Hydroxymethylation-Modified Lignin and Its Effectiveness as a Filler in Rubber Composites
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
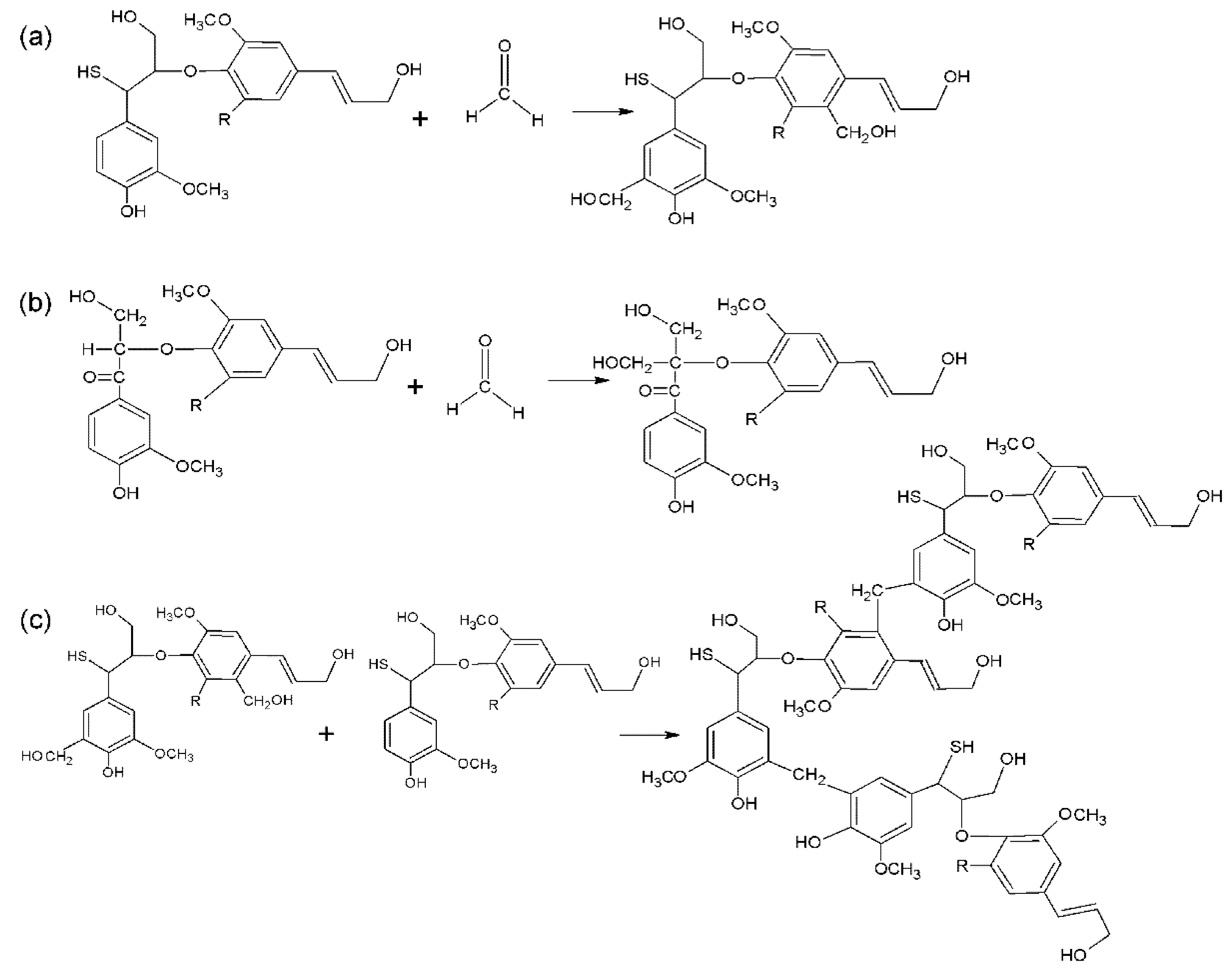
3.1. Structural Analysis
3.1.1. Fourier Transform Infrared (FTIR) Analysis
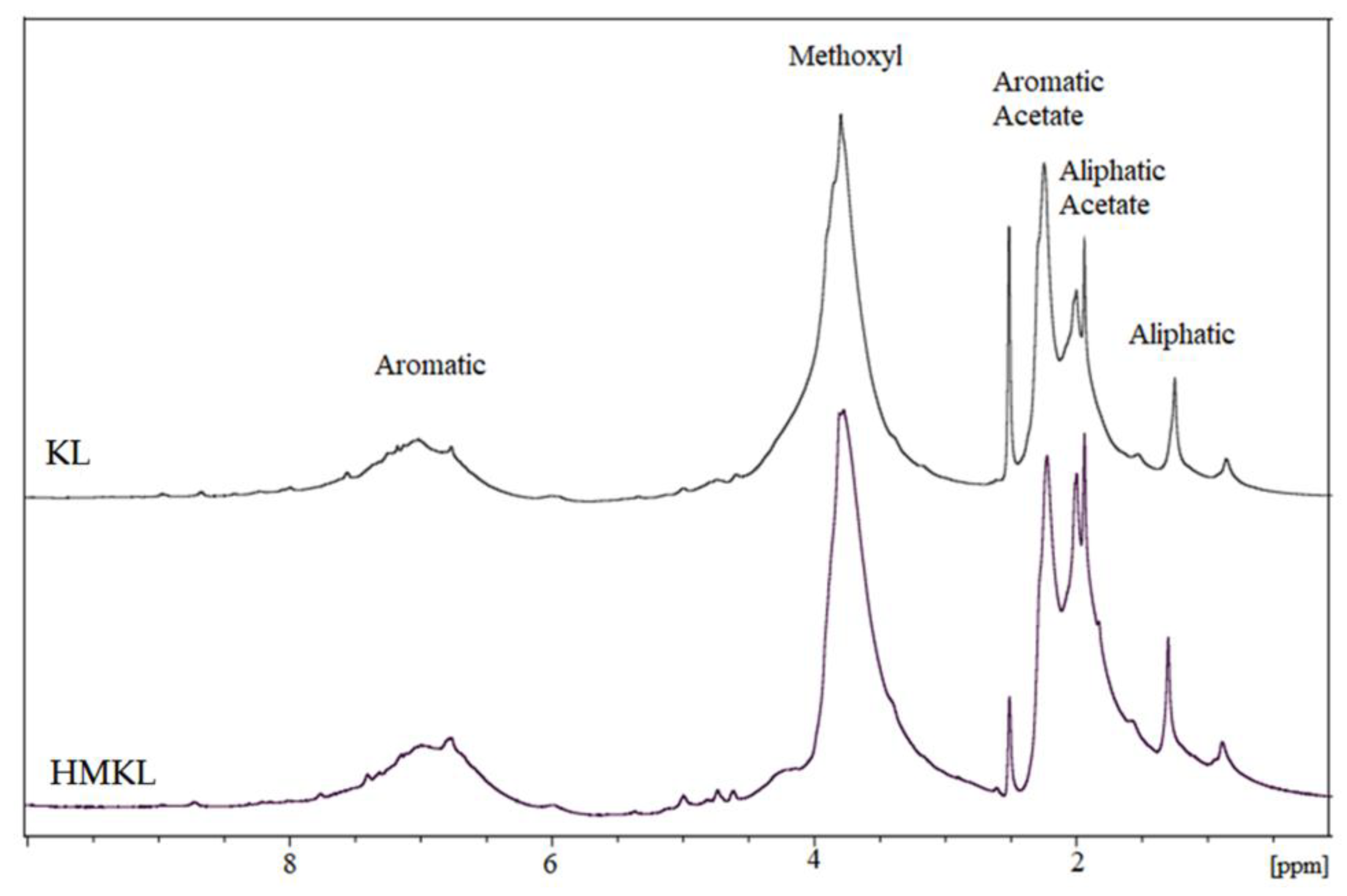
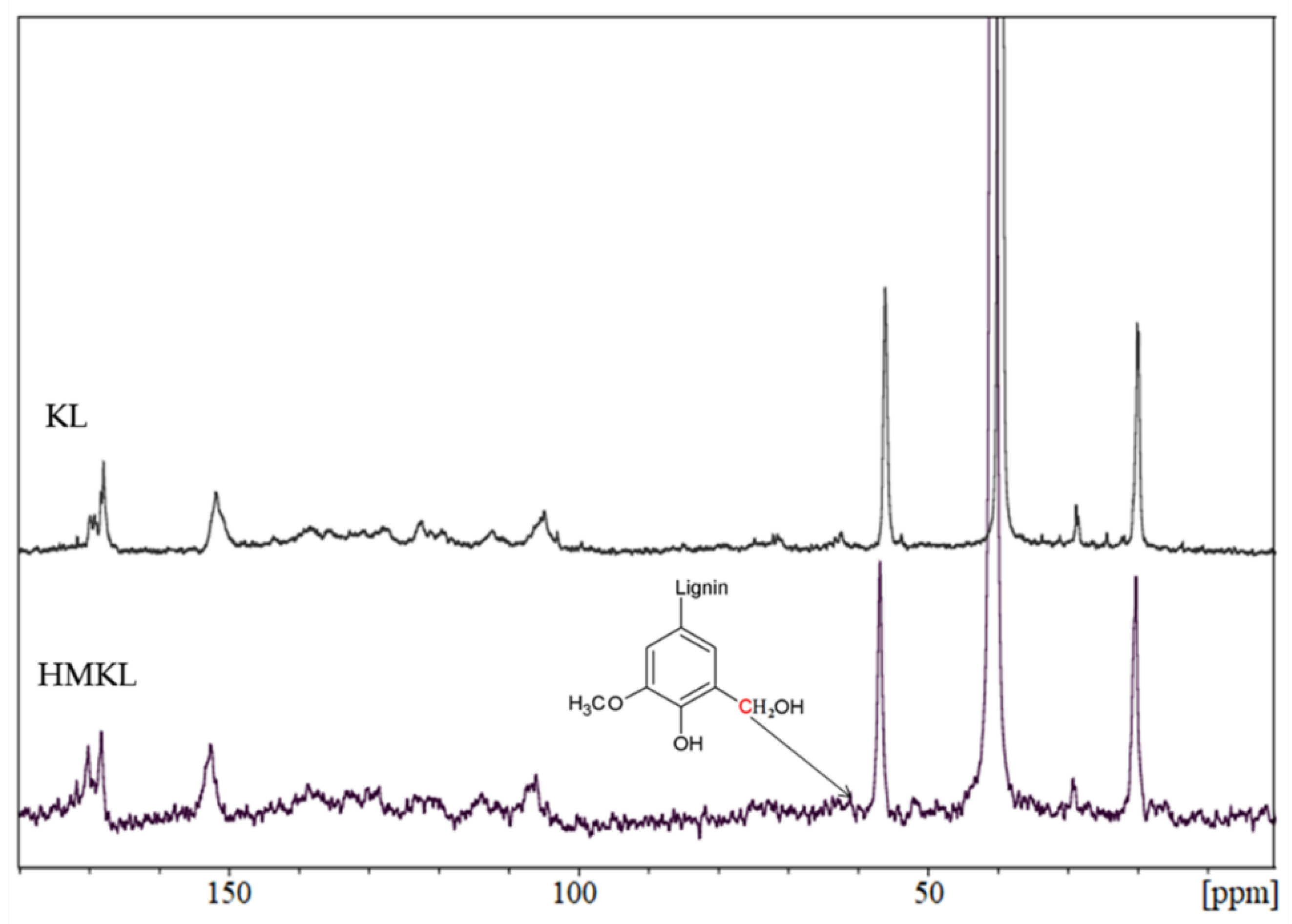
3.1.2. Nuclear Magnetic Resonance (NMR)
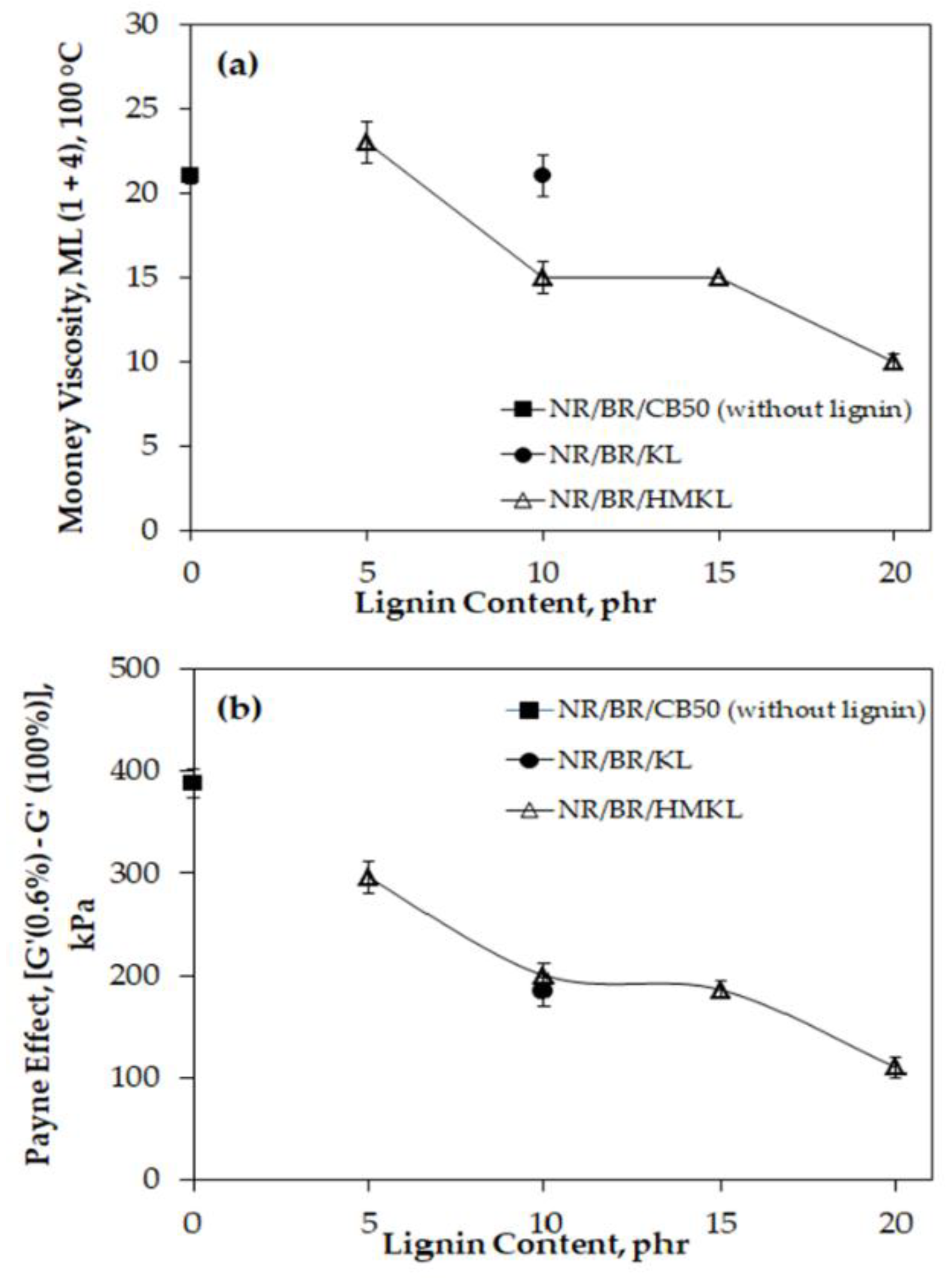
3.2. Mooney Viscosity and Payne Effect of Compounds
3.3. Curing Characteristics and Crosslink Densities
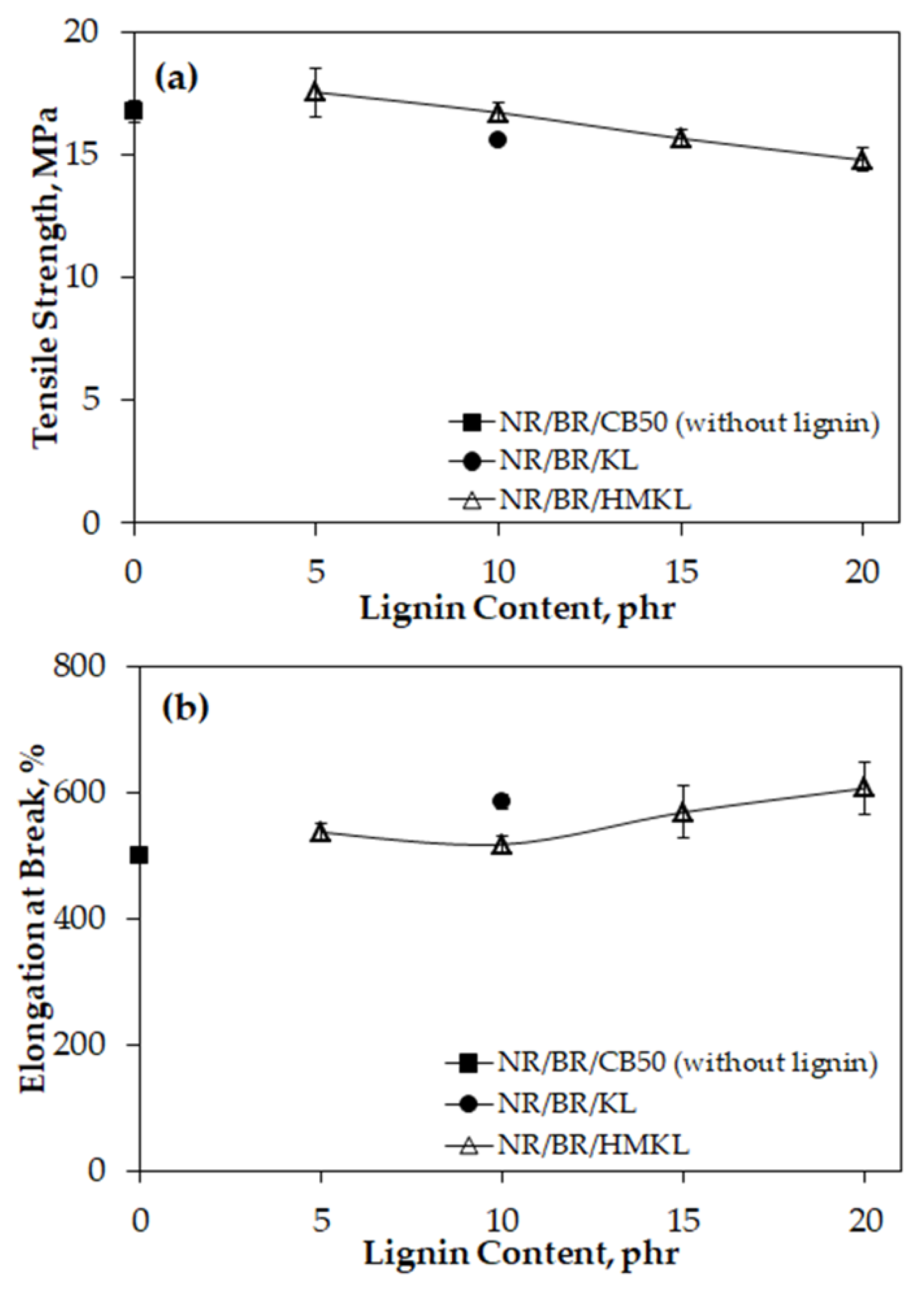
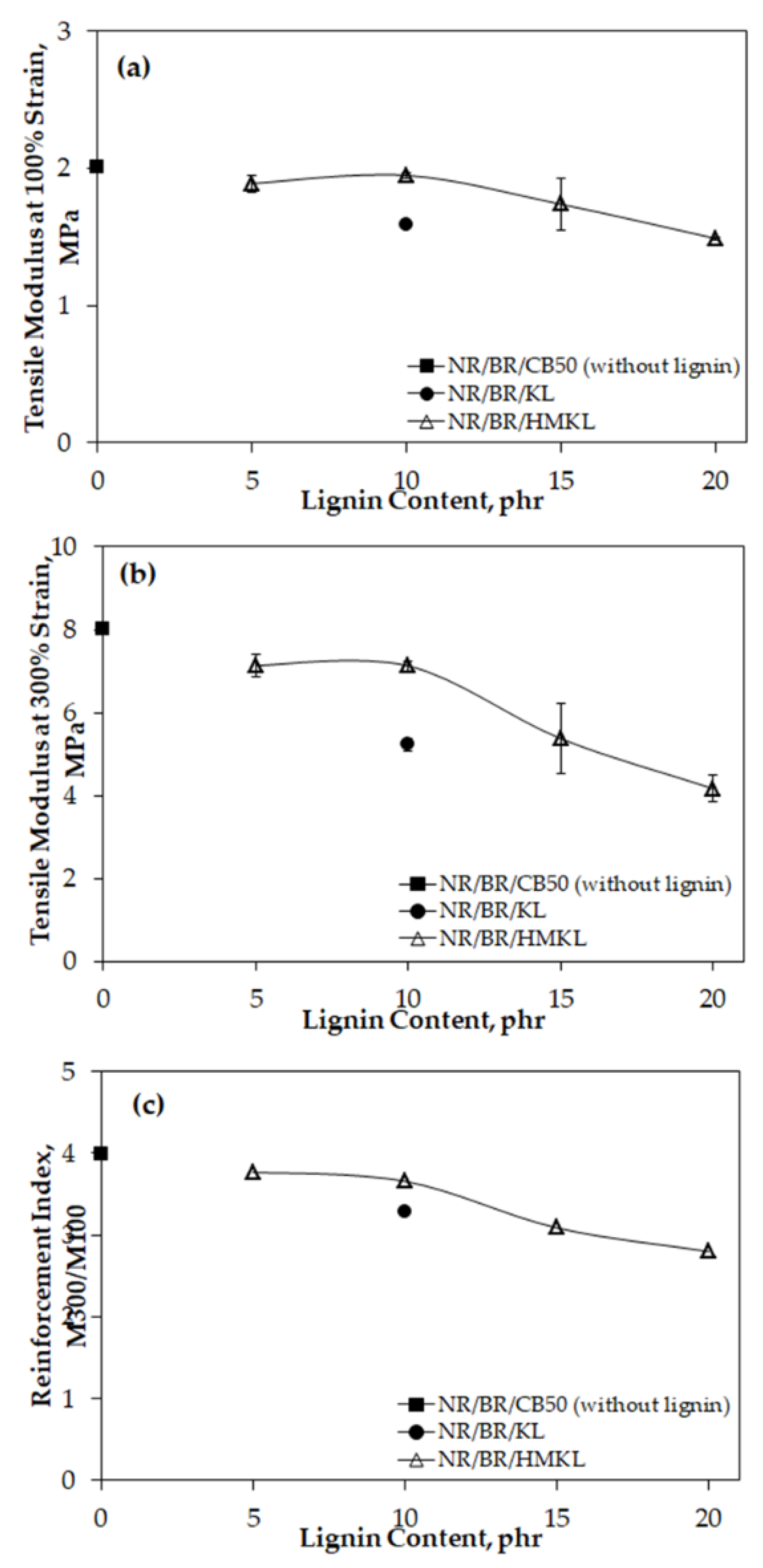
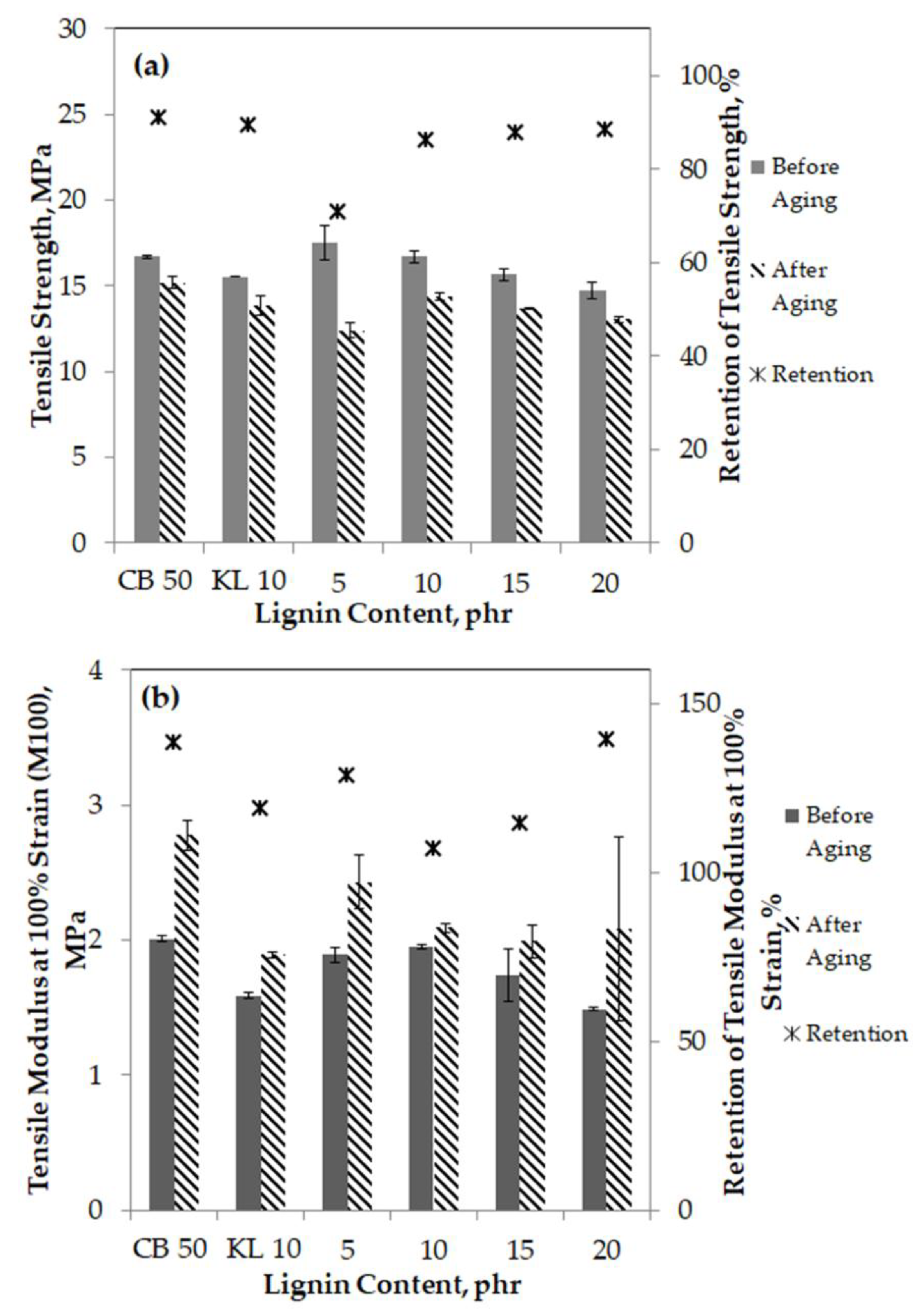
3.4. Tensile Properties
