Enhanced Production of Anti-PD1 Antibody in CHO Cells through Transient Co-Transfection with Anti-Apoptotic Gene Bcl-xL Combined with Rapamycin
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Plasmids
2.2. Cell Culture and Transfection
2.3. Analysis of Apoptosis
2.4. Analysis of Cell Cycle
2.5. Analysis of Protein Expression in Apoptosis and Autophagy
2.6. Production of Anti-PD1 Monoclonal Antibody
3. Results
3.1. Analysis of Cell Viability and Density
3.2. Analysis of Apoptosis
3.3. Analysis of Cell Cycle
3.4. Analysis of Protein Expression in Apoptosis and Autophagy
3.5. Analysis of Anti-PD1 Monoclonal Antibody Expression Quantity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trill, J.J.; Shatzman, A.R.; Ganguly, S. Production of monoclonal antibodies in COS and CHO cells. Curr. Opin. Biotechnol. 1995, 6, 553–560. [Google Scholar] [CrossRef]
- Zhu, J. Mammalian cell protein expression for biopharmaceutical production. Biotechnol. Adv. 2012, 30, 1158–1170. [Google Scholar] [CrossRef]
- Hacker, D.L.; De Jesus, M.; Wurm, F.M. 25 years of recombinant proteins from reactor-grown cells—Where do we go from here? Biotechnol. Adv. 2009, 27, 1023–1027. [Google Scholar] [CrossRef]
- Daramola, O.; Stevenson, J.; Dean, G.; Hatton, D.; Pettman, G.; Holmes, W.; Field, R. A high-yielding CHO transient system: Coexpression of genes encoding EBNA-1 and GS enhances transient protein expression. Biotechnol. Prog. 2014, 30, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, Y.; Hougland, M.D.; Alam, R.; Morehead, T.A.; Barnard, G.C. A high cell density transient transfection system for therapeutic protein expression based on a CHO GS-knockout cell line: Process development and product quality assessment. Biotechnol. Bioeng. 