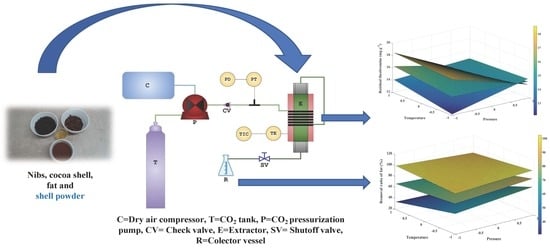
Supercritical Fluid Extraction of Fat and Caffeine with Theobromine Retention in the Cocoa Shell
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Extraction with Supercritical CO2
2.3. Statistical Analysis
2.4. Determination of Fat Using the Soxhlet Method
2.5. Calculation of the Residual Ratio and the Removal Ratio for Each Variable
2.6. Global Yield
2.7. Determination of Methylxanthines Using Visible Ultraviolet Spectroscopy
2.8. Characterization Using Fourier Transform Infrared Spectroscopy (FT–IR)
3. Results and Discussion
3.1. Fourier-Transform Infrared Spectroscopy (FT–IR)
3.2. Supercritical Fluid Extraction of Cocoa Shell Powder
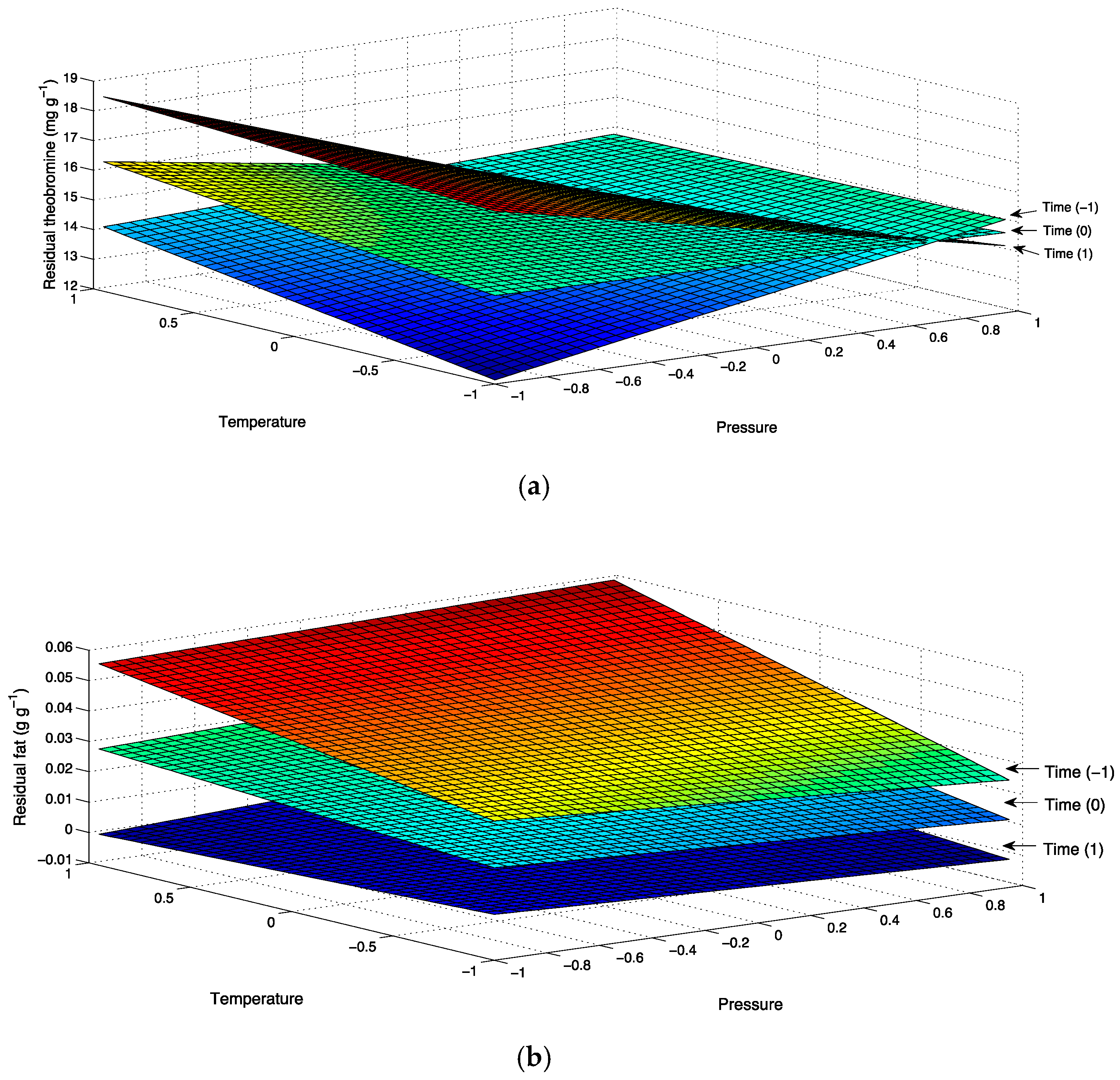
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwan, R.F.; Rose, A.H.; Board, R.G. Microbial fermentation of Cocoa Beans, with Emphasis on Enzimatic Degradation of the Pulp. J. Appl. Bacteriol. Symp. Suppl. 1995, 79, 96s–107s. [Google Scholar]
- Wood, G.A.R. Quality and Inspection. In Cocoa, 4th ed.; Wood, G.A.R., Lass, R.A., Eds.; Blackwell Science: Oxford, UK, 2001; pp. 519–520. [Google Scholar]
- Eteng, M.U.; Ettarh, R.R. Comparative effects of theobromine and cocoa extract on lipid profile in rats. Nutr. Res. 2010, 20, 1513–1517. [Google Scholar] [CrossRef]
- Sangronis, E.; Soto, M.J.; Valero, Y.; Buscema, I. Cascarilla de cacao venezolano como materia prima de infusiones. Archivos Latinoamericanos de Nutrición 2014, 64, 123–130. [Google Scholar] [PubMed]
- ICCO (2017). Production of Cocoa Beans. Available online: https://www.icco.org/statistics/production-and-grindings/production.html (accessed on 5 June 2018).
- Okiyama, D.C.G.; Navarro, S.L.B.; Rodrígues, C.E.C. Cocoa shell and its compounds: Applications in the food industry. Trends Food Sci. Technol. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- García-Alamilla, P.; Lagunes-Gálvez, L.M.; Barajas-Fernández, J.; García-Alamilla, R. Physicochemical changes of cocoa beans during roasting process. J. Food Qual. 2017, 2017. [Google Scholar] [CrossRef]
- Saltini, R.; Akkerman, R.; Frosch, S. Optimizing chocolate production through traceability: A review of the influence of farming practices on cocoa bean quality. Food Control 2013, 29, 167–187. [Google Scholar] [CrossRef]