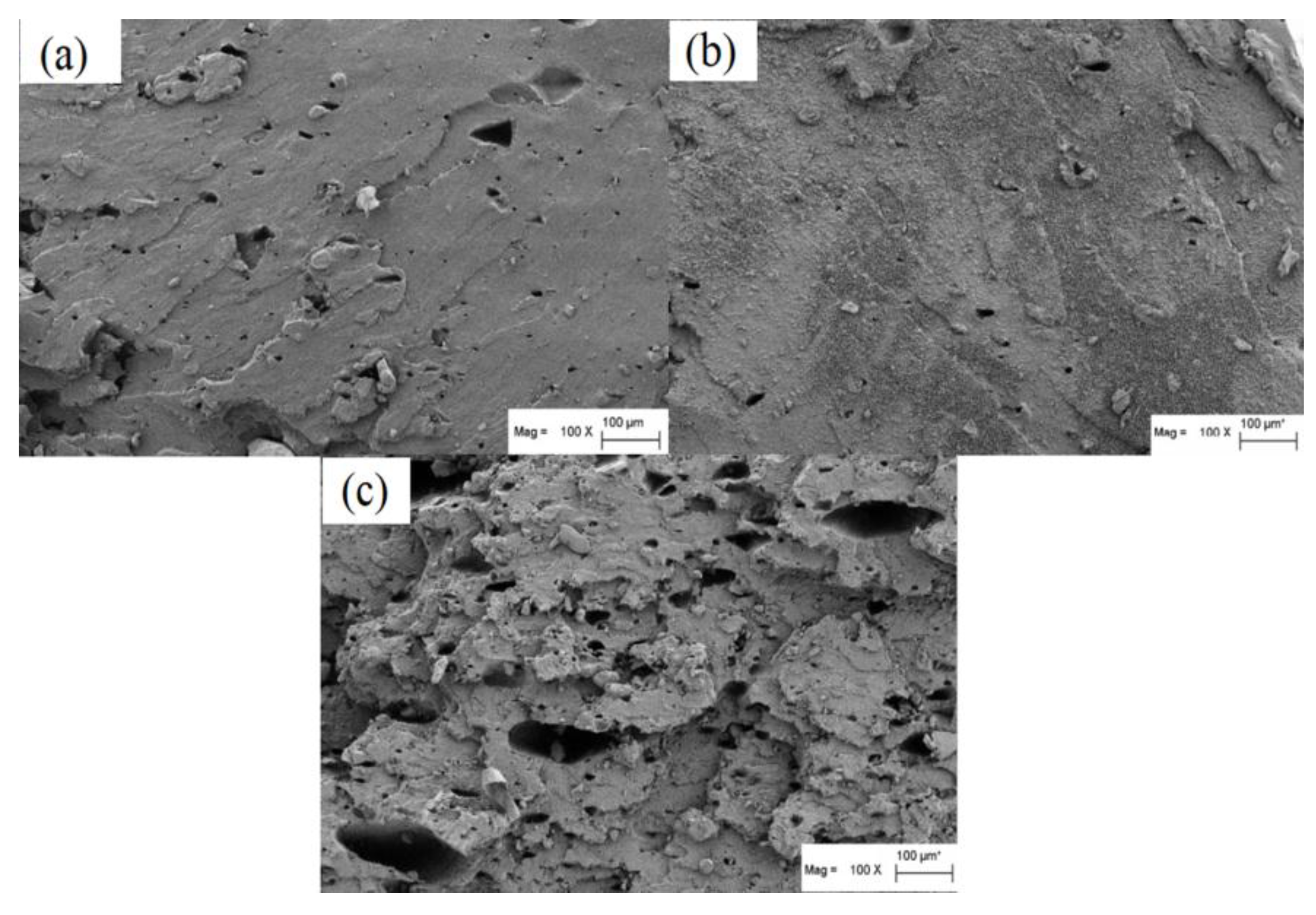
3.5. Compatibility of Lignin-Filled NR/BR
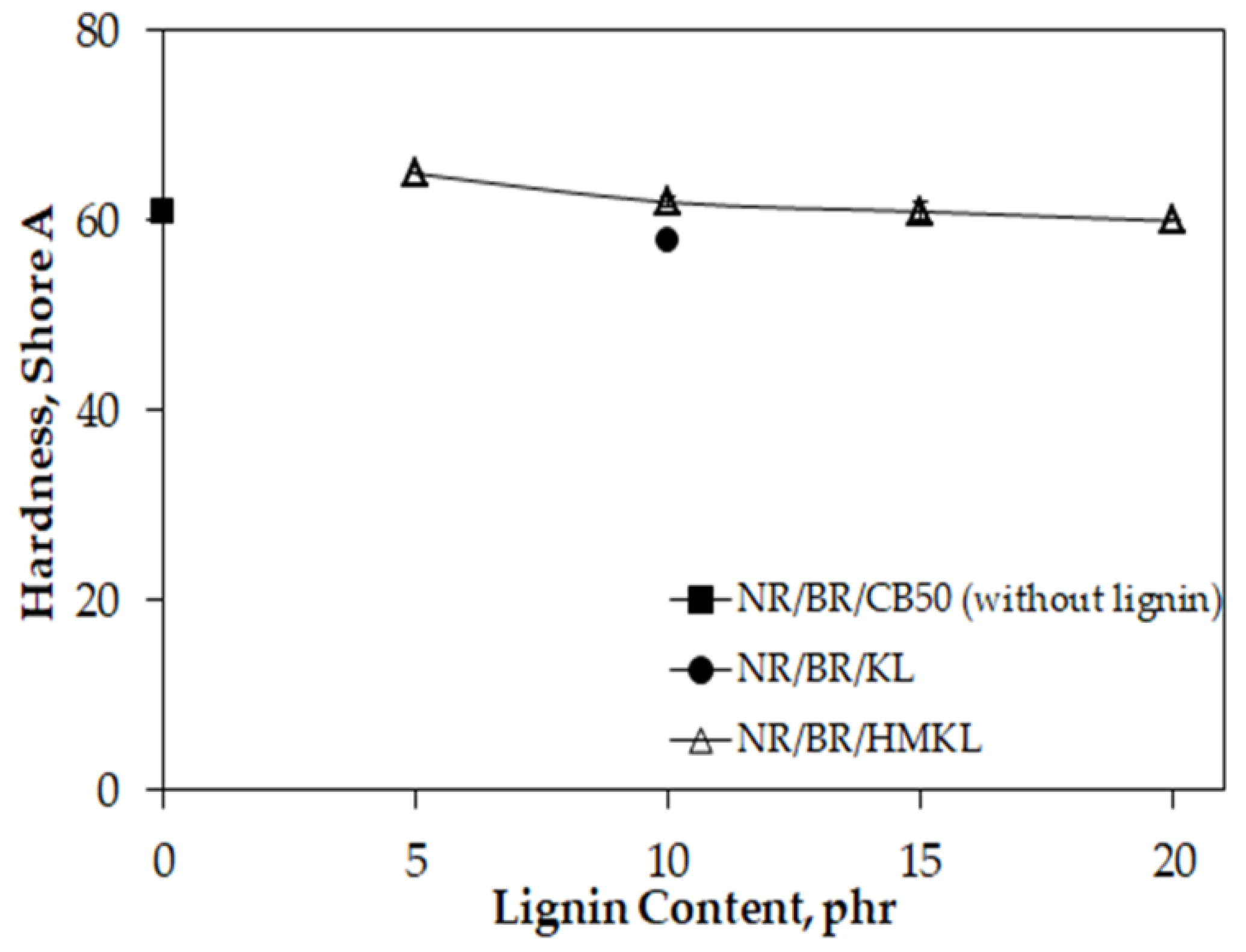
3.6. Hardness
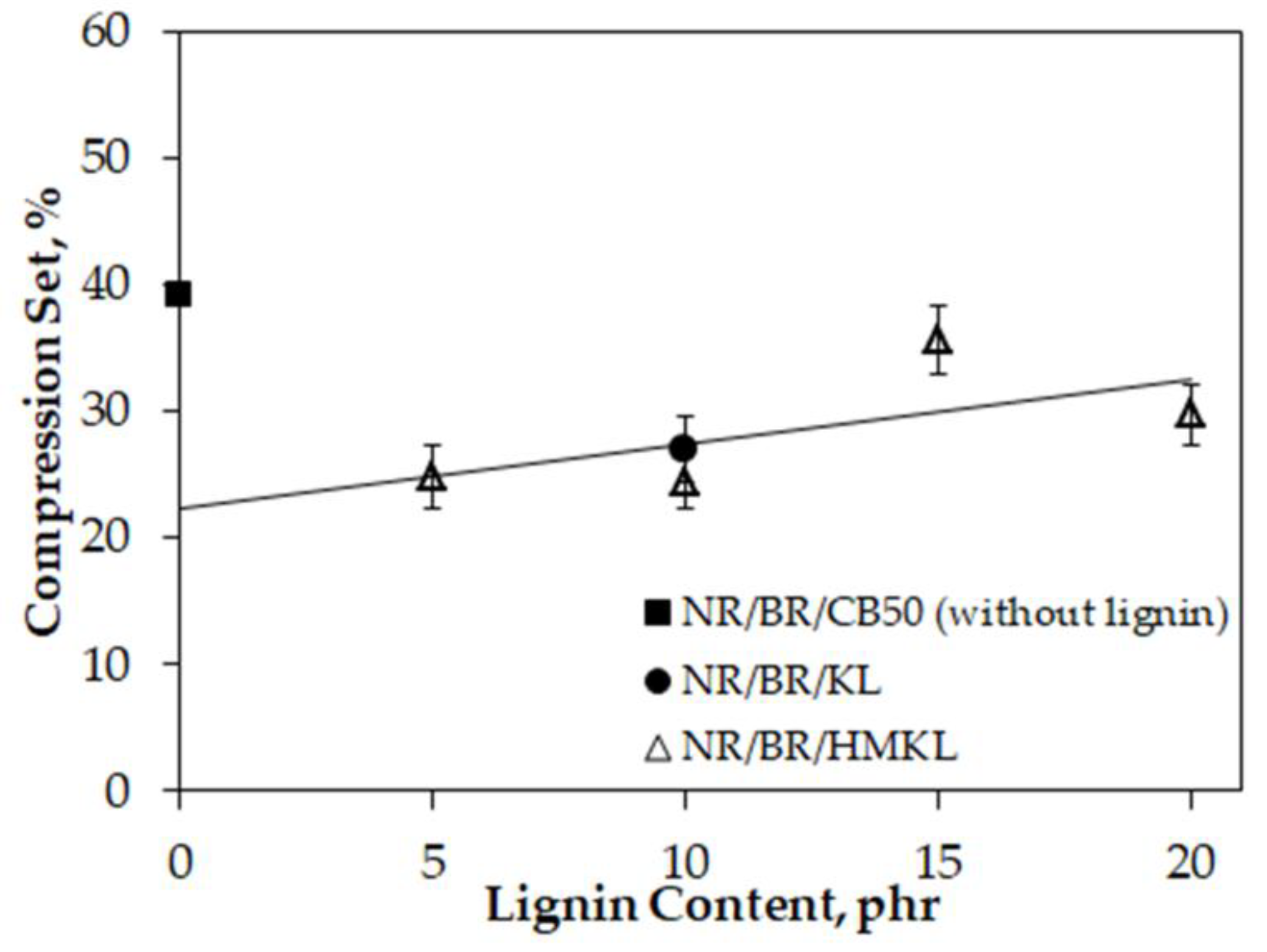
3.7. Compression Set
3.8. Flexing Resistance
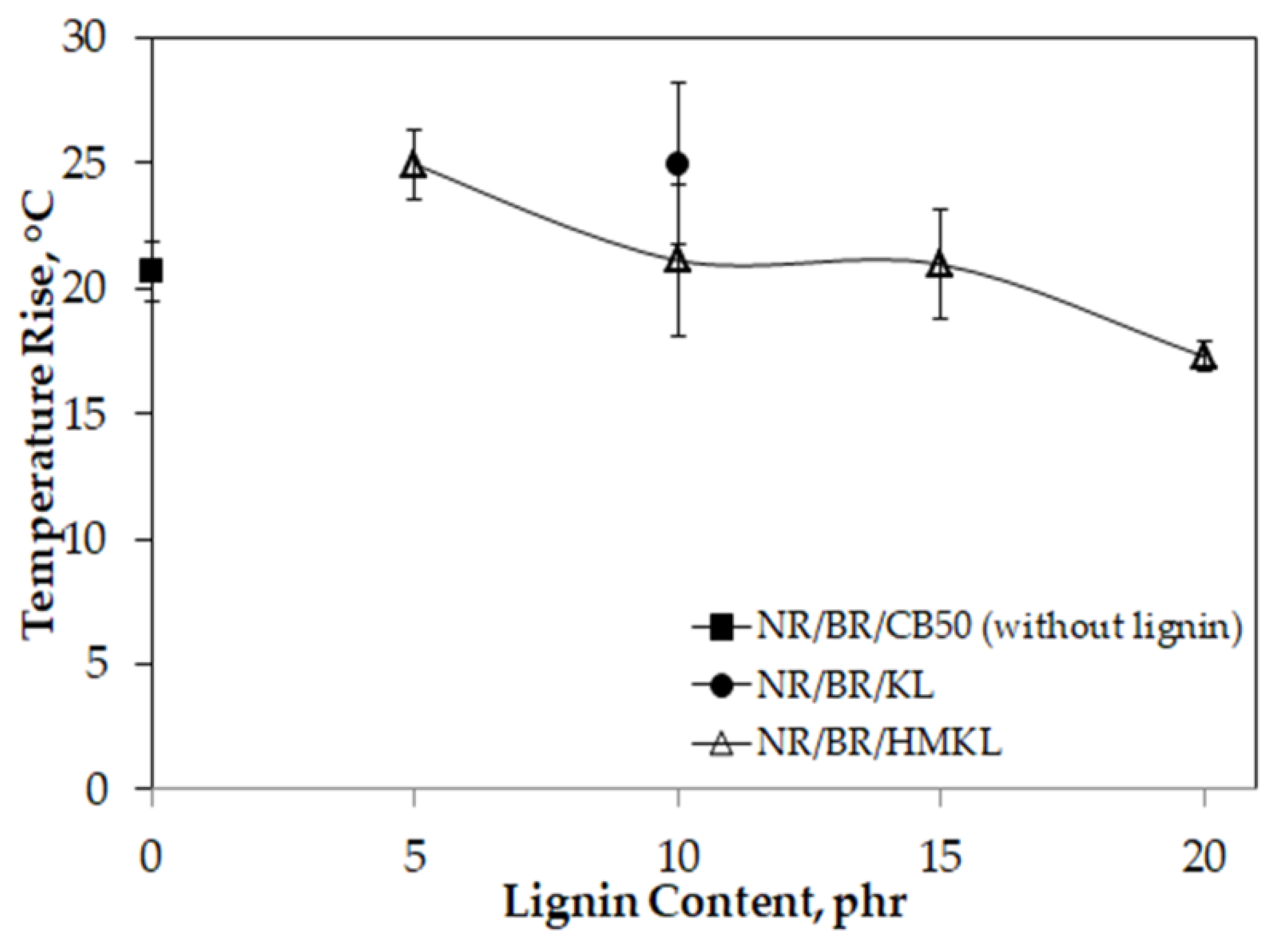
3.9. Heat Build-Up
3.10. Thermal Stability
3.10.1. Aging Resistance
3.10.2. Thermogravimetric Analysis (TGA)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ismail, H.; Ramly, F.; Othman, N. Multiwall carbon nanotube-filled natural rubber: The effects of filler loading and mixing method. Polym. Plast. Technol. Eng. 2010, 49, 260–266. [Google Scholar] [CrossRef]
