The Effect of Extraction Methods on Preliminary Structural Properties and Antioxidant Activities of Polysaccharides from Lactarius vividus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction of LVPs by Various Methods
2.2.1. Ultrasonic-Assisted Extraction
2.2.2. Microwave-Assisted Extraction
2.2.3. Enzyme-Assisted Extraction
2.2.4. Hot Water Extraction
2.3. Yield Calculation of LVPs
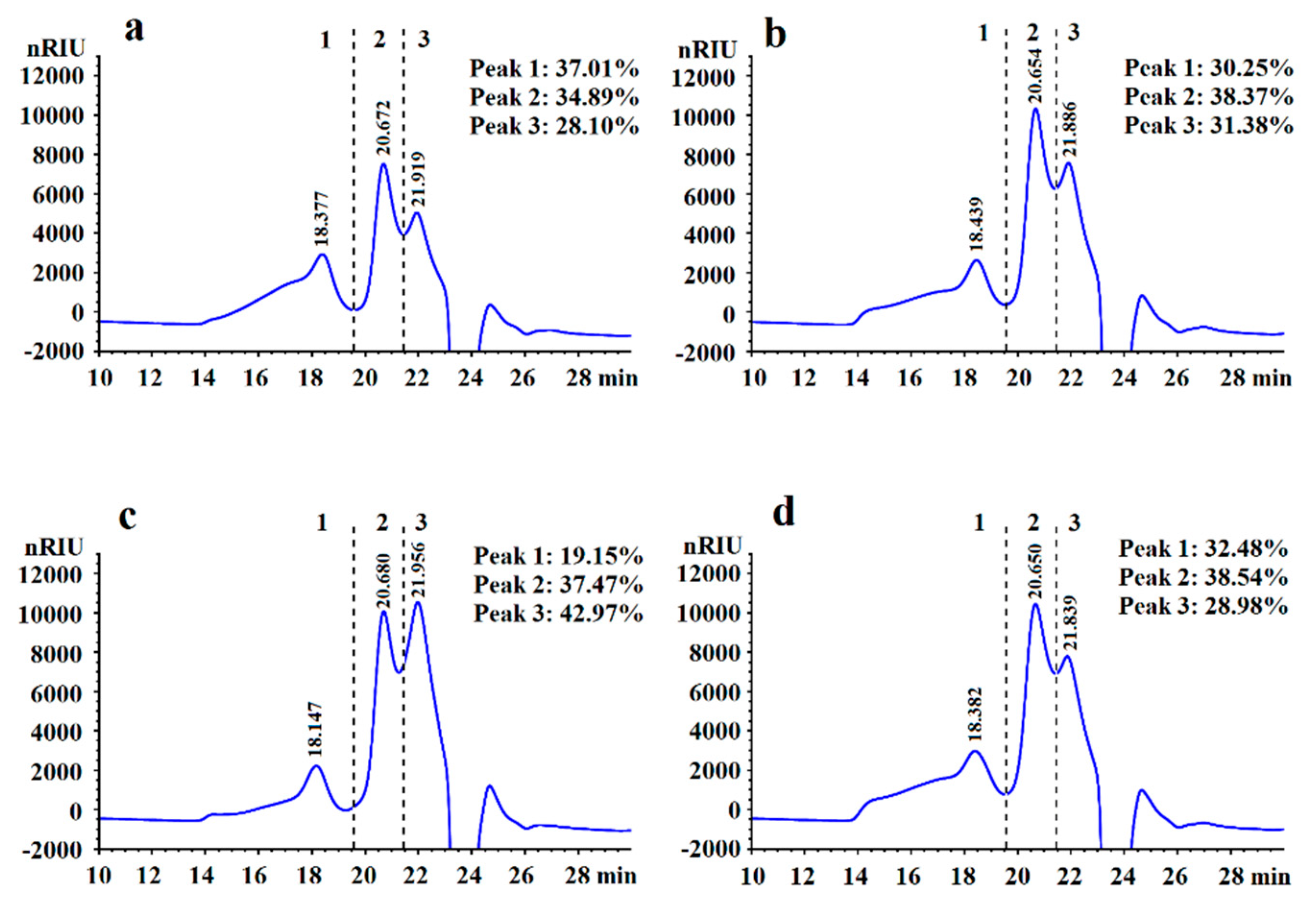
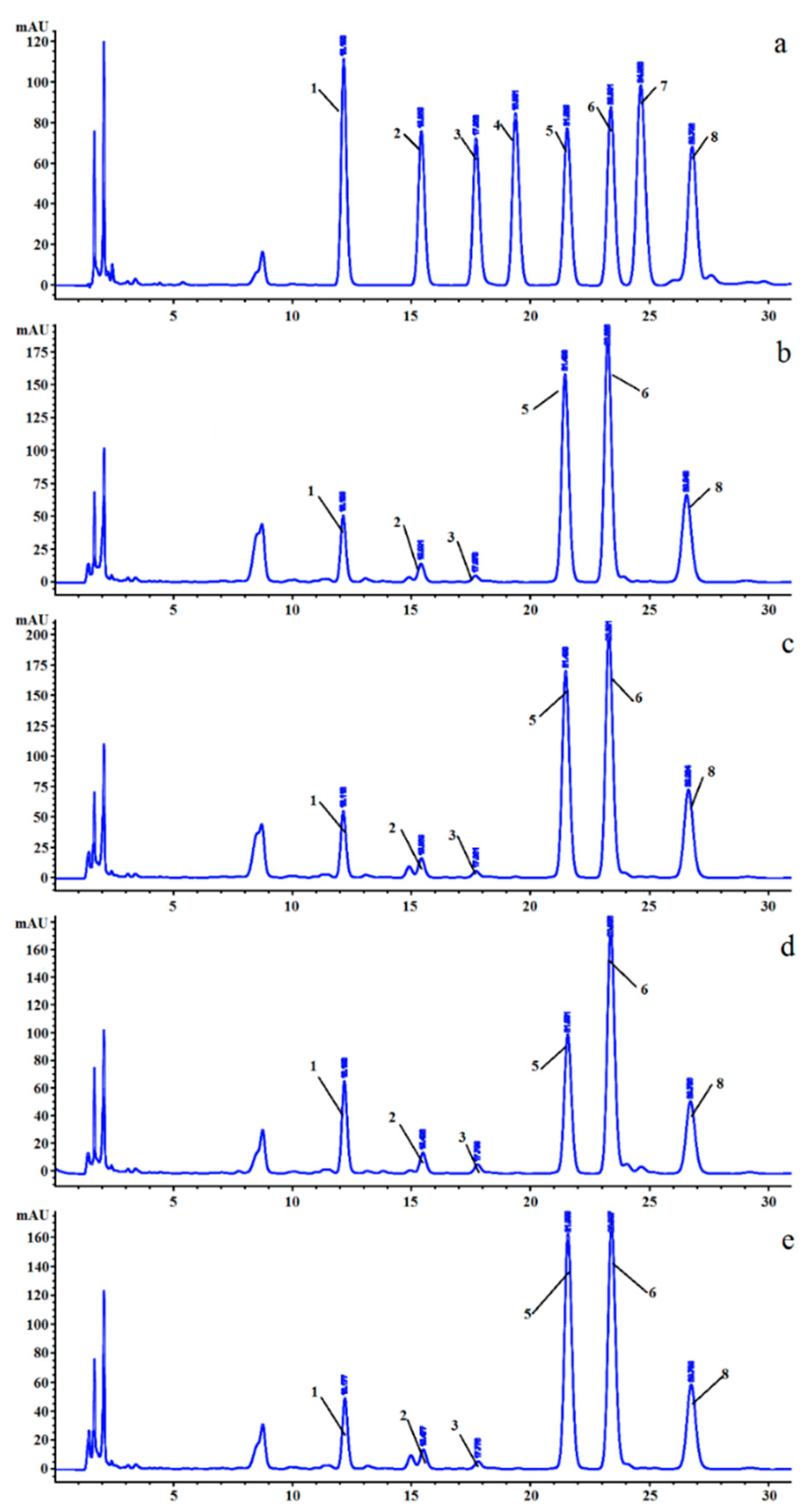
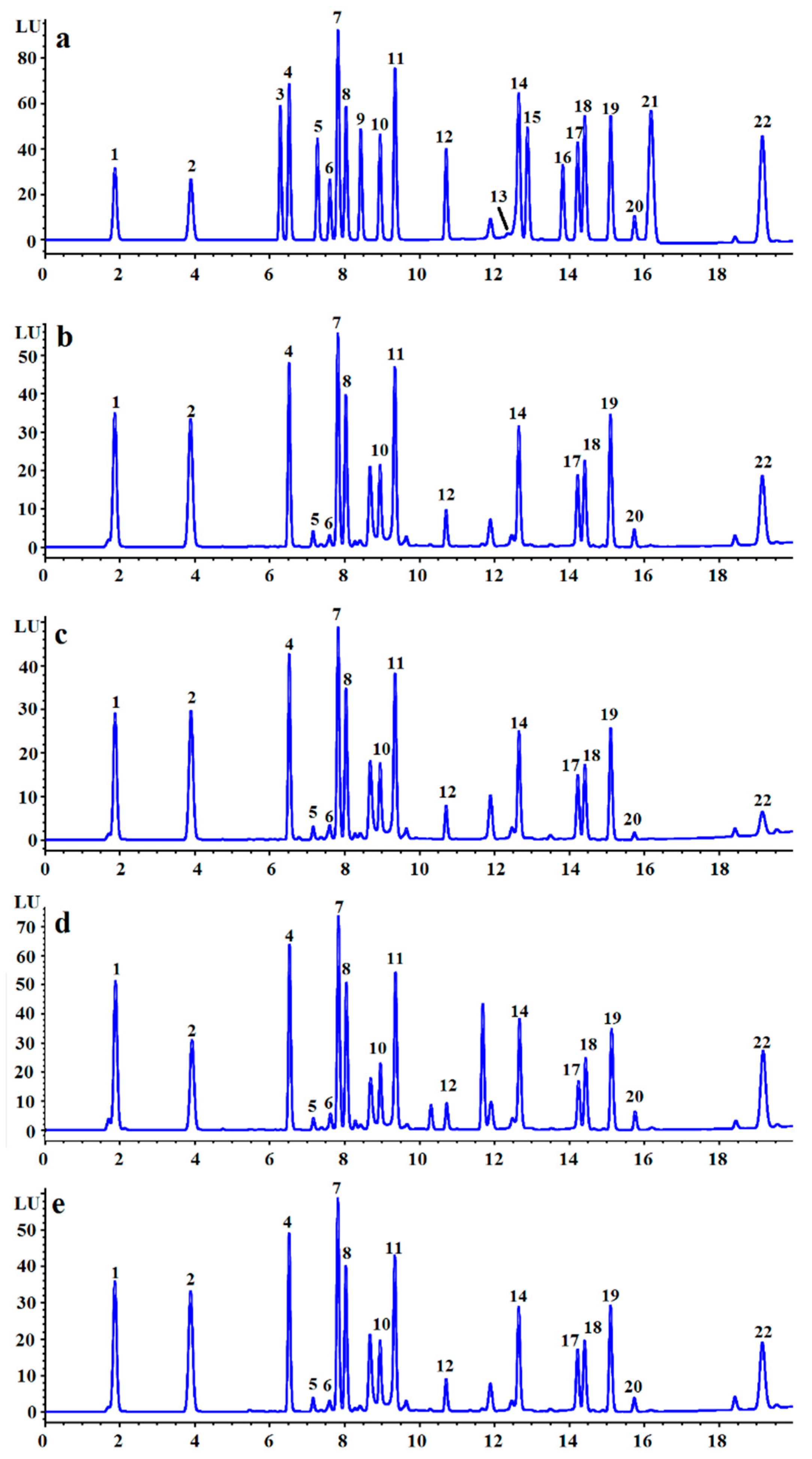
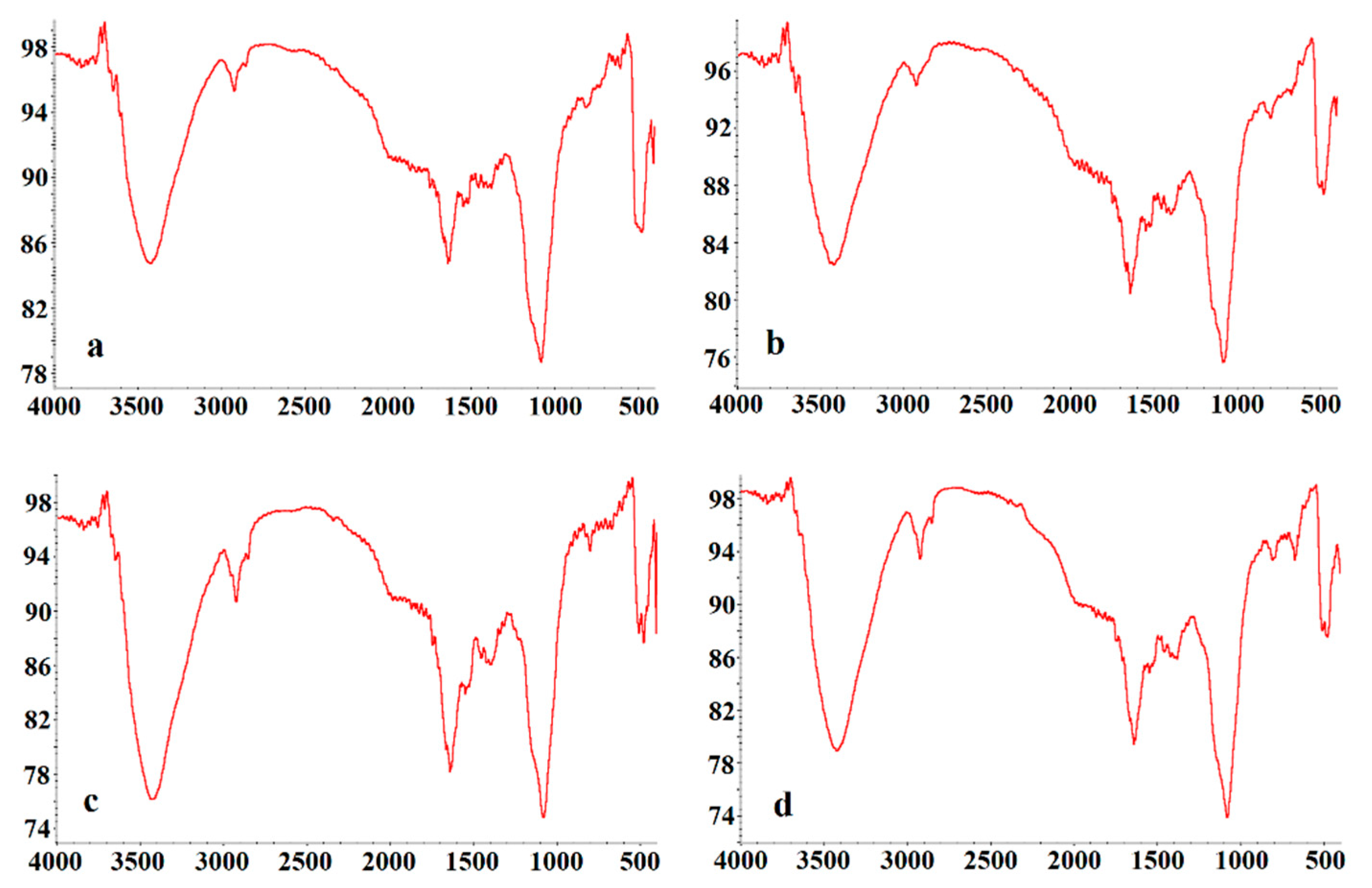
2.4. Preliminary Structural Properties of LVPs
2.5. Radical Scavenging Assays of LVPs
2.6. Metal Ion Chelating Assays of LVPs
2.7. Statistical Analysis
3. Results
3.1. Yields and Preliminary Structure Properties of LVPs
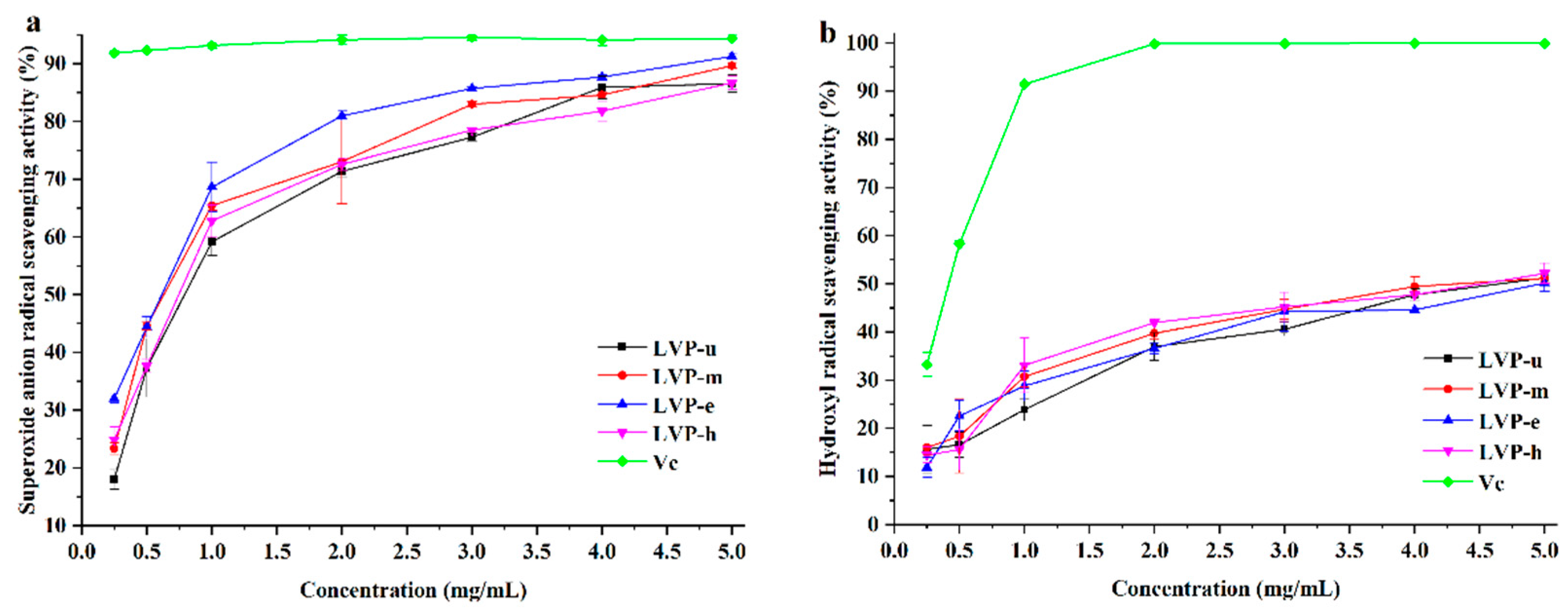
3.2. Radical Scavenging Activities of LVPs
3.3. Metal Ion Chelating Abilities of LVPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. PharmaNutrition 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Beulah, G.H.; Margret, A.A.; Nelson, J. Marvelous medicinal mushrooms. Int. J. Pharm. Biol. Sci. 2013, 3, 611–615. [Google Scholar]
- Finimundy, T.C.; Dillon, A.J.P.; Henriques, J.A.P.; Ely, M.R. A review on general nutritional compounds and pharmacological properties of the Lentinula edodes mushroom. Food Nutr. Sci. 2014, 5, 1095–1105. [Google Scholar]
- Heleno, S.A.; Barros, L.; Martins, A.; Morales, P.; Fernández-Ruiz, V.; Glamoclija, J.; Sokovic, M.; Ferreira, I.C.F.R. Nutritional value, bioactive compounds, antimicrobial activity and bioaccessibility studies with wild edible mushrooms. LWT Food Sci. Technol. 2015, 63, 799–806. [Google Scholar] [CrossRef]
