Recombinant Proteins for Industrial versus Pharmaceutical Purposes: A Review of Process and Pricing
Abstract
1. Introduction
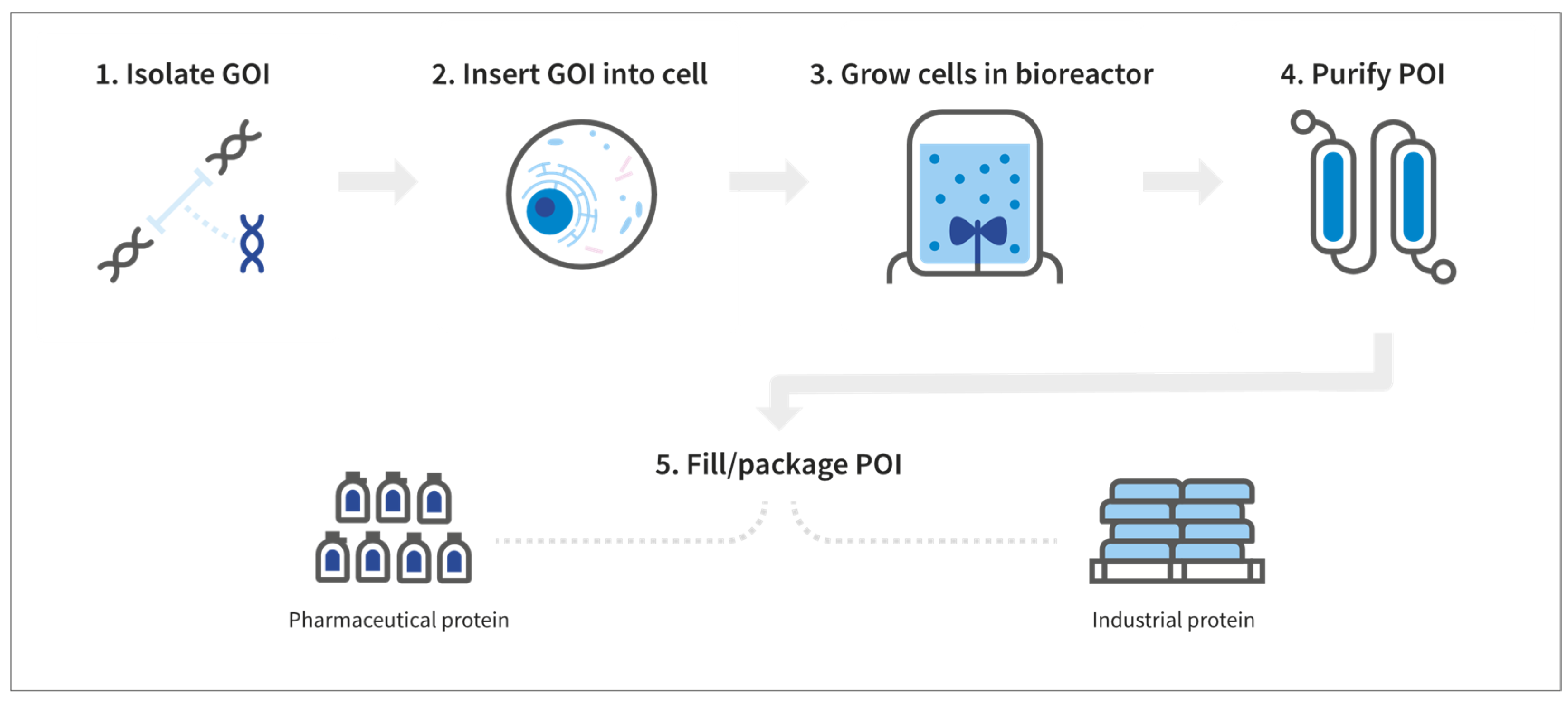
2. The Basics of Manufacturing Recombinant Proteins
3. Differences in Manufacturing Pharmaceutical and Industrial Recombinant Proteins
3.1. DNA Vector Construction and Gene Transfer to Host Systems
3.2. Cell Host Systems
3.3. Growth Media
3.4. Bioreactors and the Production Process
3.5. Downstream Processing and Formulation
3.6. Quality Control
3.7. Manufacturing Summary
4. Clinical Trials, R&D, and Patents
5. Marketing and Liability
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
- rFVIII: 7.5 × 103 IU/mg × 106 mg/kg = 7.5 × 109 IU/kg × $1.28/IU = $9.6 × 109/kg
- rFVII: $2.07/mcg × 109 mcg/kg = $2.07 × 109/kg
- Rituximab: $95/mL × 1 mL/10 mg × 106 mg/kg = $9.5 × 106/kg
- rHGH: $55/0.4 mg × 106 mg/kg = $1.37 × 108/kg
- eculizumab: $230/mL × 1 mL/10 mg × 106 mg/kg = $2.3 × 107/kg
- rHepatitis B surface antigen: $27/5 mcg × 109 mcg/kg = $ 5.4 × 109/kg
References
- Arrans-Otaegui, A.; Carretero, L.; Ramsey, M.; Fuller, D.; Richter, T. Archaeobotanical evidence reveals the origins of bread 14,400 years ago in northeastern Jordan. Proc. Natl. Acad. Sci. USA 2018, 115, 7925–7930. [Google Scholar] [CrossRef]
- Hayden, B.; Canuel, N.; Shanse, J. What Was Brewing in the Natufian? An Archaeological Assessment of Brewing Technology in the Epipaleolithic. J. Archaeol. Method Theory 2013, 20, 102–150. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv. 2009, 27, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Kirk, O.; Borchert, T.; Fuglsang, C. Industrial enzyme applications. Curr. Opin. Biotechnol. 2002, 13, 345–351. [Google Scholar] [CrossRef]
- Pham, P.V. Medical Biotechnology: Techniques and Applications. In Omics Technologies and Bio-Engineering. Towards Improving Quality of Life, 1st ed.; Barh, D., Azevedo, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 449–469. [Google Scholar]
