Role of Counterions and Nature of Spacer on Foaming Properties of Novel Polyoxyethylene Cationic Gemini Surfactants
Abstract
:1. Introduction
2. Materials and Methods
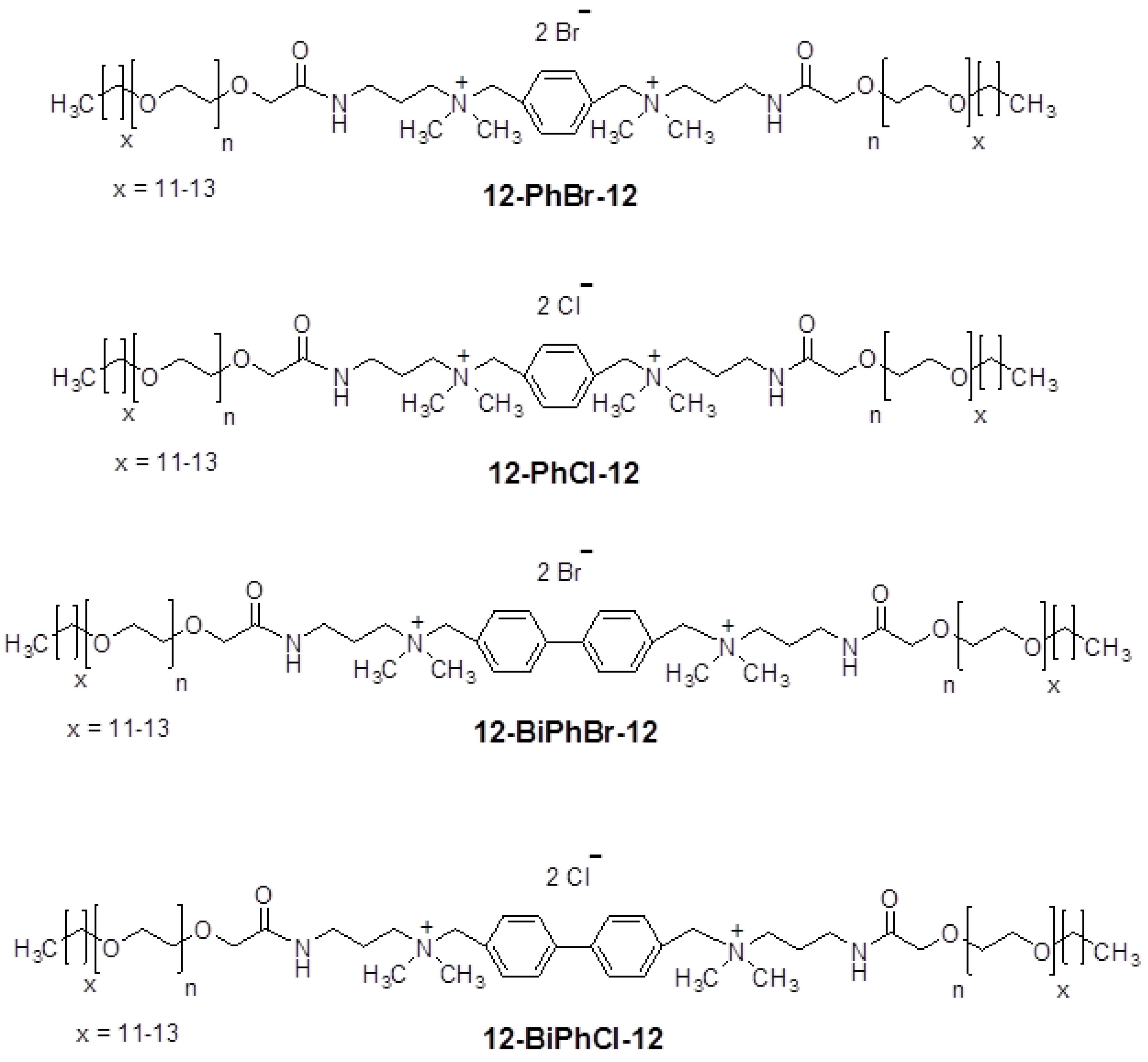