- Chihara, G.; Maeda, Y.; Hamuro, J.; Sasaki, T.; Fukuoka, F. Inhibition of mouse sarcoma 180 by polysaccharides from Lentinus edodes (Berk.) Sing. Nature 1969, 222, 687–688. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Li, F.; Huang, L.; Xue, D.; Liu, W.; Xu, C. Chemical characterization and antioxidant activity of polysaccharide extract from spent mushroom substrate of Pleurotus eryngii. J. Taiwan Inst. Chem. Eng. 2016, 69, 48–53. [Google Scholar] [CrossRef]
- Wang, M.; Konishi, T.; Gao, Y.; Xu, D.; Gao, Q. Anti-gastric ulcer activity of polysaccharide fraction isolated from mycelium culture of Lion’s Mane medicinal mushroom, Hericium Erinaceus (Higher basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.R.; Liu, Z.K.; Liu, F.; Ng, T.B. Antihyperglycaemic mechanisms of an aceteoside polymer from rose flowers and a polysaccharide–protein complex from abalone mushroom. Nat. Prod. Res. 2015, 29, 558–561. [Google Scholar] [CrossRef]
- Song, H.; Zhang, Q.; Zhang, Z.; Wang, J. In vitro antioxidant activity of polysaccharides extracted from Bryopsis plumosa. Carbohydr. Polym. 2010, 80, 1057–1061. [Google Scholar] [CrossRef]
- Jia, S.; Li, F.; Liu, Y.; Ren, H.; Gong, G.; Wang, Y.; Wu, S. Effects of extraction methods on the antioxidant activities of polysaccharides from Agaricus blazei Murrill. Int. J. Biol. Macromol. 2013, 62, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, X.; Wan, M.; Chen, J.; Li, S.; Cao, M.; Zhang, D. Effect of extraction methods on property and bioactivity of water-soluble polysaccharides from Amomum villosum. Carbohydr. Polym. 2015, 117, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lv, G.; He, W.; Shi, L.; Pan, H.; Fan, L. Effects of extraction methods on the antioxidant activities of polysaccharides obtained from Flammulina velutipes. Carbohydr. Polym. 