- Powell, J.S.; Josephson, N.C.; Quon, D.; Ragni, M.V.; Cheng, G.; Li, E.; Jiang, H.; Li, L.; Dumont, J.A.; Goyal, J.; et al. Safety and prolonged activity of recombinant factor VIII Fc fusion protein in hemophilia A patients. Blood 2012, 119, 3031–3037. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.; Milne, A.; Kayani, K.; Ojha, U. How the discovery of rituximab impacted the treatment of B-cell non-Hodgkin’s lymphomas. J. Blood Med. 2019, 10, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Pranchevicius, M.C.; Vieira, T. Production of recombinant immunotherapeutics for anticancer treatment: The role of bioengineering. Bioengineered 2013, 4, 305–312. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P. Microbial enzymes: Industrial progress in 21st century. 3 Biotech 2016, 6, 174. [Google Scholar] [CrossRef]
- Vojcic, L.; Pitzler, C.; Koerfer, G.; Jakob, F.; Martinez, R.; Maurer, K.H.; Schwaneberg, U. Advances in protease engineering for laundry detergents. New Biotechnol. 2015, 32, 629–634. [Google Scholar] [CrossRef]
- Maurer, K.H. Detergent proteases. Curr. Opin. Biotechnol. 2004, 15, 330–334. [Google Scholar] [CrossRef]
- Von der Osten, C.; Branner, S.; Hastrup, S.; Hedegaard, L.; Rasmussen, M.D.; Bisgaard-Frantzen, H.; Carlsen, S.; Mikkelsen, J.M. Protein engineering of subtilisins to improve stability in detergent formulations. J. Biotechnol. 1993, 28, 55–63. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, J.; Bao, J. Cost evaluation of cellulase enzyme for industrial-scale cellulosic ethanol production based on rigorous Aspen Plus modelling. Bioprocess Biosyst. Eng. 2016, 39, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.A.A.; Freitas, S. Techno-economic analysis of the industrial production of a low-cost enzyme using E. coli: The case of recombinant β-glucosidase. Biotechnol. Biofuels 2018, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- 2019 ASP Drug Pricing Files. Centers for Medicare and Medicaid Services. CMS.gov. Available online: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2019ASPFiles.html (accessed on 21 January 2019).