- Pickenhagen, W.; Dietrich, P.; Keil, B.; Polonsky, J.; Nouaille, F.; Lederer, E. Identification of the bitter principle of cocoa. Helv. Chim. Acta 1975, 58, 1078–1086. [Google Scholar] [CrossRef]
- Moratalla, R. Neurobiología de las metilxántinas. Trastor Adict. 2008, 10, 201–207. [Google Scholar] [CrossRef]
- Arnaud, M.J. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. In Methylxanthines, 1st ed.; Fredholm, B.B., Ed.; Handbook of experimental pharmacology 200; Springer: Berlin/Heidelberg, Germany, 2011; p. 203. [Google Scholar]
- Grases, F.; Rodríguez, A.; Costa-Bauza, A. Theobromine inhibits uric acid crystallization. A potential application in the treatment of uric acid nephrolithiasis. PLoS ONE. 2014, 9, e111184. [Google Scholar] [CrossRef]
- Finney-Brown, T. Reviews of articles on medicinal herbs. Aust. J. Med. Herb. 2011, 23, 146–149. [Google Scholar]
- Neufingerl, N.; Zebregs, Y.E.M.P.; Schuring, E.A.H.; Trautwein, E.A. Effect of cocoa and theobromine consumption on serum HDL-cholesterol concentrations: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Baggott, M.J.; Childs, E.; Hart, A.B.; de Bruin, E.; Palmer, A.A.; Wilkinson, J.E.; de Wit, H. Psychopharmacology of theobromine in healthy volunteers. Psychopharmacology 2013, 228, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Sansone, R.; Ottaviani, J.I.; Rodriguez-Mateos, A.; Heinen, Y.; Noske, D.; Spencer, J.P.; Crozier, A.; Merx, M.W.; Kelm, M.; Schroeter, H.; et al. Methylxantines enhance the effects of cocoa flavanols on cardiovascular function: Randomized, double-masked controlled studies. Am. J. Clin. Nutr. 2017, 105, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barbero, G.; Pinedo, C.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; García-Barroso, C. Optimization of ultrasound-assisted extraction of bioactive compounds from jabuticaba (Myrciaria cauliflora) fruit through a Box-Behnken experimental design. Food Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Chan, C.-H.; See, T.-Y.; Yusoff, R.; Ngoh, G.-C.; Kow, K.-W. Extraction of bioactives from Orthosiphon stamineus using microwave and ultrasound-assisted techniques: Process optimization and scale up. Food Chem. 2017, 221, 1382–1387. [Google Scholar] [CrossRef]