2015, 112, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Steger, K.; Brady, J.; Wang, W.; Duskin, M.; Donato, K.; Peshwa, M. CHO-S antibody titers >1 gram/liter using flow electroporation-mediated transient gene expression followed by rapid migration to high-yield stable cell lines. J. Biomol. Screen. 2015, 20, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.K.; Barkowski-Clark, S.; Altman, R.; Johnson, K.; Sun, F.; Zmuda, J.; Liu, C.Y.; Kita, A.; Schulz, R.; Neill, A.; et al. A high density CHO-S transient transfection system: Comparison of ExpiCHO and Expi293. Protein Expr. Purif. 2017, 134, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Ma, W.; Meade, C.L.; Tam, A.S.; Llewellyn, E.; Cornell, R.; Cote, K.; Scarcelli, J.J.; Marshall, J.K.; Tzvetkova, B.; et al. Transient CHO expression platform for robust antibody production and its enhanced N-glycan sialylation on therapeutic glycoproteins. Biotechnol. Prog. 2019, 35, e2724. [Google Scholar] [CrossRef]
- Danial, N.N. BCL-2 family proteins: Critical checkpoints of apoptotic cell death. Clin. Cancer Res. 2007, 13, 7254–7263. [Google Scholar] [CrossRef]
- Baek, E.; Noh, S.M.; Lee, G.M. Anti-apoptosis engineering for improved protein production from CHO cells. Methods Mol. Biol. 2017, 1603, 71–85. [Google Scholar] [PubMed]
- Lee, S.; Lee, G.M. Bcl-2 overexpression in CHO cells improves polyethylenimine-mediated gene transfection. Process Biochem. 2013, 48, 1436–1440. [Google Scholar] [CrossRef]