2013, 98, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhu, Y.; Zhang, L.; Yang, R.; Zhou, Y. Extraction, preliminary structural characterization, and antioxidant activities of polysaccharides from Salvia miltiorrhiza Bunge. Carbohydr. Polym. 2012, 87, 1348–1353. [Google Scholar] [CrossRef]
- Wang, X.; Nuytinck, J.; Verbeken, A. Lactarius vividus sp. nov. (Russulaceae, Russulales), a widely distributed edible mushroom in central and southern China. Phytotaxa 2015, 231, 63–72. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, H.; Xu, Z.; Zheng, B.; Lin, Y.; Gan, C.; Lo, Y.M. Ultrasonic-assisted extraction and antioxidant activity of polysaccharides recovered from white button mushroom (Agaricus bisporus). Carbohydr. Polym. 2012, 88, 522–529. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, G.; Pan, H.; Shi, L.; Fan, L. Optimization of the microwave-assisted extraction process for polysaccharides in himematsutake (Agaricus blazei Murrill) and evaluation of their antioxidant activities. Food Sci. Technol. Res. 2011, 17, 461–470. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Q.; Mao, G.; Zou, Y.; Feng, W.; Zheng, D.; Wang, W.; Zhou, L.; Zhang, T.; Yang, J.; et al. Optimization of enzyme-assisted extraction and characterization of polysaccharides from Hericium erinaceus. Carbohydr. Polym. 2014, 101, 606–613. [Google Scholar] [CrossRef]