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.; Cravotto, G.; Binello, A.; Lorenzo, J.M.; Shpigelman, A. Innovative “Green” and Novel Strategies for the Extraction of Bioactive Added Value Compounds from Citrus Wastes—A Review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef]
- Hauthal, W.H. Advances with supercritical fluids. Chemosphere 2001, 43, 123–135. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef]
- Knez, Ž.; Markočič, E.; Leitgeb, M.; Primožič, M.; Hrnčič, M.K.; Škerget, M. Industrial applications of supercritical fluids: A review. Energy 2014, 77, 235–243. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Pardo-Castaño, C.; Velásquez, M.; Bolaños, G. Simple models for supercritical extraction of natural matter. J. Supercrit. Fluids 2015, 97, 165–173. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.P.; Mendiola, J.A.; Ibáñez, E.; Herrero, M. Supercritical fluid extraction. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Reedijk, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–17. [Google Scholar]
- Valadez-Carmona, L.; Ortiz-Moreno, A.; Ceballos-Reyes, G.; Mendiola, J.A.; Ibáñez, E. Valorization of cacao pod husk through supercritical fluid extraction of phenolic compounds. J. Supercrit. Fluids 2018, 131, 99–105. [Google Scholar] [CrossRef]
- Asep, E.K.; Jinap, S.; Tan, T.J.; Rahman, R.A.; Harcharan, S.; Hamid, N. The effects of particle size, fermentation and roasting of cocoa nibs on supercritical fluid extraction of cocoa butter. J. Food Eng. 2008, 85, 450–458. [Google Scholar] [CrossRef]
- Sharif, K.M.; Rahman, M.M.; Azmir, J.; Mohamed, A.; Jahurul, M.H.A.; Sahena, F.; Zaidul, I.S.M. Experimental design of supercritical fluid extraction—A review. J. Food Eng. 2014, 105–116. [Google Scholar] [CrossRef]
- Fernandez-Trujillo, J.P. Supercritical CO2 extraction of sweet and hot paprika. Grasas y Aceites 2008, 59, 7–15. [Google Scholar] [CrossRef]
- Phan, T.; Bruner, G. Extraction of oil and minor compounds from oil palm fruit with supercritical carbon dioxide. Processes 2019, 7, 107. [Google Scholar] [CrossRef]