- Zustiak, M.P.; Jose, L.; Xie, Y.; Zhu, J.; Betenbaugh, M.J. Enhanced transient recombinant protein production in CHO cells through the co-transfection of the product gene with Bcl-xL. Biotechnol. J. 2014, 9, 1164–1174. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.; Zong, H.; Ding, K.; Yuan, Y.; Bai, J.; Zhou, Y.; Zhang, B.; Zhu, J. Enhanced production of anti-PD1 antibody in CHO cells through transient co-transfection with anti-apoptotic genes Bcl-x L and Mcl-1. Bioprocess Biosyst. Eng. 2018, 41, 633–640. [Google Scholar] [CrossRef]
- Ashford, T.P. Cytoplasmic components in hepatic cell lysosomes. J. Cell Biol. 1962, 12, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Edros, R.; Mcdonnell, S.; Al-Rubeai, M. The relationship between mTOR signalling pathway and recombinant antibody productivity in CHO cell lines. BMC Biotechnol. 2014, 14, 15. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, G.M. Rapamycin treatment inhibits CHO cell death in a serum-free suspension culture by autophagy induction. Biotechnol. Bioeng. 2012, 109, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Alsayyari, A.A.; Dalm, C.; Hageman, J.A.; Wijffels, R.H.; Martens, D.E. Transcriptome analysis of CHO cell size increase during a fed-batch process. Biotechnol. J. 2019, 14, 1800156. [Google Scholar] [CrossRef] [PubMed]
- Balcarcel, R.R.; Stephanopoulos, G. Rapamycin reduces hybridoma cell death and enhances monoclonal antibody production. Biotechnol. Bioeng. 2001, 76, 1–10. [Google Scholar] [CrossRef]
- Dadehbeigi, N.; Dickson, A.J. Chemical manipulation of the mTORC1 pathway in industrially relevant CHOK1 cells enhances production of therapeutic proteins. Biotechnol. J. 2015, 10, 1041–1050. [Google Scholar] [CrossRef]