- Pan, H.; Chen, Y.; Yu, P. Advanced Strategies for Improving the Production of Industrial Enzymes in Heterologous Host Systems. Enzym. Eng. 2013, 2, 2. [Google Scholar]
- Yang, Z.; Zhang, Z. Engineering strategies for enhanced production of protein and bio-products in Pichia pastoris: A review. Biotechnol. Adv. 2018, 36, 182–195. [Google Scholar] [CrossRef]
- De Jesus, M.; Wurm, F. Manufacturing recombinant proteins in kg-ton quantities using animal cells in bioreactors. Eur. J. Pharm. Biopharm. 2011, 78, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Goeddel, D.V.; Kleid, D.G.; Bolivar, F.; Heyneker, H.L.; Yansura, D.G.; Crea, R.; Hirose, T.; Kraszewski, A.; Itakura, K.; Riggs, A.D. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc. Natl. Acad. Sci. USA 1979, 76, 106–110. [Google Scholar] [CrossRef]
- Gaglione, R.; Pane, K.; Dell’Olmo, E.; Cafaro, V.; Pizzo, E.; Olivieri, G.; Notomista, E.; Arciello, A. Cost-effective production of recombinant peptides in Escherichia coli. New Biotechnol. 2019, 51, 39–48. [Google Scholar] [CrossRef]
- Khootama, A.; Putri, D.N.; Hermansyah, H. Techno-economic analysis of lipase enzyme production from Aspergillus niger using agro-industrial waste by solid state fermentation. Energy Procedia 2018, 153, 143–148. [Google Scholar] [CrossRef]
- Chartrain, M.; Chu, L. Development and Production of Commercial Therapeutic Monoclonal Antibodies in Mammalian Cell Expression Systems: An Overview of the Current Upstream Technologies. Curr. Pharm. Biotechnol. 2008, 9, 447–467. [Google Scholar] [CrossRef]
- Saraswat, M.; Musante, L.; Ravidà, A.; Shortt, B.; Byrne, B.; Holthöfer, H. Preparative Purification of Recombinant Proteins: Current Status and Future Trends. BioMed Res. Int. 2013, 2013, 312709. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.; Wuchterl, D.; López, M.; Minshall, B.; Prusti, R.; Boclair, D.; Peterson, J.; Allen, C. The Biomanufacturing of Biotechnology Products. In Biotechnology Entrepreneurship; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 351–385. [Google Scholar]
- Frokjaer, S.; Otzen, D.E. Protein drug stability: A formulation challenge. Nat. Rev. Drug Discov. 2005, 4, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Peraman, R.; Bhadraya, K.; Reddy, Y.P. Analytical Quality by Design: A Tool for Regulatory Flexibility and Robust Analytics. Int. J. Anal. Chem. 2015, 2015, 868727. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceutical CGMPS for the 21st Century—A Risk-Based Approach. Available online: https://www.fda.gov/about-fda/center-drug-evaluation-and-research/pharmaceutical-quality-21st-century-risk-based-approach-progress-report (accessed on 3 May 2019).
- Monroe, T.; Mcrogers, R.; Larson, P.J.; Jiang, R. Manufacturing challenges in the commercial production of recombinant coagulation factor VIII. Haemophilia 2002, 8, 1–5. [Google Scholar]
- Quality Guidelines. International Council for Harmonisation. Available online: https://www.ich.org/products/guidelines.html (accessed on 3 May 2019).