- Hou, X.; Zhan, N.; Xiong, S.; Li, S.; Yang, B. Extraction of BaChu mushroom polysaccharides and preparation of a compound beverage. Carbohydr. Polym. 2008, 73, 289–294. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Xu, Z.; Li, X.; Feng, S.; Liu, J.; Zhou, L.; Yuan, M.; Ding, C. Characteristics and bioactivities of different molecular weight polysaccharides from camellia seed cake. Int. J. Biol. Macromol. 2016, 91, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, Q.; Bao, C.; Fan, J. Comparison of free total amino acid compositions and their functional classifications in 13 wild edible mushrooms. Molecules 2017, 22, 350. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Giese, E.C.; Gascon, J.; Anzelmo, G.; Barbosa, A.M.; da Cunha, M.A.A.; Dekker, R.F.H. Free-radical scavenging properties and antioxidant activities of botryosphaeran and some other β-D-glucans. Int. J. Biol. Macromol. 2015, 72, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Kalın, P.; Gülçin, I.; Gören, A. Antioxidant activity and polyphenol content of cranberries (Vaccinium macrocarpon). Rec. Nat. Prod. 2015, 9, 496–502. [Google Scholar]
- Chi, A.; Shen, Z.; Zhu, W.; Sun, Y.; Kang, Y.; Guo, F. Characterization of a protein-bound polysaccharide from Herba Epimedii and its metabolic mechanism in chronic fatigue syndrome. J. Ethnopharmacol. 2017, 203, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-K.; Wang, Y.-Y.; Qiu, W.-Y.; Wu, L.-X.; Ding, Z.-C.; Cai, W.-D. Purification, structural characterization and bioactivity evaluation of a novel proteoglycan produced by Corbicula fluminea. Carbohydr. Polym. 2017, 176, 11–18. [Google Scholar] [CrossRef]
- Kang, J.-H.; Jang, J.-E.; Mishra, S.K.; Lee, H.-J.; Nho, C.W.; Shin, D.; Jin, M.; Kim, M.K.; Choi, C.; Oh, S.H. Ergosterol peroxide from Chaga mushroom (Inonotus obliquus) exhibits anti-cancer activity by down-regulation of the β-catenin pathway in colorectal cancer. J. Ethnopharmacol. 2015, 173, 303–312. [Google Scholar] [CrossRef]