- Merabet, S.; Robert, D.; Weber, J.V.; Bouhelassa, M.; Benkhanouche, S. Photocatalytic degradation of indole in UV/TiO2: Optimization and modelling using the response surface methodology (RSM). Environ. Chem. Lett. 2009, 7, 45–49. [Google Scholar] [CrossRef]
- Tantriratna, P.; Wirojanagud, W.; Neramittagapong, S.; Wantala, K.; Grisdanurak, N. Optimization for UV-phothocatalytic degradation of paraquat over titanium dioxide supported or rice husk silica using Box-Behnken design. Indian J. Chem. Technol. 2011, 18, 363–371. [Google Scholar]
- Singh, K.P.; Singh, A.K.; Singh, U.V.; Verma, P. Optimizing removal of ibuprofen from water by magnetic nanocomposite using Box-Behnken design. Environ. Sci. Pollut. Res. 2012, 19, 724–738. [Google Scholar] [CrossRef]
- Carrara, V.S.; Filho, L.C.; García, V.A.S.; Faioes, V.S.; Cunha-Júnior, E.F.; Torres-Santos, E.C.; Cortez, D.A.G. Supercritical fluid extraction of Pyrrolidine alkaloid form leaves of Piper amalago L. J. Evid Based Complementary Alternat. Med. 2017, 2017. [Google Scholar] [CrossRef]
- Xu, X.; Gao, Y.; Liu, G.; Wang, Q.; Zhao, J. Optimization of supercritical carbon dioxide extraction of sea buckthorn (Hippophae thamnoides L.) oil using response surface methodology. LWT Food Sci. Technol. 2008, 41, 1223–1231. [Google Scholar] [CrossRef]
- Montgomery, D.C. Response surface methods and designs. In Design and Analysis of Experiments; Wiley: New York, NY, USA, 2001; p. 455. [Google Scholar]
- Edzuan, A.M.F.; Majid, N.A.A.; Bong, H.L. Physical and Chemical Property Changes of Coffee Beans during Roasting. Am. J. Chem. 2015, 5, 56–60. [Google Scholar] [CrossRef]
- Gallignani, M.; Torres, M.; Ayala, C.; Brunetto, M. Determinación de cafeína en café mediante espectrometría infrarroja de transformada de Fourier. Rev. Tec. Fac. Ing. Uni. Zulia. 2008, 31, 159–168. [Google Scholar]
- NMX-F-615-NORMEX-2004. Alimentos-determinación de extracto etéreo (método soxhlet) en alimentos-método de prueba (cancels NMX-F-089-S-1978). Mexico City, Federal District. 2004. Available online: https://www.colpos.mx/bancodenormas/nmexicanas/NMX-F-089-S-1978.PDF (accessed on 10 May 2019).