- Attarwala, H. TGN1412: From Discovery to Disaster. J. Young Pharm. 2010, 2, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.J.; Zhang, H.; Anderson, G.; Alexander, G.C. Estimated Costs of Pivotal Trials for Novel Therapeutic Agents Approved by the US Food and Drug Administration, 2015–2016. JAMA Intern. Med. 2018, 178, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Grootendorst, P.; Lexchin, J.; Cunningham, C.; Greyson, D. The cost of drug development: A systematic review. Health Policy 2011, 100, 4–17. [Google Scholar] [CrossRef]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar]
- Prasad, V.; Mailankody, S. Research and Development Spending to Bring a Single Cancer Drug to Market and Revenues After Approval. JAMA Intern. Med. 2017, 177, 1569–1575. [Google Scholar] [CrossRef]
- Tulum, O.; Lazonick, W. Financialized corporations in a national innovation system: The US pharmaceutical industry. Int. J. Political Econ. 2018, 47, 281–316. [Google Scholar] [CrossRef]
- Schwartz, L.M.; Woloshin, S. Medical Marketing in the United States, 1997–2016. JAMA 2019, 321, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.-A.; Lexchin, J. The Cost of Pushing Pills: A New Estimate of Pharmaceutical Promotion Expenditures in the United States. PLoS Med. 2008, 5, e1. [Google Scholar] [CrossRef] [PubMed]
- The R&D Smokescreen: The Prioritization of Marketing & Sales in the Pharmaceutical Industry. Institute for Health and Socio-Economic Policy/National Nurses United. Available online: https://nurses.3cdn.net/e74ab9a3e937fe5646_afm6bh0u9.pdf (accessed on 3 May 2019).
- 2018 PhRMA Annual Membership Survey. Available online: https://www.phrma.org/report/2018-phrma-annual-membership-survey2018 (accessed on 3 May 2019).
- Lexchin, J. Pharmaceutical company spending on research and development and promotion in Canada, 2013–2016: A cohort analysis. J. Pharm. Policy Pract. 2018, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Enzyme Market Type, Source, Reaction Type, and Application-Global Opportunity Analysis and Industry Forecast, 2017–2024. Available online: https://www.researchandmarkets.com/reports/4580579/enzymes-market-type-source-reaction-type-and#relb0–4520168 (accessed on 21 May 2019).
- Helland, E.; Lakdawalla, D.; Malani, A.; Seabury, S. Unintended Consequences of Product Liability: Evidence from the Pharmaceutical Market. National Bureau of Economic Research. Available online: http://www.nber.org/papers/w20005 (accessed on 21 May 2019).
| Pharmaceutical Protein | |||
| Product | Cell Line | Application | Retail Price per Kg |
| Rituximab | Hamster | Lymphoma | $9,500,000.00 |
| Eculizumab | Murine Myeloma | PNH | $23,000,000.00 |
| rHGH | E. coli | GH deficiency | $137,000,000.00 |
| rFVIIa | Hamster | Hemophilia with Inhibitor | $2,070,000,000.00 |
| rHepatitis B Surface Antigen | S. cerevisiae | vaccine | $5,400,000,000.00 |
| rFVIII | Hamster | Hemophilia | $9,600,000,000.00 |
| Industrial Protein | |||
| Product | Cell Line | Application | Retail Price per Kg |
| Cellulase | T. reesei | Fuel Ethanol | $10.00 |
| rβ-Glucosidase | E. coli | Fuel Ethanol | $37.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puetz, J.; Wurm, F.M. Recombinant Proteins for Industrial versus Pharmaceutical Purposes: A Review of Process and Pricing. Processes 2019, 7, 476. https://doi.org/10.3390/pr7080476
Puetz J, Wurm FM. Recombinant Proteins for Industrial versus Pharmaceutical Purposes: A Review of Process and Pricing. Processes. 2019; 7(8):476. https://doi.org/10.3390/pr7080476
Chicago/Turabian StylePuetz, John, and Florian M. Wurm. 2019. "Recombinant Proteins for Industrial versus Pharmaceutical Purposes: A Review of Process and Pricing" Processes 7, no. 8: 476. https://doi.org/10.3390/pr7080476
APA StylePuetz, J., & Wurm, F. M. (2019). Recombinant Proteins for Industrial versus Pharmaceutical Purposes: A Review of Process and Pricing. Processes, 7(8), 476. https://doi.org/10.3390/pr7080476