- Xu, S.H.; Gu, J.; Luo, Y.F.; Jia, D.M. Effects of partial replacement of silica with surface modified nanocrystalline cellulose on properties of natural rubber nanocomposites. Express Polym. Lett. 2012, 6, 14–25. [Google Scholar] [CrossRef]
- Cummings, S.; Zhang, Y.; Smeets, N.; Cunningham, M.; Dubé, M.A. On the use of starch in emulsion polymerizations. Processes 2019, 7, 140. [Google Scholar] [CrossRef]
- Rattanasom, N.; Prasertsri, S. Mechanical properties, gas permeability and cut growth behavior of natural rubber vulcanizates: Influence of clay types and clay/carbon black ratios. Polym. Test. 2012, 31, 645–653. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothan, L.A.; John, M.; Narine, S.S.; Thomas, S.; Anandjiwala, R. Physicomechanical properties of nanocomposites based on cellulose nanofibre and natural rubber latex. Cellullose 2013, 20, 417–427. [Google Scholar] [CrossRef]
- Rajisha, K.R.; Maria, H.J.; Pothan, L.A.; Ahmad, Z.; Thomas, S. Preparation and characterization of potato starch nanocrystal reinforced natural rubber nanocomposites. Int. J. Biol. Macromol. 2014, 67, 147–153. [Google Scholar] [CrossRef]
- Jong, L. Modulus enhancement of natural rubber through the dispersion size reduction of protein/fiber aggregates. Ind. Crops Prod. 2014, 55, 25–32. [Google Scholar] [CrossRef]
