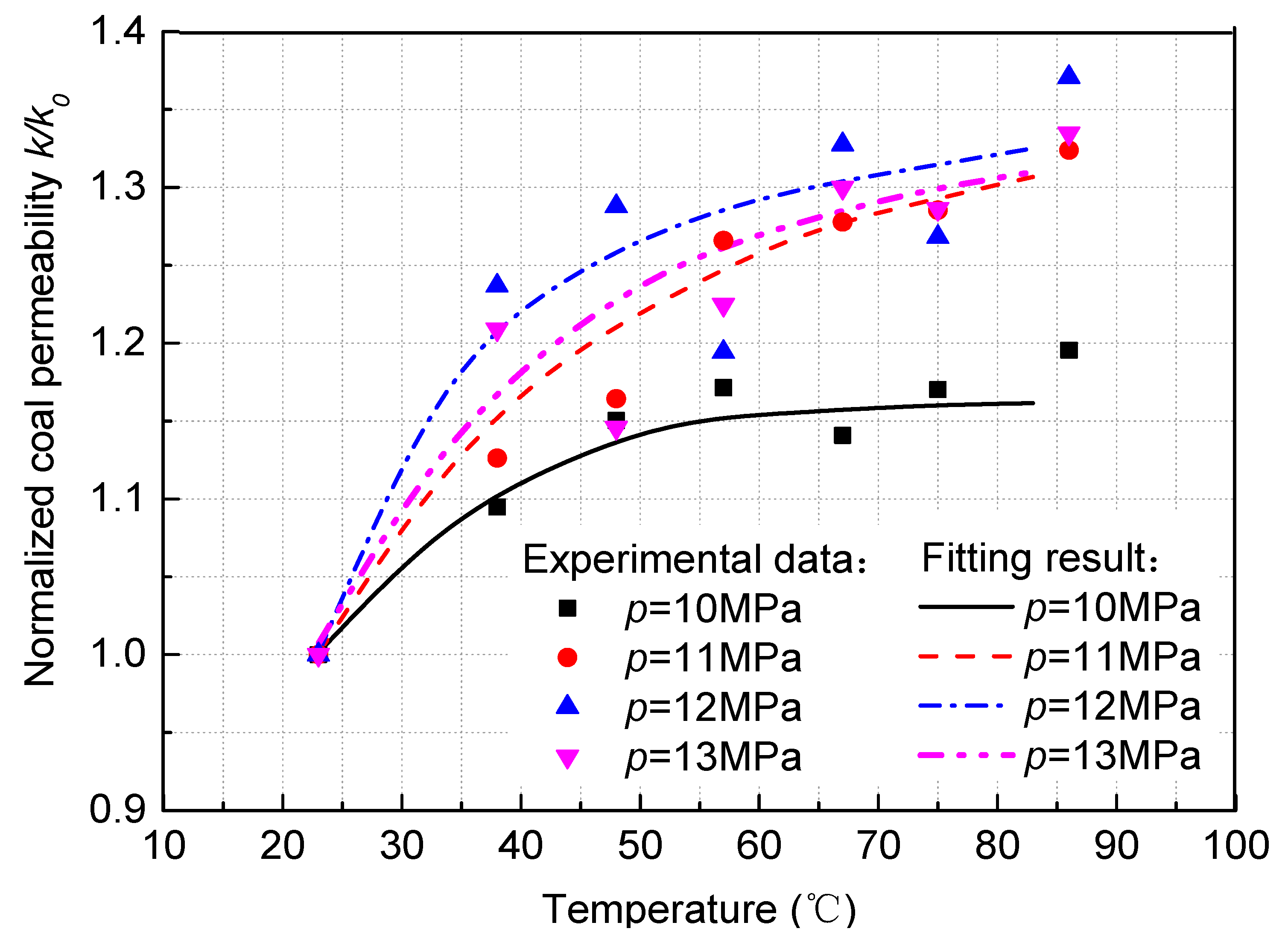
| Coal Samples | Fitting Parameters | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | R2 | ||
| Gas pressure (MPa) | 10 | 5.00 × 10−2 | 0.09 | 6.14 × 10−4 | 1.05 × 10−5 | 0.915 |
| 11 | 4.92 × 10−2 | 0.11 | 8.23 × 10−4 | 1.03 × 10−5 | 0.854 | |
| 12 | 4.83 × 10−2 | 0.08 | 7.01 × 10−4 | 1.16 × 10−5 | 0.975 | |
| 13 | 4.76 × 10−2 | 0.10 | 1.50× 10−3 | 1.50 × 10−5 | 0.962 | |
| Variable | Parameter | Value Used | Sources |
|---|---|---|---|
| Young’s modulus of coal, (MPa) | 2713 | [45] | |
| Young’s modulus of coal grains, (MPa) | 4070 | [45] | |
| Poisson’s ratio of coal | 0.339 | [45] | |
| Density of coal, (kg/m3) | 1.25 × 103 | [41] | |
| Density of gas at standard condition, (kg/m3) | 0.717 | - | |
| Sorption coefficient for volumetric strain, (kg/m3) | 0.0156 | [41] | |
| Initial permeability of coal, (mD) | 0.001 | [31] | |
| Initial porosity of coal | 0.02 | Given | |
| Dynamic viscosity coefficient of gas, (Pa·s) | 1.84 × 10−5 | [41] | |
| Langmuir volume constant, (m3/kg) | 0.048 | [46] | |
| Langmuir pressure constant, (MPa) | 1.57 | [46] | |
| Pressure coefficient, (MPa−1) | 0.07 | [44] | |
| Temperature coefficient, (K−1) | 0.02 | [44] | |
| Temperature at standard condition, (K) | 273 | - | |
| Temperature of coal seam, (K) | 298 | Given | |
| Initial value of gas pressure, (MPa) | 3.5 | Given | |
| Pressure at standard condition, (MPa) | 0.103 | - | |
| Thermal expansion coefficient, (K−1) | 2.4 × 10−5 | [45] | |
| Specific heat capacity of coal, (kJ/(kg·K)) | 1.25 | [44] | |
| Specific heat capacity of gas, (kJ/(kg·K)) | 1.62 | [44] | |
| Effective thermal conductivity of coal, (J/(m·s·K)) | 0.2 | [44] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, T.; Wang, Y.; He, X.; Chen, P. Mathematical Modeling and Simulation on the Stimulation Interactions in Coalbed Methane Thermal Recovery. Processes 2019, 7, 526. https://doi.org/10.3390/pr7080526
Teng T, Wang Y, He X, Chen P. Mathematical Modeling and Simulation on the Stimulation Interactions in Coalbed Methane Thermal Recovery. Processes. 2019; 7(8):526. https://doi.org/10.3390/pr7080526
Chicago/Turabian StyleTeng, Teng, Yingheng Wang, Xiang He, and Pengfei Chen. 2019. "Mathematical Modeling and Simulation on the Stimulation Interactions in Coalbed Methane Thermal Recovery" Processes 7, no. 8: 526. https://doi.org/10.3390/pr7080526
APA StyleTeng, T., Wang, Y., He, X., & Chen, P. (2019). Mathematical Modeling and Simulation on the Stimulation Interactions in Coalbed Methane Thermal Recovery. Processes, 7(8), 526. https://doi.org/10.3390/pr7080526