- Pan, H.; Han, Y.; Huang, J.; Yu, X.; Jiao, C.; Yang, X.; Dhaliwal, P.; Xie, Y.; Yang, B.B. Purification and identification of a polysaccharide from medicinal mushroom Amauroderma rude with immunomodulatory activity and inhibitory effect on tumor growth. Oncotarget 2015, 6, 17777–17791. [Google Scholar] [CrossRef]
- Chihara, G.; Hamuro, J.; Maeda, Y.Y.; Arai, Y.; Fukuoka, F. Fractionation and purification of the polysaccharides with marked antitumor activity, especially lentinan, from Lentinus edodes (Berk.) Sing.(an edible mushroom). Cancer Res. 1970, 30, 2776–2781. [Google Scholar] [PubMed]
- Yoon, S.-J.; Yu, M.-A.; Pyun, Y.-R.; Hwang, J.-K.; Chu, D.-C.; Juneja, L.R.; Mourao, P.A. The nontoxic mushroom Auricularia auricula contains a polysaccharide with anticoagulant activity mediated by antithrombin. Thromb. Res. 2003, 112, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Mao, J.-W.; Zhang, A.-Q.; Wang, Y.-J.; Sun, P.-L. Purification, chemical characterization, and antioxidant activity of a polysaccharide from the fruiting bodies of sanghuang mushroom (Phellinus baumii Pilát). Food Sci. Biotechnol. 2013, 22, 301–307. [Google Scholar] [CrossRef]
- Yan, J.-K.; Wang, W.-Q.; Wu, J.-Y. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: A review. J. Funct. Foods 2014, 6, 33–47. [Google Scholar] [CrossRef]
- Prakash Maran, J.; Mekala, V.; Manikandan, S. Modeling and optimization of ultrasound-assisted extraction of polysaccharide from Cucurbita moschata. Carbohydr. Polym. 2013, 92, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Z.; Wei, X. Sugar compositions, α-glucosidase inhibitory and amylase inhibitory activities of polysaccharides from leaves and flowers of Camellia sinensis obtained by different extraction methods. Int. J. Biol. Macromol. 