- Kobori, K.; Maruta, Y.; Mineo, S.; Shigematsu, T.; Hirayama, M. Polyphenol-retaining decaffeinated cocoa powder obtained by supercritical carbon dioxide extraction and its antioxidant activity. Foods 2013, 2, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Jiménez, L.; Canízares-Macías, M.P. Ultrasound-assisted method for extraction of theobromine and caffeine from cacao seeds and chocolate products. Food Bioproc. Tech. 2013, 6, 3522–3529. [Google Scholar] [CrossRef]
- Cardoso, N.F.; Lima, E.C.; Pinto, I.S.; Amavisca, C.V.; Royer, B.; Pinto, R.B.; Pereira, S.F.P. Application of cupuassu shell as biosorbent for the removal of textile dyes from aqueous solution. J. Environ. Manag. 2011, 92, 1237–1247. [Google Scholar] [CrossRef]
- Coates, J.P.; Shelley, P.H. Infrared Spectroscopy in Process Analysis. Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Marsiglia, D.E.; Ojeda, K.A.; Ramírez, M.C.; Sánchez, E. Pectin extraction from cocoa pod husk (Theobroma cacao L.) by hydrolysis with citric and acetic acid. Int. J. Chemtech. Res. 2016, 9, 497–507. [Google Scholar]
- Li, S.; Hartland, S. Influence of co-solvents on solubility and selectivity in extraction of xanthines and cocoa butter from cocoa beans with supercritical CO2. J. Supercrit. Fluids 1992, 5, 7–12. [Google Scholar] [CrossRef]
- Kopcak, U.; Mohamed, R.S. Caffeine solubility in supercritical carbon dioxide/co-solvent mixtures. J. Supercrit. Fluids 2005, 34, 209–214. [Google Scholar] [CrossRef]
- Cornelio-Santiago, H.P.; Gonçalvez, C.B.; de Oliveria, N.A.; de Oliveira, A.L. Supercritical CO2 extraction of oil from green coffee beans: Solubility, triacylglycerol composition, thermophysical properties and thermodynamic modelling. J. Supercrit. Fluids 2017, 128, 386–394. [Google Scholar] [CrossRef]
- Noor-Soffalina, S.S.; Jinap, S.; Nazamid, S.; Nazimah, S.A.H. Effect of polyphenol and pH on cocoa Maillard related flavor precursors in a lipidic model system. Int. J. Food Sci. Technol. 2009, 44, 168–180. [Google Scholar] [CrossRef]
- Asep, E.K.; Jinap, S.; Russly, A.R.; Jahurul, M.H.A.; Ghafoor, K.; Zaidul, I.S.M. The effect of flow rate at different pressures and temperatures on cocoa butter extracted from cocoa nib using supercritical carbon dioxide. J. Food Sci. Technol. 2016, 53, 2287–2297. [Google Scholar] [CrossRef] [PubMed][Green Version]


| Natural Variables | Concentration in the Residue | Residual Ratio | Removal Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treat | P (psi) | T (K) | Time (min) | Rg | Th (mg·g −¹) | Caf (mg·g −¹) | Fat (g·g −¹) | Th (%) | Caf (%) | Fat (%) |
| 1 | 2000 | 333 | 90 | 3.66 | 17.593 | 0.563 | 0.011 | 106.89 | 32.67 | 86.99 |
| 2 | 2000 | 313 | 30 | 11.65 | 14.810 | 0.000 | 0.025 | 82.53 | 100.00 | 72.50 |
| 3 | 2000 | 333 | 30 | 8.61 | 12.113 | 0.000 | 0.009 | 69.81 | 100.00 | 89.60 |
| 4 | 2000 | 313 | 90 | 5.20 | 11.158 | 0.438 | 0.047 | 66.71 | 48.48 | 43.95 |
| 5 | 6000 | 333 | 90 | 8.11 | 13.189 | 0.280 | 0.010 | 76.43 | 68.10 | 88.97 |
| 6 | 6000 | 333 | 30 | 7.45 | 13.922 | 0.000 | 0.072 | 81.26 | 100.00 | 16.24 |