2.1. Synthesis of Surfactants
2.2. Solution Preparation
2.3. Foaming Properties
3. Results and Discussion
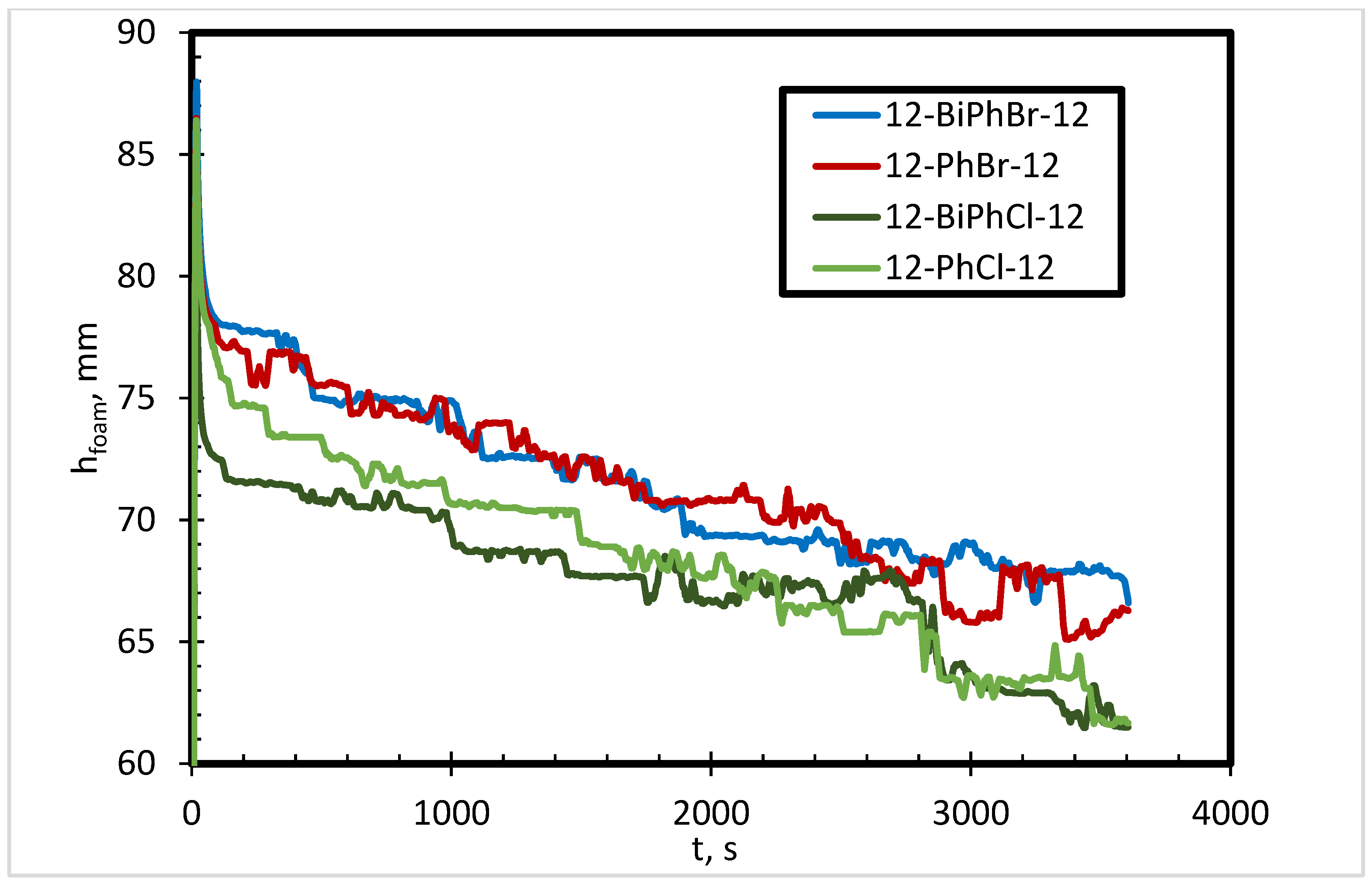
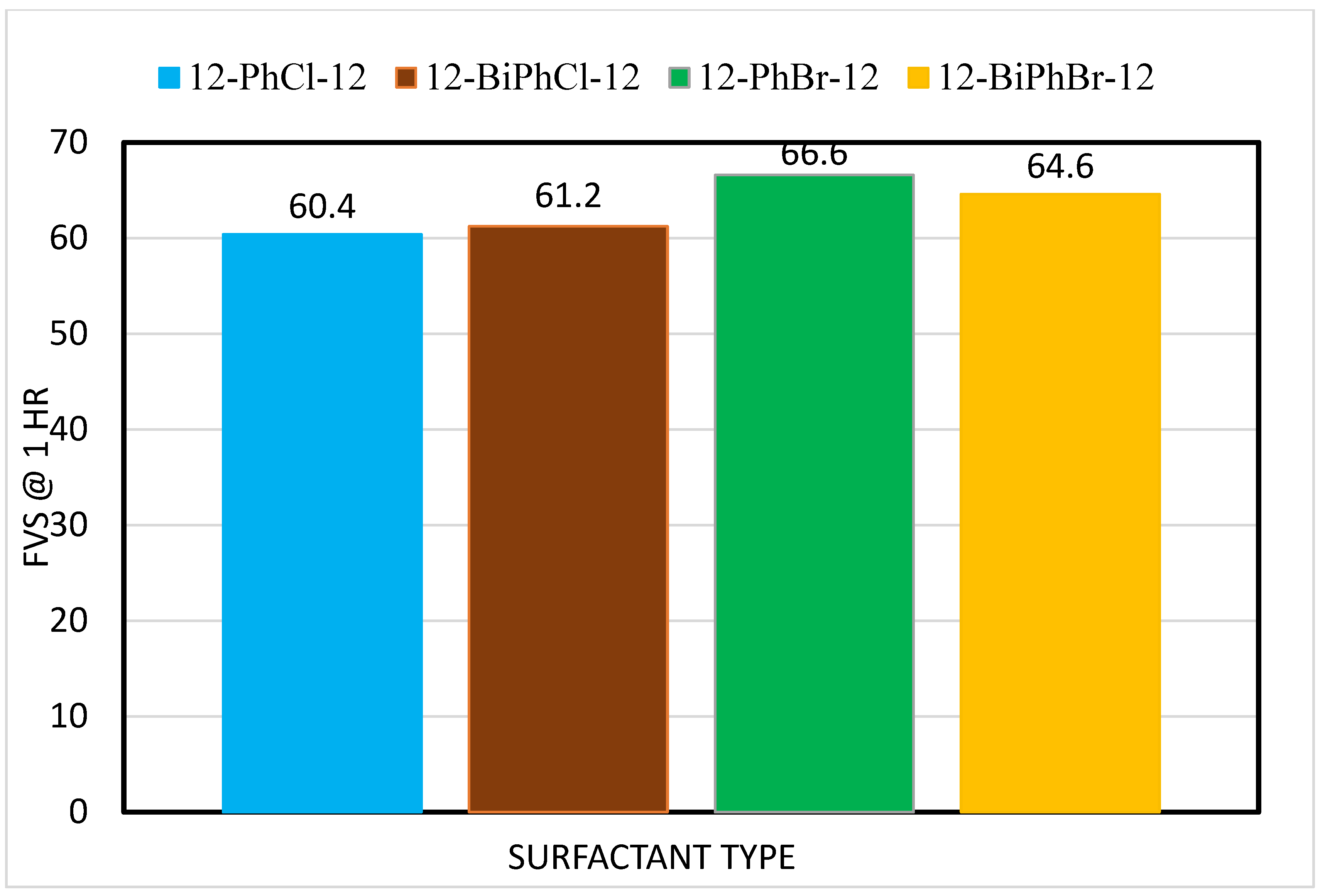
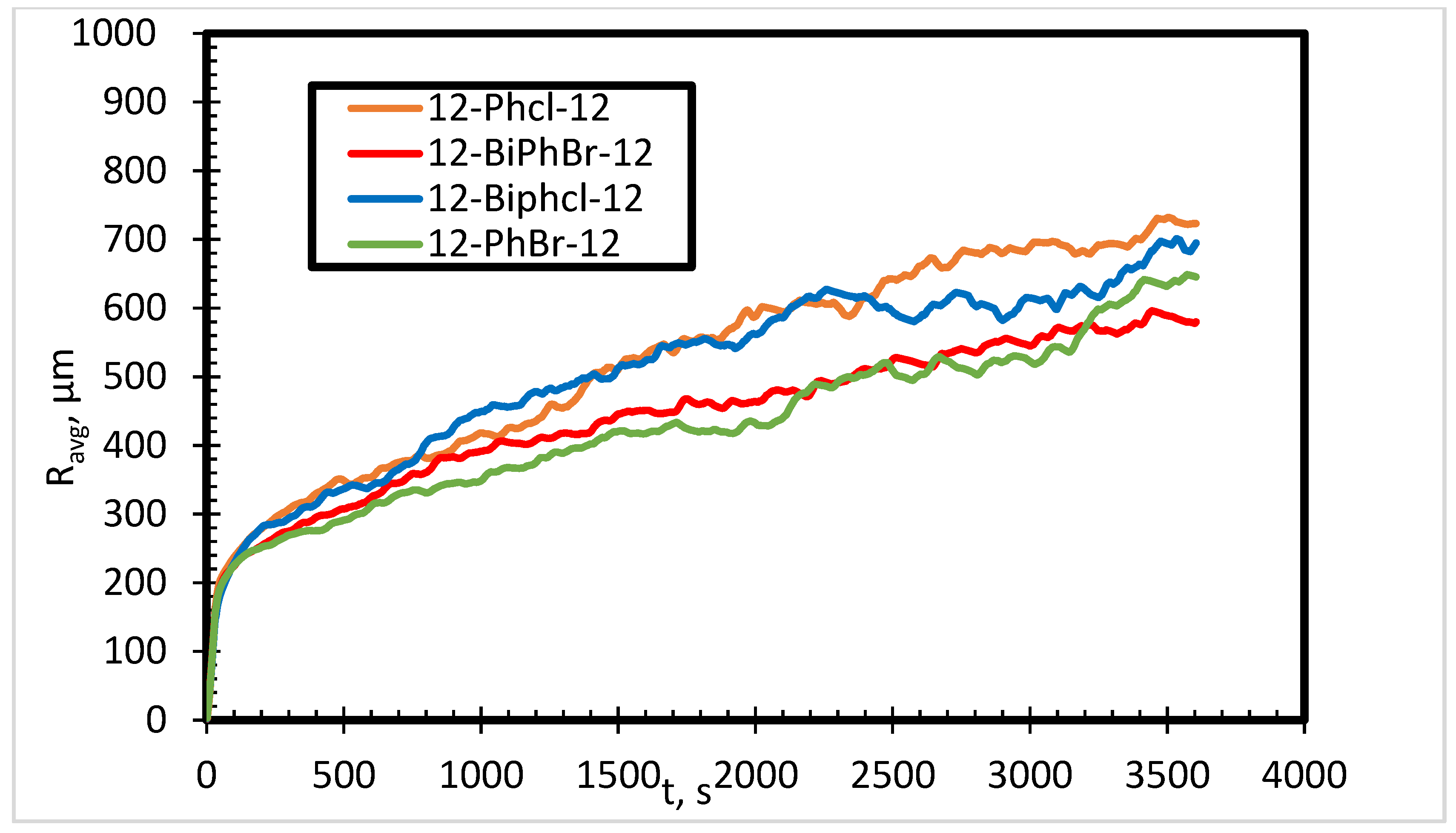