2010, 47, 534–539. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X.; Kong, W.; Yang, H.; Su, Y.; Zhang, L.; Zhang, Y.; Yang, Y.; Ding, L.; Liu, G. Antioxidant activities of malt extract from barley (Hordeum vulgare L.) toward various oxidative stress in vitro and in vivo. Food Chem. 2010, 118, 84–89. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Castegna, A.; Pocernich, C.B.; Drake, J.; Scapagnini, G.; Calabrese, V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J. Nutr. Biochem. 2002, 13, 444–461. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Tan, J.B.L.; Lim, Y.Y. Critical analysis of current methods for assessing the in vitro antioxidant and antibacterial activity of plant extracts. Food Chem. 2015, 172, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Kong, F.; Li, N.; Zhang, D.; Yan, C.; Lv, H. Purification, structural characterization and in vitro antioxidant activity of a novel polysaccharide from Boshuzhi. Carbohydr. Polym. 2016, 147, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Yuan, L.; Cai, D.; Jiao, L.; Zhang, L. Characterization and antioxidant activities of the polysaccharides from mycelium of Phellinus pini and culture medium. Carbohydr. Polym. 2015, 117, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Zhong, J.; Ye, X.; Lu, S.; Wang, W.; Zhang, R.; Xu, J.; Chen, S.; Liu, D. Ultrasonic-assisted enzymatic extraction of polysaccharide from Corbicula fluminea: Characterization and antioxidant activity. LWT Food Sci. Technol. 2015, 60, 1113–1121. [Google Scholar] [CrossRef]