- Ding, K.; Han, L.; Zong, H.; Chen, J.; Zhang, B.; Zhu, J. Production process reproducibility and product quality consistency of transient gene expression in HEK293 cells with anti-PD1 antibody as the model protein. Appl. Microbiol. Biotechnol. 2017, 101, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Mizushima, N.; Yamamoto, A.; Oshitani-Okamoto, S.; Ohsumi, Y.; Yoshimori, T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J. Cell Sci. 2004, 117 Pt 13, 2805–2812. [Google Scholar] [CrossRef]
- Dai, C.; Ciccotosto, G.D.; Cappai, R.; Wang, Y.; Tang, S.; Hoyer, D.; Schneider, E.K.; Velkov, T.; Xiao, X. Rapamycin confers neuroprotection against colistin-induced oxidative stress, mitochondria dysfunction, and apoptosis through the activation of autophagy and mTOR/Akt/CREB signaling pathways. ACS Chem. Neurosci. 2018, 9, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Lalaoui, N.; Lindqvist, L.M.; Sandow, J.J.; Ekert, P.G. The molecular relationships between apoptosis, autophagy and necroptosis. Semin. Cell Dev. Biol. 2015, 39, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Sever, O.N.; Demir, O.G. Autophagy: Cell death or survive mechanism. J. Oncol. Sci. 2017, 3, 37–44. [Google Scholar] [CrossRef]
- Levine, B.; Sinha, S.C.; Kroemer, G. Bcl-2 family members: Dual regulators of apoptosis and autophagy. Autophagy 2008, 4, 600–606. [Google Scholar] [CrossRef]
- Noble, C.G.; Dong, J.M.; Manser, E.; Song, H. Bcl-xL and UVRAG cause a monomer-dimer switch in Beclin1. J. Biol. Chem. 2008, 283, 26274–26282. [Google Scholar] [CrossRef]

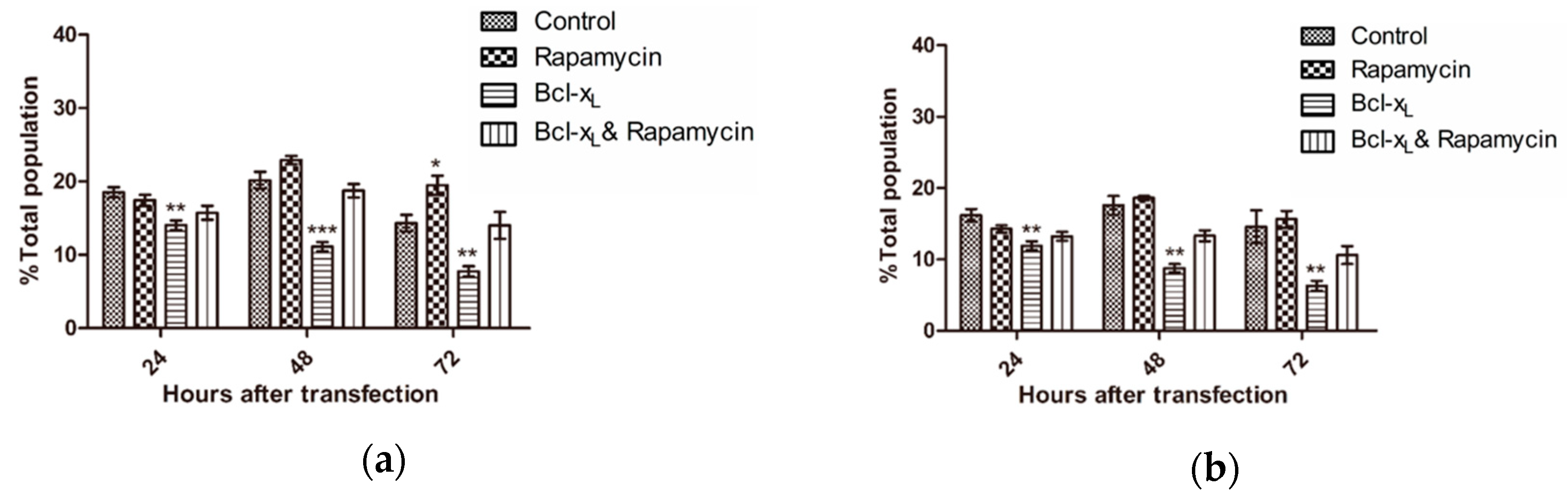
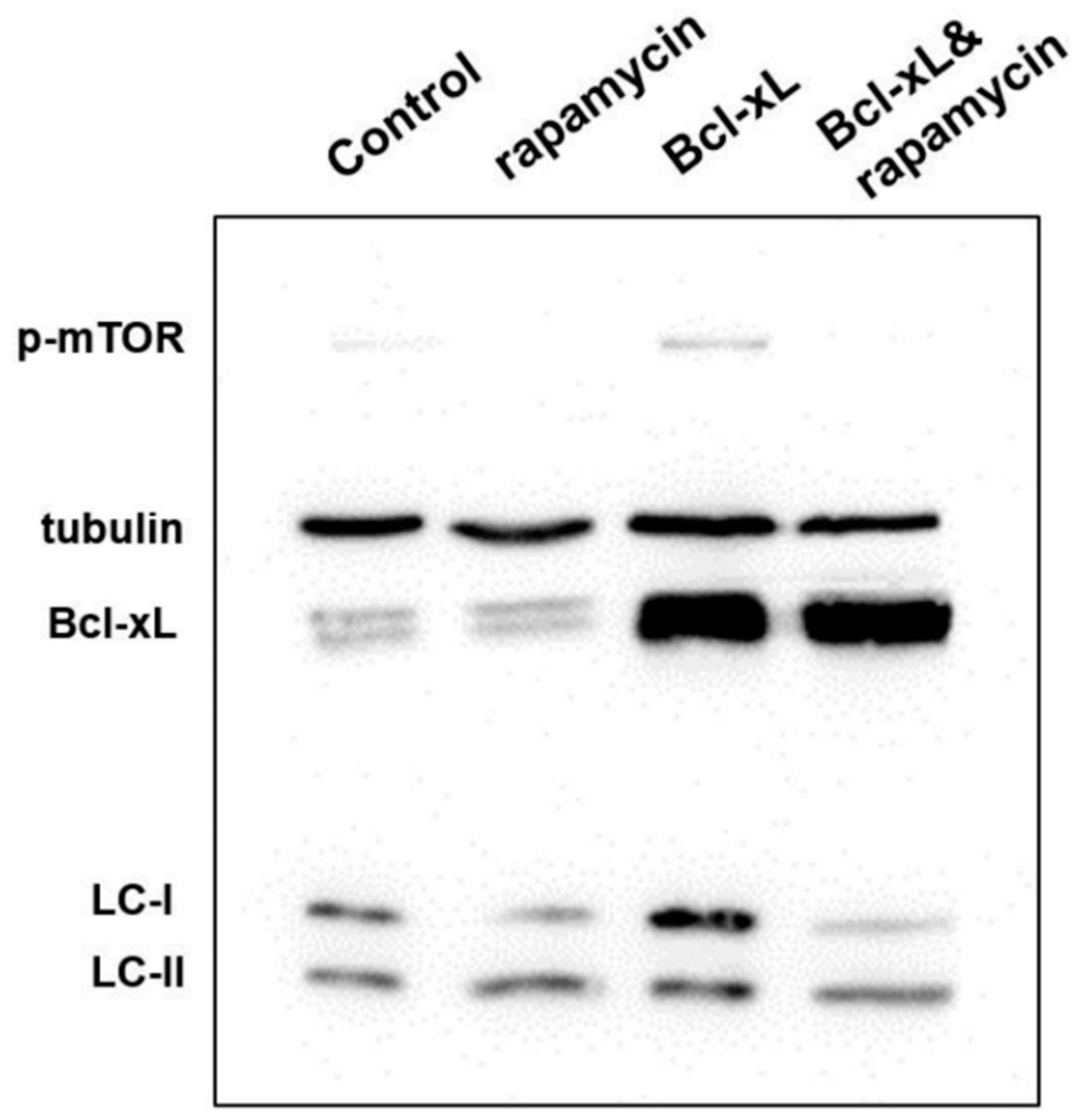
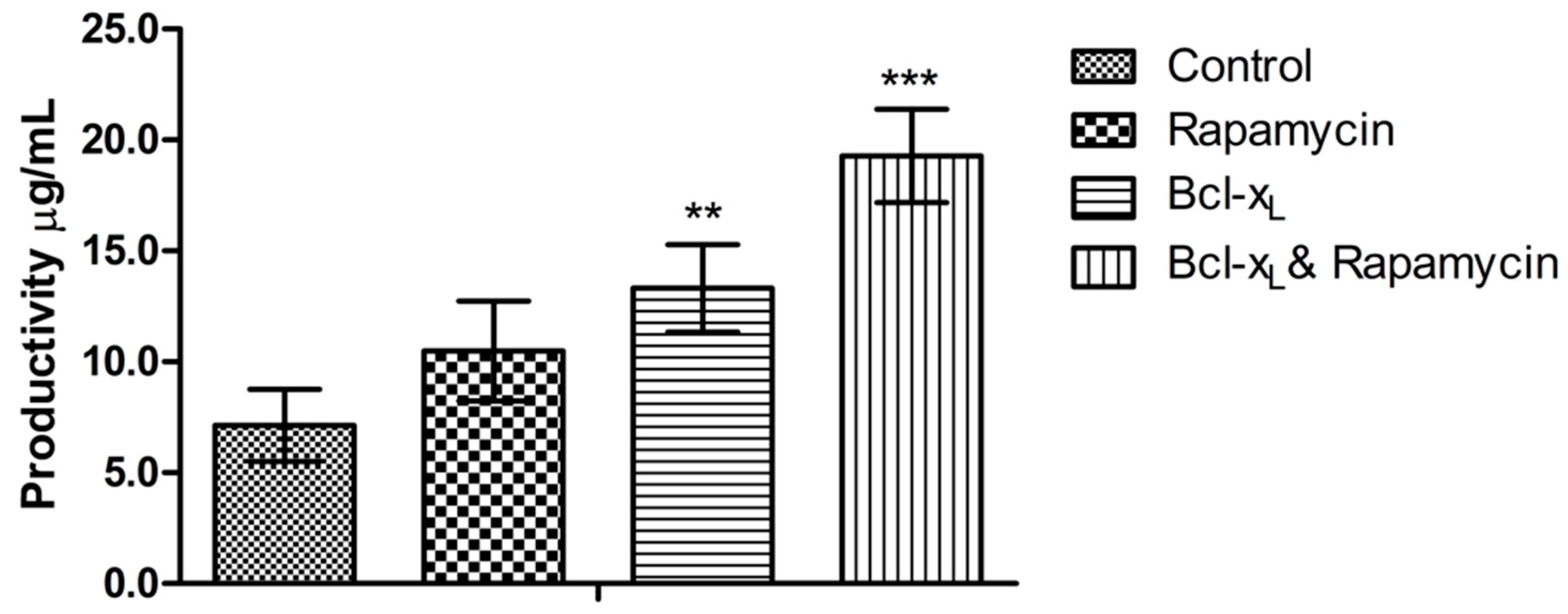
| Day | Cell Cycle | Control | Rapamycin | Bcl-xL | Bcl-xL & Rapamycin |
|---|---|---|---|---|---|
| D2 | G1 | 53.2 ± 1.0 | 61.5 ± 1.4 | 49.7 ± 0.8 | 59.6 ± 1.9 |
| S | 25.9 ± 3.1 | 17.8 ± 1.9 | 26.1 ± 2.6 | 25.3 ± 1.5 | |