| 7 | 6000 | 313 | 90 | 6.31 | 17.415 | 0.071 | 0.005 | 102.90 | 91.80 | 94.73 |
| 8 | 6000 | 313 | 30 | 7.89 | 13.813 | 0.107 | 0.057 | 80.24 | 87.82 | 34.14 |
| 9 | 4000 | 323 | 60 | 15.30 | 15.778 | 0.113 | 0.043 | 84.28 | 88.17 | 54.26 |
| 10 | 4000 | 323 | 60 | 11.45 | 15.309 | 0.047 | 0.011 | 85.50 | 94.82 | 88.05 |
| 11 | 4000 | 323 | 60 | 6.68 | 16.557 | 0.000 | 0.006 | 97.45 | 100.00 | 92.53 |
| 12 | 4000 | 323 | 60 | 7.20 | 15.793 | 0.122 | 0.009 | 92.43 | 86.00 | 90.02 |
| 13 | 4000 | 323 | 60 | 10.99 | 16.885 | 0.170 | 0.009 | 94.79 | 81.26 | 90.32 |
| Sum of squares | Source | Regression | Linear | Non-Linear | Residual | Lack of Fit | Pure Error | Total | R² |
|---|---|---|---|---|---|---|---|---|---|
| Residue | Th | 36.40 | 9.96 * | 26.43 | 12.40 | 10.76 * | 1.64 | 48.81 | 0.75 |
| Caf | 0.185 | 0.119 | 0.065 | 0.178 | 0.170 * | 0.008 | 0.364 | 0.51 | |
| Fat | 0.004 | 0.0039 * | 0.000 | 0.002 | 0.001 | 0.001 | 0.006 | 0.71 | |
| Residual ratio | Th | 1457.10 * | 540.92 | 916.14 * | 310.52 | 177.23 | 133.29 | 1767.6 | 0.82 |
| Removal ratio | Caf | 5994.70 | 5355.00 * | 639.66 | 2325.30 | 1280.00 | 1045.30 | 8320.0 | 0.72 |
| Fat | 2635.80 | 1697.50 | 938.27 | 2746.50 | 2527.6 * | 218.92 | 5382.3 | 0.49 |
| Coefficients | β0 | β1 | β2 | β3 | β12 | β13 | β23 | |
|---|---|---|---|---|---|---|---|---|
| Residue | Theobromine | 14.949 | −0.743 | 0.108 | 0.825 | −0.599 | −1.683 * | −0.333 |
| Caffeine | 0.152 | −0.112 | −0.014 | 0.046 | −0.055 | 0.023 | 0.067 | |
| Fat | 0.024 | −0.001 | 0.008 | −0.021 * | 0.004 | 0.001 | −0.007 | |
| Residual Ratio | Theobromine | 13.753 | 5.838 | −1.205 | −5.666 | 3.175 | 10.049 * | 1.860 |
| Removal Ratio | Caffeine | 72.486 | 0.937 | −9.147 | 24.183 * | −4.761 | −1.724 | 7.370 |
| Fat | 83.008 | 13.417 | 1.515 | −5.466 | 6.461 | −2.509 | −8.321 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Alejo, F.A.; Barajas-Fernández, J.; Olán-Acosta, M.d.l.Á.; Lagunes-Gálvez, L.M.; García-Alamilla, P. Supercritical Fluid Extraction of Fat and Caffeine with Theobromine Retention in the Cocoa Shell. Processes 2019, 7, 385. https://doi.org/10.3390/pr7060385
González-Alejo FA, Barajas-Fernández J, Olán-Acosta MdlÁ, Lagunes-Gálvez LM, García-Alamilla P. Supercritical Fluid Extraction of Fat and Caffeine with Theobromine Retention in the Cocoa Shell. Processes. 2019; 7(6):385. https://doi.org/10.3390/pr7060385
Chicago/Turabian StyleGonzález-Alejo, Fanny Adabel, Juan Barajas-Fernández, María de los Ángeles Olán-Acosta, Laura Mercedes Lagunes-Gálvez, and Pedro García-Alamilla. 2019. "Supercritical Fluid Extraction of Fat and Caffeine with Theobromine Retention in the Cocoa Shell" Processes 7, no. 6: 385. https://doi.org/10.3390/pr7060385
APA StyleGonzález-Alejo, F. A., Barajas-Fernández, J., Olán-Acosta, M. d. l. Á., Lagunes-Gálvez, L. M., & García-Alamilla, P. (2019). Supercritical Fluid Extraction of Fat and Caffeine with Theobromine Retention in the Cocoa Shell. Processes, 7(6), 385. https://doi.org/10.3390/pr7060385