- Zhang, W.; Song, D.; Xu, D.; Wang, T.; Chen, L.; Duan, J. Characterization of polysaccharides with antioxidant and immunological activities from Rhizoma Acori Tatarinowii. Carbohydr. Polym. 2015, 133, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chang, S.C.; Inbaraj, B.S.; Chen, B.H. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. and evaluation of antioxidant activity. Food Chem. 2010, 120, 184–192. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, T.; Jin, Z.-Y.; Xu, X.-M.; Wang, J.-H.; Zha, X.-Q.; Chen, H.-Q. Structural characterisation, physicochemical properties and antioxidant activity of polysaccharide from Lilium lancifolium Thunb. Food Chem. 2015, 169, 430–438. [Google Scholar] [CrossRef]
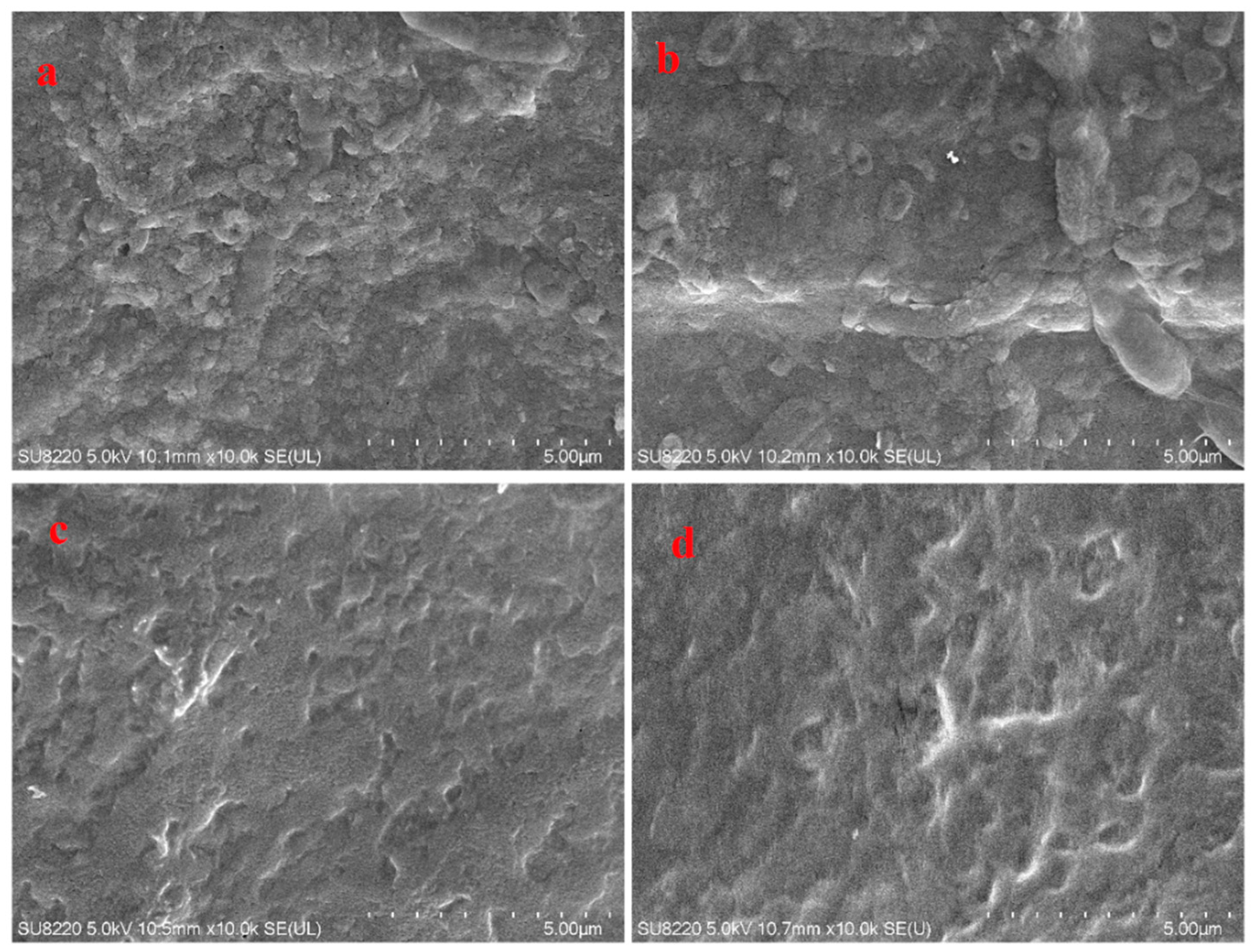

| Polysaccharides Yield (%) | Total Carbohydrate Content (%) | Protein Content (%) | |
|---|---|---|---|
| LVP-u | 3.74 ± 0.14 a | 57.59 ± 1.90 a | 25.69 ± 0.76 c |
| LVP-m | 3.09 ± 0.19 c | 55.34 ± 1.65 b | 28.16 ± 0.25 b |
| LVP-e | 3.33 ± 0.13 b,c | 49.95 ± 1.92 c | 38.19 ± 0.92 a |
| LVP-h | 3.63 ± 0.20 a,b | 59.64 ± 1.04 a | 28.93 ± 0.40 b |
| LVP-u | LVP-m | LVP-e | LVP-h | |
|---|---|---|---|---|
| Monosaccharide composition (Percentage mole ratio) | ||||
| Mannose | 8.41 | 7.66 | 11.78 | 7.71 |
| Rhamnose | 2.60 | 2.72 | 3.23 | 2.60 |
| Glucuronic acid | 0.75 | 0.82 | 1.78 | 1.32 |
| Glucose | 33.01 | 32.51 | 24.23 | 35.09 |
| Galactose | 37.23 | 39.10 | 43.20 | 37.71 |
| Fucose | 18.00 | 17.18 | 15.78 | 15.56 |
| Amino acid composition (Percentage mole ratio) | ||||
| Asparaginic acid | 12.27 | 12.23 | 14.24 | 12.57 |
| Glutamic acid | 12.52 | 13.89 | 9.30 | 12.71 |
| Serine | 9.65 | 10.96 | 10.75 | 10.16 |
| Glutamine | 0.61 | 0.60 | 0.50 | 0.59 |