| G2/M | 15.8 ± 0.8 | 16.8 ± 1.2 | 19.6 ± 1.5 | 14.5 ± 1.9 | |
| D3 | G1 | 46.6 ± 2.6 | 56.7 ± 1.0 | 46.9 ± 1.9 | 56.9 ± 2.7 |
| S | 31.3 ± 3.4 | 26.0 ± 2.6 | 28.6 ± 2.3 | 21.0 ± 3.0 | |
| G2/M | 18.2 ± 1.0 | 14.5 ± 3.0 | 22.2 ± 1.1 | 17.7 ± 1.7 | |
| D4 | G1 | 42.9 ± 1.5 | 56.4 ± 2.5 | 46.5 ± 1.2 | 53.6 ± 2.6 |
| S | 32.3 ± 2.2 | 23.0 ± 4.2 | 35.0 ± 2.9 | 27.1 ± 1.3 | |
| G2/M | 24.2 ± 1.0 | 16.2 ± 2.0 | 17.9 ± 2.0 | 17.3 ± 1.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, X.; Wang, L.; Zong, H.; Yuan, Y.; Han, L.; Li, X.; Xu, C.; Zhang, J.; Zhu, J.; et al. Enhanced Production of Anti-PD1 Antibody in CHO Cells through Transient Co-Transfection with Anti-Apoptotic Gene Bcl-xL Combined with Rapamycin. Processes 2019, 7, 329. https://doi.org/10.3390/pr7060329
Li Y, Zhang X, Wang L, Zong H, Yuan Y, Han L, Li X, Xu C, Zhang J, Zhu J, et al. Enhanced Production of Anti-PD1 Antibody in CHO Cells through Transient Co-Transfection with Anti-Apoptotic Gene Bcl-xL Combined with Rapamycin. Processes. 2019; 7(6):329. https://doi.org/10.3390/pr7060329
Chicago/Turabian StyleLi, Yunxia, Xinyu Zhang, Lei Wang, Huifang Zong, Yuan Yuan, Lei Han, Xi Li, Chenxiao Xu, Jingyi Zhang, Jianwei Zhu, and et al. 2019. "Enhanced Production of Anti-PD1 Antibody in CHO Cells through Transient Co-Transfection with Anti-Apoptotic Gene Bcl-xL Combined with Rapamycin" Processes 7, no. 6: 329. https://doi.org/10.3390/pr7060329
APA StyleLi, Y., Zhang, X., Wang, L., Zong, H., Yuan, Y., Han, L., Li, X., Xu, C., Zhang, J., Zhu, J., & Zhang, B. (2019). Enhanced Production of Anti-PD1 Antibody in CHO Cells through Transient Co-Transfection with Anti-Apoptotic Gene Bcl-xL Combined with Rapamycin. Processes, 7(6), 329. https://doi.org/10.3390/pr7060329