- Jong, L. Influence of protein hydrolysis on the mechanical properties of natural rubber composites reinforced with soy protein particles. Ind. Crops Prod. 2015, 65, 102–109. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; Abächerli, A.; Semke, H.; Malherbe, R.; Käuper, P.; Nadif, A.; van Dam, J.E.G. Analytical protocols for characterization of sulphur-free lignin. Ind. Crops Prod. 2004, 19, 271–281. [Google Scholar] [CrossRef]
- Saito, K.; Kato, T.; Tsuiji, Y.; Fukushima, K. Identifying the characteristic secondary ions of lignin polymer using ToF-SIMS. Biomacromolecules 2005, 6, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Lora, J.H.; Glasser, W.G. Recent industrial applications of lignin: A sustainable alternative to nonrenewable materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Abejόn, R.; Pérez-Acebo, H.; Clavijo, L. Alternatives for chemical and biochemical lignin volarization: Hot topics from a bibliometric analysis of the research published during the 2000–2016 period. Processes 2018, 6, 98. [Google Scholar] [CrossRef]
- Abejόn, R.; Rabadán, J.; Lanza, S.; Abejόn, A.; Garea, A.; Irabien, A. Supported ionic liquid membranes for separation of lignin aqueous solutions. Processes 2018, 6, 143. [Google Scholar] [CrossRef]
- Deka, H.; Mohanty, A.; Misra, M. Renewable-resource-based green blends from poly(furfuryl alcohol) bioresin and lignin. Macromol. Mater. Eng. 2014, 299, 552–559. [Google Scholar] [CrossRef]