| Histidine | 1.76 | 2.50 | 1.76 | 1.85 |
| Glycine | 11.53 | 12.85 | 12.71 | 12.57 |
| Threonine | 8.44 | 9.40 | 8.95 | 8.80 |
| Arginine | 4.62 | 4.92 | 3.98 | 4.43 |
| Alanine | 11.19 | 11.75 | 10.43 | 10.72 |
| Tyrosine | 1.30 | 1.37 | 1.042 | 1.26 |
| Valine | 5.56 | 5.76 | 5.71 | 5.12 |
| Phenylalanine | 3.92 | 3.80 | 3.05 | 3.83 |
| Isoleucine | 4.39 | 4.35 | 4.07 | 3.97 |
| Leucine | 3.40 | 3.23 | 2.87 | 2.96 |
| Lysine | 4.36 | 0.71 | 4.85 | 3.77 |
| Proline | 4.46 | 1.67 | 5.79 | 4.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Feng, S.; Qu, J.; Yuan, M.; Yang, R.; Zhou, L.; Chen, T.; Ding, C. The Effect of Extraction Methods on Preliminary Structural Properties and Antioxidant Activities of Polysaccharides from Lactarius vividus. Processes 2019, 7, 482. https://doi.org/10.3390/pr7080482
Xu Z, Feng S, Qu J, Yuan M, Yang R, Zhou L, Chen T, Ding C. The Effect of Extraction Methods on Preliminary Structural Properties and Antioxidant Activities of Polysaccharides from Lactarius vividus. Processes. 2019; 7(8):482. https://doi.org/10.3390/pr7080482
Chicago/Turabian StyleXu, Zhou, Shiling Feng, Jipeng Qu, Ming Yuan, Ruiwu Yang, Lijun Zhou, Tao Chen, and Chunbang Ding. 2019. "The Effect of Extraction Methods on Preliminary Structural Properties and Antioxidant Activities of Polysaccharides from Lactarius vividus" Processes 7, no. 8: 482. https://doi.org/10.3390/pr7080482
APA StyleXu, Z., Feng, S., Qu, J., Yuan, M., Yang, R., Zhou, L., Chen, T., & Ding, C. (2019). The Effect of Extraction Methods on Preliminary Structural Properties and Antioxidant Activities of Polysaccharides from Lactarius vividus. Processes, 7(8), 482. https://doi.org/10.3390/pr7080482