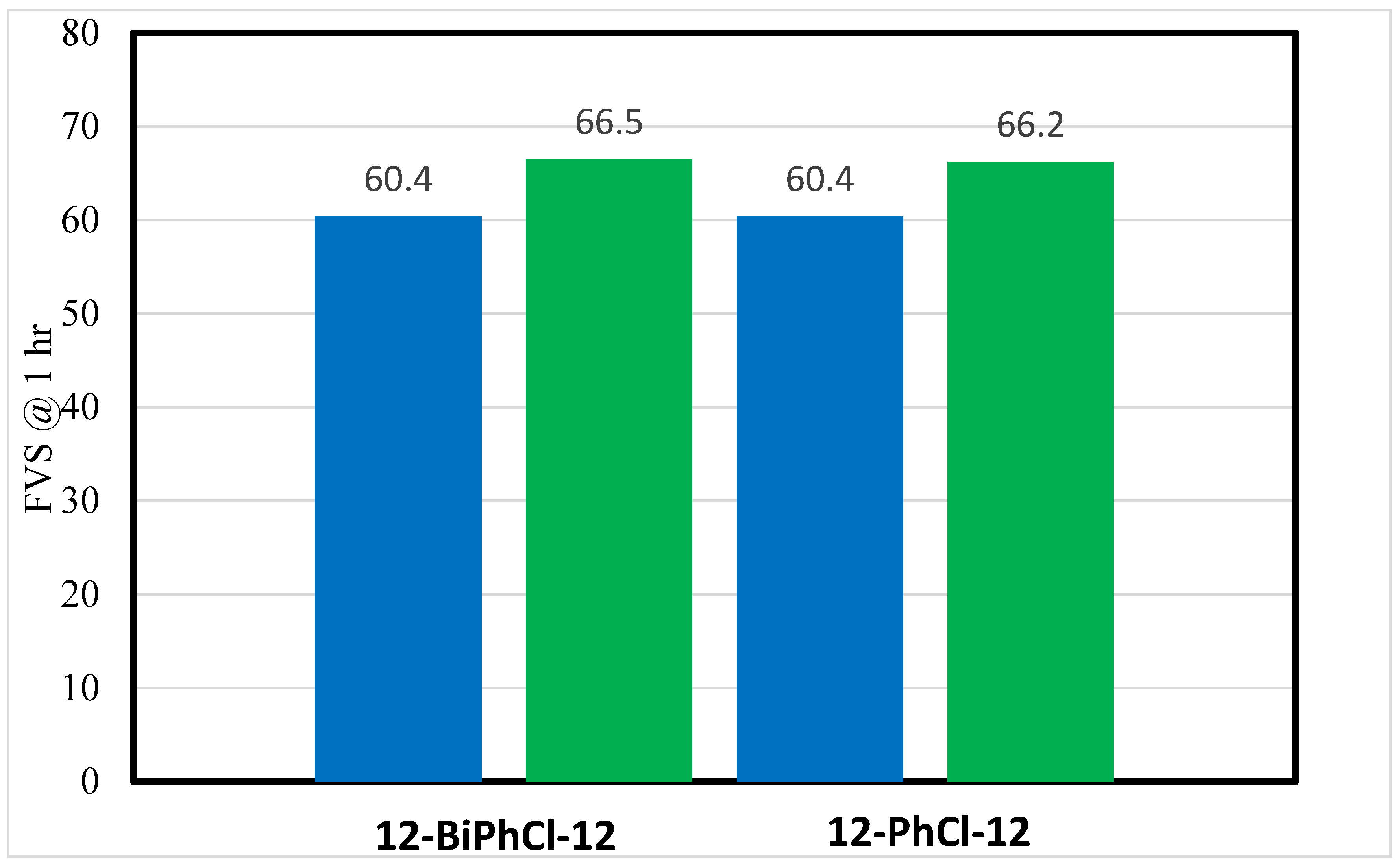
3.1. Effects of Spacer Nature and Counterions
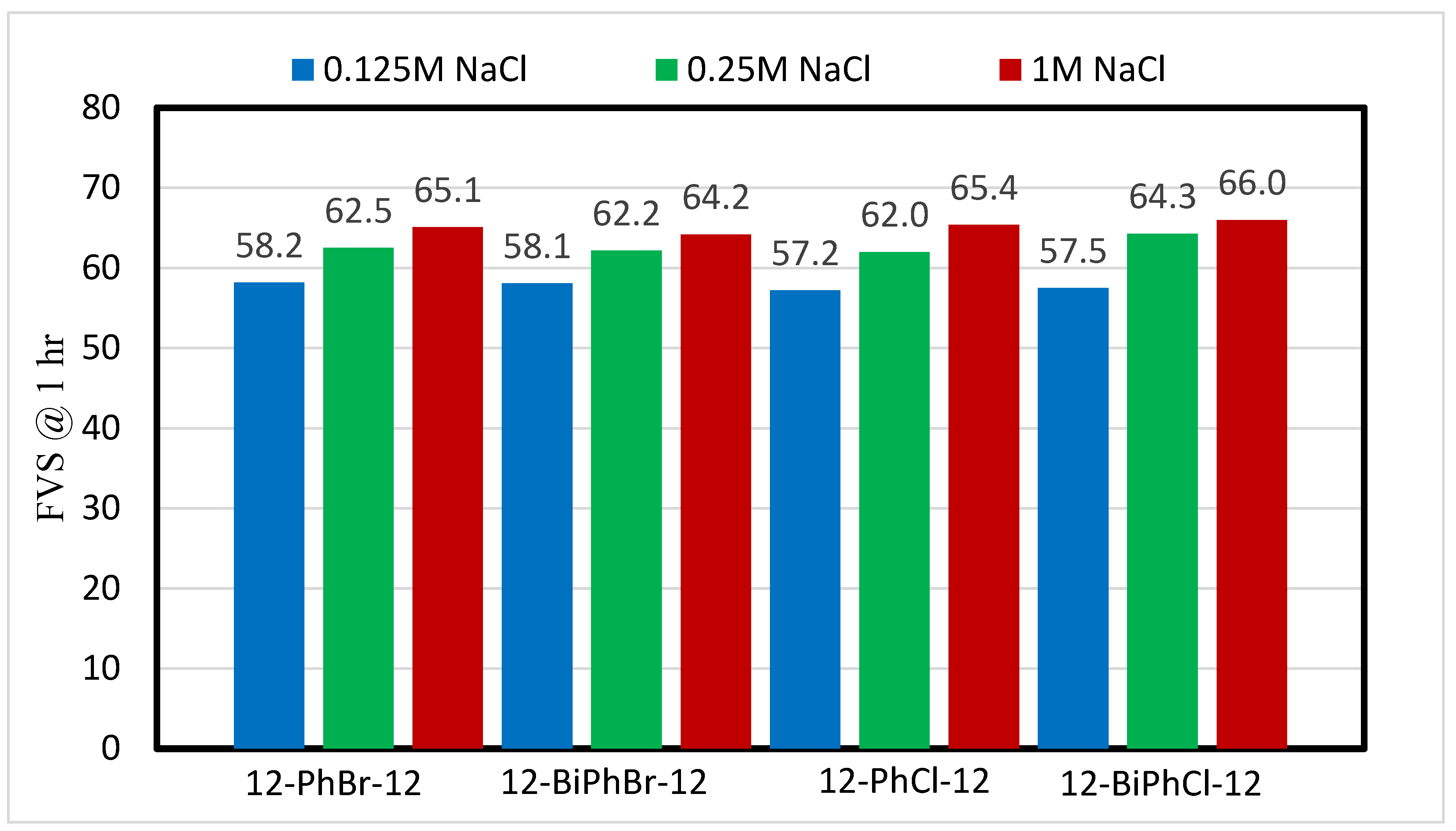
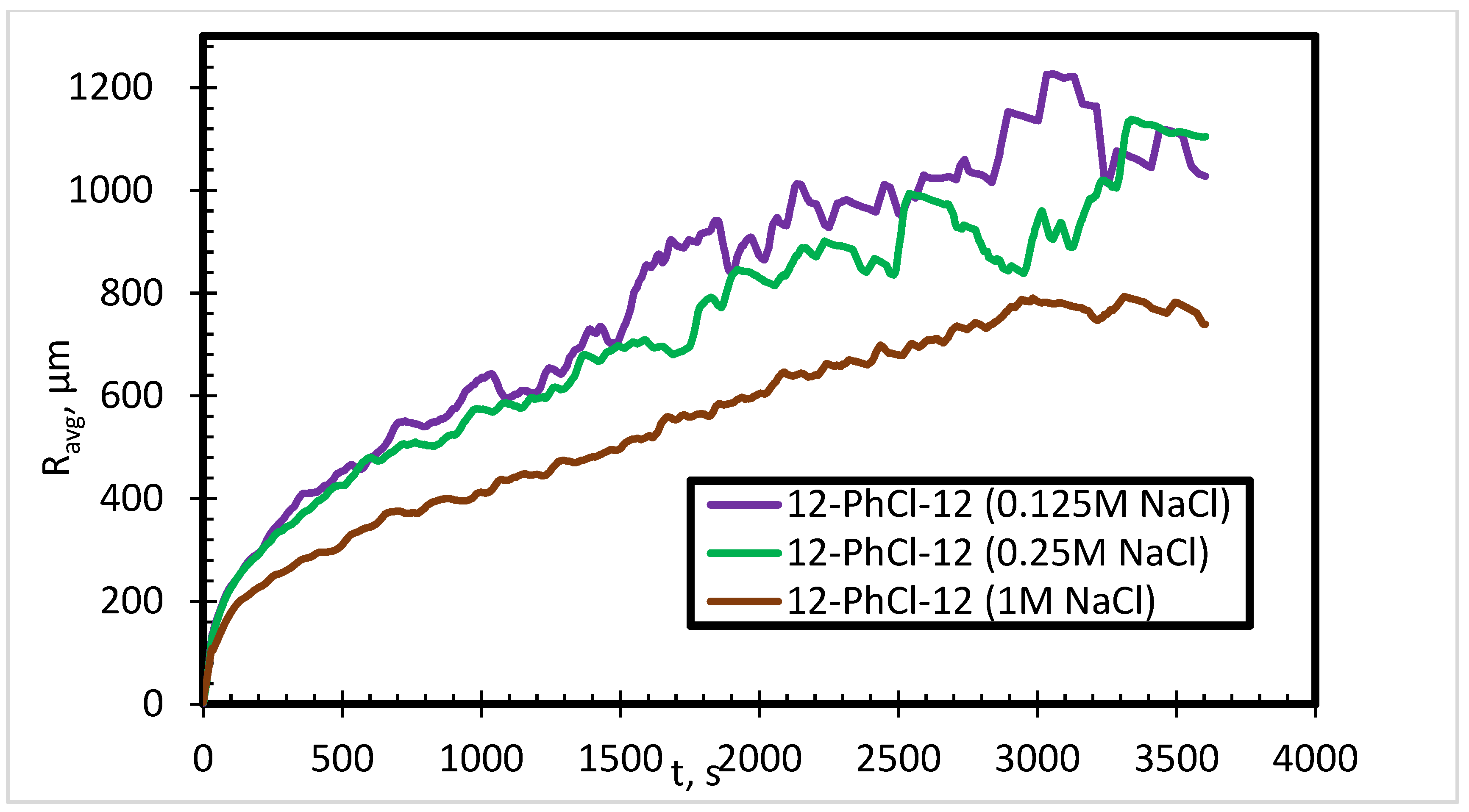
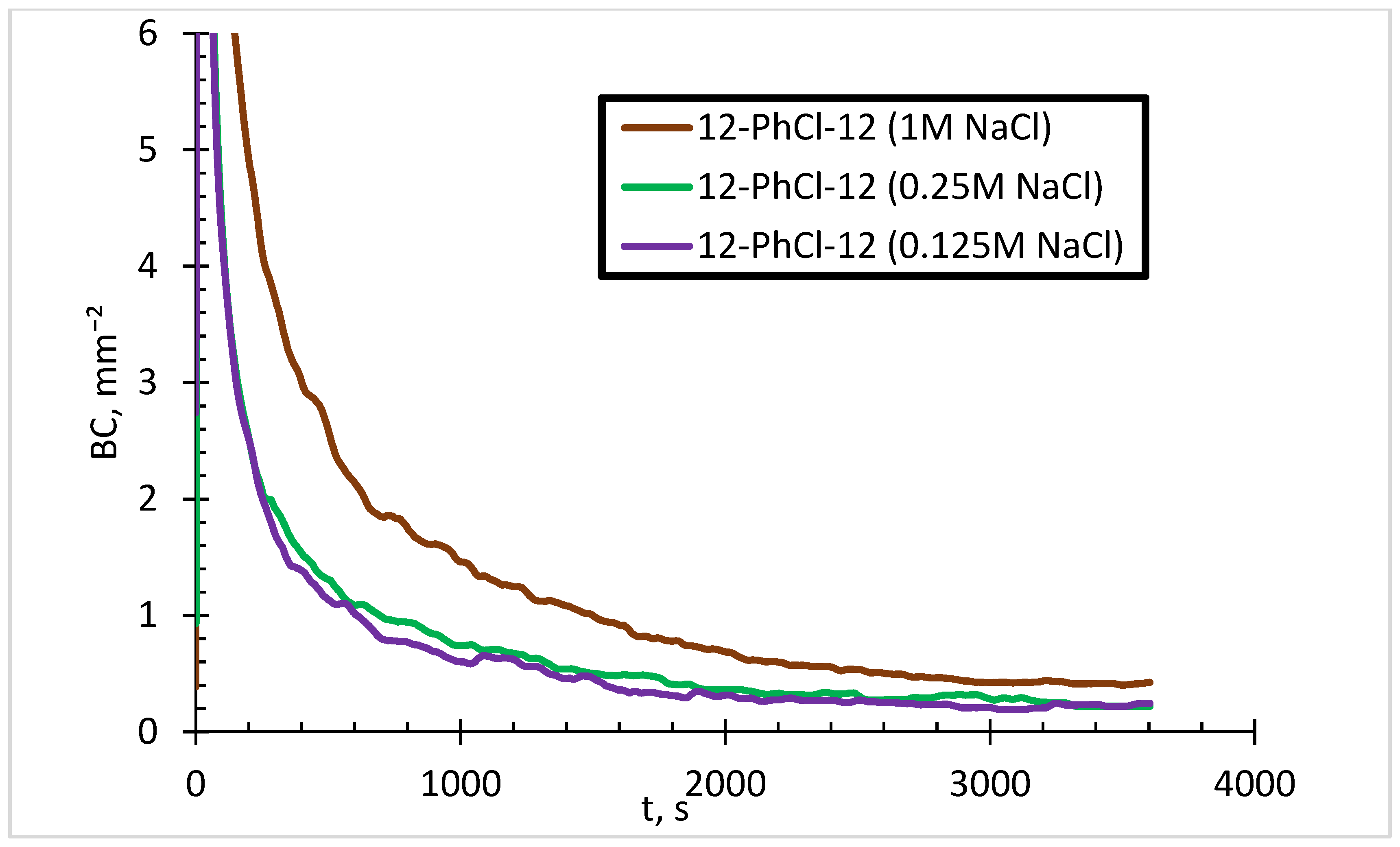
3.2. Effect of Salinity
4. Conclusions
- 1-
- All newly synthesized surfactants in this study were stable in deionized water, NaCl brine, and seawater.
- 2-
- The type of spacer and counterion have no impact on the foamability (initial foam volume produced) of these surfactants in deionized water and saline water.
- 3-
- The presence of bromide counterions generates more stable foam as compared to chloride counterions in deionized water.
- 4-
- The effect of counterions on foam stability becomes insignificant in the presence of salts.
- 5-
- The number of aromatic phenyl rings in the spacer has no significant effect on foam stability in deionized and saline water.
- 6-
- The addition of salts reduces the FVS, but with further increase in the salt concentration, the FVS increases.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Menger, F.M.; Keiper, J.S. Gemini surfactants. Angew. Chem. Int. Ed. 2000, 39, 1906–1920. [Google Scholar] [CrossRef]
- Pal, N.; Saxena, N.; Mandal, A. Studies on the physicochemical properties of synthesized tailor-made gemini surfactants for application in enhanced oil recovery. J. Mol. Liquids 2018, 258, 211–224. [Google Scholar] [CrossRef]
- Pal, N.; Kumar, N.; Verma, A.; Ojha, K.; Mandal, A. Performance Evaluation of Novel Sunflower Oil-based Gemini Surfactant (s) with different Spacer Lengths: Application in Enhanced Oil Recovery. Energy Fuels 2018, 32, 11344–11361. [Google Scholar] [CrossRef]
- Kamal, M.S.; Hussain, S.S.; Fogang, L.T.; Sultan, A.S. Impact of Spacer and Hydrophobic Tail on Interfacial and Rheological Properties of Cationic Amido-Amine Gemini Surfactants for EOR Application. Tenside Surf. Deterg. 2018, 55, 491–497. [Google Scholar] [CrossRef]
- Taleb, K.; Mohamed-Benkada, M.; Benhamed, N.; Saidi-Besbes, S.; Grohens, Y.; Derdour, A. Benzene ring containing cationic gemini surfactants: Synthesis, surface properties and antibacterial activity. J. Mol. Liquids 2017, 241, 81–90. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Huo, S.; Deng, Q.; Yan, T.; Ding, L.; Zhang, C.; Meng, L.; Lu, Q. Synthesis and Surface Properties of Novel Gemini Imidazolium Surfactants. J. Surf. Deterg. 2014, 17, 1107–1116. [Google Scholar] [CrossRef]
- Dong, Z.; Zheng, Y.; Zhao, J. Synthesis, physico-chemical properties and enhanced oil recovery flooding evaluation of novel zwitterionic gemini surfactants. J. Surf. Deterg. 2014, 17, 1213–1222. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Galedarzadeh, M.; Shadizadeh, S.R. Wettability Alteration in Carbonate Rocks by Implementing New Derived Natural Surfactant: Enhanced Oil Recovery Applications. Trans. Porous Med. 2015, 106, 645–667. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Shadizadeh, S. Experimental and Theoretical Study of a New Plant Derived Surfactant Adsorption on Quartz Surface: Kinetic and Isotherm Methods. J. Dispers. Sci. Technol. 2015, 36, 441–452. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Shadizadeh, S.R. Adsorption of novel nonionic surfactant and particles mixture in carbonates: Enhanced oil recovery implication. Energy Fuels 2012, 26, 4655–4663. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Zendehboudi, S.; Sha, A.; James, L. Nonionic Surfactant for Enhanced Oil Recovery from Carbonates: Adsorption Kinetics and Equilibrium. Ind. Eng. Chem. Res. 2012, 51, 9894–9905. [Google Scholar] [CrossRef]
- Sharma, H.; Panthi, K.; Mohanty, K.K. Surfactant-less alkali-cosolvent-polymer floods for an acidic crude oil. Fuel 2018, 215, 484–491. [Google Scholar] [CrossRef]
- Abdulredha, M.M.; Hussain, S.A.; Abdullah, L.C. Separation Emulsion via Non-Ionic Surfactant: An Optimization. Processes 2019, 7, 382. [Google Scholar] [CrossRef]
- Udy, J.; Hansen, B.; Maddux, S.; Petersen, D.; Heilner, S.; Stevens, K.; Lignell, D.; Hedengren, J. Review of field development optimization of waterflooding, eor, and well placement focusing on history matching and optimization algorithms. Processes 2017, 5, 34. [Google Scholar]
- Huang, B.; Wang, J.; Zhang, W.; Fu, C.; Wang, Y.; Liu, X. Screening and Optimization of Demulsifiers and Flocculants Based on ASP Flooding-Produced Water. Processes 2019, 7, 239. [Google Scholar] [CrossRef]
- Ter Minassian-Saraga, L. Thin films including layers: Terminology in relation to their preparation and characterization (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 1667–1738. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Muruganathan, R.; Rossen, W.; Krastev, R. Effect of gas type on foam film permeability and its implications for foam flow in porous media. Adv. Coll. Interface Sci. 2011, 168, 71–78. [Google Scholar] [CrossRef]
- Sánchez, C.C.; Patino, J.M.R. Interfacial, foaming and emulsifying characteristics of sodium caseinate as influenced by protein concentration in solution. Food Hydrocoll. 2005, 19, 407–416. [Google Scholar] [CrossRef]
- Langevin, D. Influence of interfacial rheology on foam and emulsion properties. Adv. Coll. Interface Sci. 2000, 88, 209–222. [Google Scholar] [CrossRef]
- Sakai, T.; Kaneko, Y. The effect of some foam boosters on the foamability and foam stability of anionic systems. J. Surf. Deterg. 2004, 7, 291–295. [Google Scholar] [CrossRef]
- Acharya, D.P.; Gutiérrez, J.M.; Aramaki, K.; Aratani, K.-I.; Kunieda, H. Interfacial properties and foam stability effect of novel gemini-type surfactants in aqueous solutions. J. Coll. Interface Sci. 2005, 291, 236–243. [Google Scholar] [CrossRef]
- Trujillo-Cayado, L.; Ramírez, P.; Pérez-Mosqueda, L.; Alfaro, M.; Muñoz, J. Surface and foaming properties of polyoxyethylene glycerol ester surfactants. Coll. Surf. A Physicochem. Eng. Asp. 2014, 458, 195–202. [Google Scholar] [CrossRef]
- Hussain, S.S.; Kamal, M.S.; Morteza, M. Effect of aromatic spacer groups and counter ions on aqueous micellar and thermal properties of the synthesized ethoxylated quaternary ammonium gemini Surfactants. Langmuir 1995, 11, 1448–1456. [Google Scholar]
- Nikolov, A.; Wasan, D.; Huang, D.; Edwards, D. The effect of oil on foam stability: mechanisms and implications for oil displacement by foam in porous media. In Proceedings of the SPE annual technical conference and exhibition, New Orleans, LA, USA, 5–8 October 1986. [Google Scholar]

| Ions | SW (ppm) |
|---|---|
| Na+ | 18,300 |
| Ca++ | 650 |
| Mg++ | 2110 |
| Cl− | 32,200 |
| 120 | |
| 4290 | |
| TDS | 57,670 |
| Evolution of Foam | |||||
|---|---|---|---|---|---|
| Start of Foaming | 15 min | 30 min | 60 min | ||
| Surfactant in Deionized Water | 12-PhBr-12 |  | 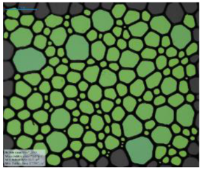 |  | 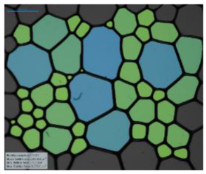 |
| 12-BiPhBr-12 | 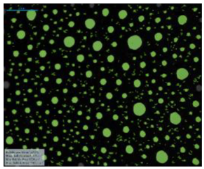 |  |  |  | |
| 12-PhCl-12 | 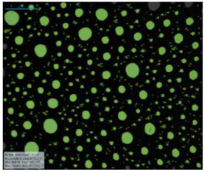 |  |  | 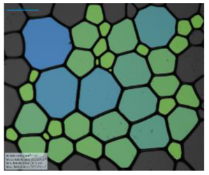 | |
| 12-BiPhCl-12 | 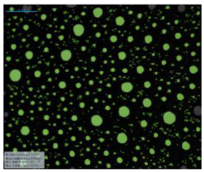 | 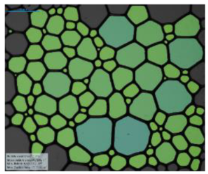 | 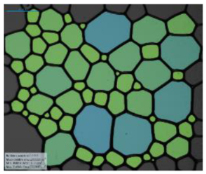 | 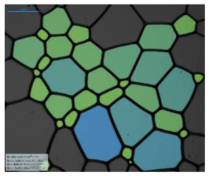 | |
| Evolution of Foam | |||||
|---|---|---|---|---|---|
| Start of Foaming | 15 min | 30 min | 60 min | ||
| Salinity Effect of 12-PhCl-12 | 0.125 M NaCl |  | 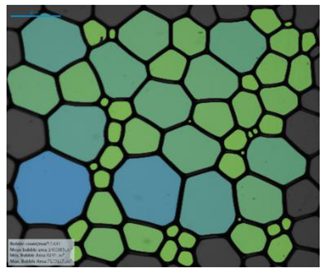 |  | 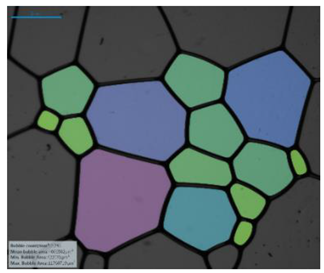 |
| 0.25 M NaCl | 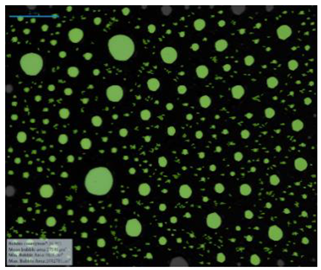 | 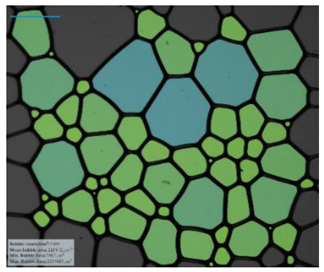 | 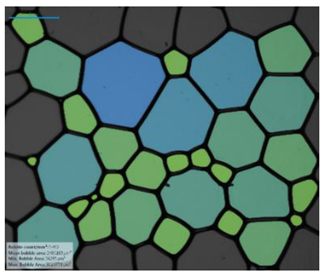 |  | |
| 1 M NaCl | 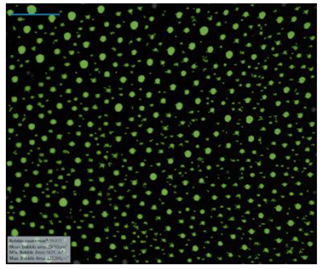 | 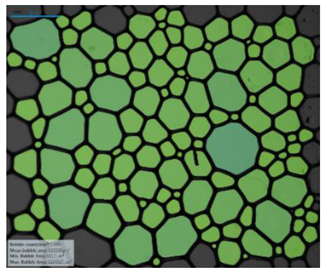 |  | 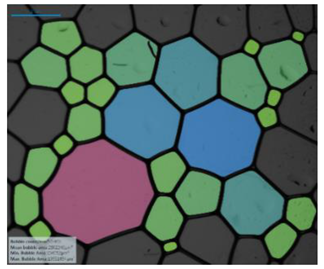 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalam, S.; Kamal, M.S.; Patil, S.; Hussain, S.M.S. Role of Counterions and Nature of Spacer on Foaming Properties of Novel Polyoxyethylene Cationic Gemini Surfactants. Processes 2019, 7, 502. https://doi.org/10.3390/pr7080502
Kalam S, Kamal MS, Patil S, Hussain SMS. Role of Counterions and Nature of Spacer on Foaming Properties of Novel Polyoxyethylene Cationic Gemini Surfactants. Processes. 2019; 7(8):502. https://doi.org/10.3390/pr7080502
Chicago/Turabian StyleKalam, Shams, Muhammad Shahzad Kamal, Shirish Patil, and S. M. Shakil Hussain. 2019. "Role of Counterions and Nature of Spacer on Foaming Properties of Novel Polyoxyethylene Cationic Gemini Surfactants" Processes 7, no. 8: 502. https://doi.org/10.3390/pr7080502
APA StyleKalam, S., Kamal, M. S., Patil, S., & Hussain, S. M. S. (2019). Role of Counterions and Nature of Spacer on Foaming Properties of Novel Polyoxyethylene Cationic Gemini Surfactants. Processes, 7(8), 502. https://doi.org/10.3390/pr7080502