- Sahoo, S.; Misra, M.; Mohanty, A.K. Biocomposites from switchgrass and lignin hybrid and poly(butylene succinate) bioplastic: Studies on reactive compatibilization and performance evaluation. Macromol. Mater. Eng. 2014, 299, 178–189. [Google Scholar] [CrossRef]
- Miao, C.; Hamad, W.Y. Controlling lignin particle size for polymer blend applications. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Ten, E.; Vermerris, W. Recent developments in polymers derived from industrial lignin. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Bahl, K.; Jana, S.C. Surface modification of lignosulfonates for reinforcement of styrene-butadiene rubber compounds. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Frigerio, P.; Zoia, L.; Orlandi, M.; Hanel, T.; Castellani, L. Application of sulphur-free lignins as a filler for elastomers: Effect of hexamethylenetetramine treatment. BioResources 2014, 9, 1387–1400. [Google Scholar] [CrossRef]
- Bahl, K.; Miyoshi, T.; Jana, C. Hybrid fillers of lignin and carbon black for lowering of viscoelastic loss in rubber compounds. Polymer 2014, 55, 3825–3835. [Google Scholar] [CrossRef]
- Yu, P.; He, H.; Jia, Y.; Tian, S.; Chen, J.; Jia, D.; Luo, Y. A comprehensive study on lignin as a green alternative of silica in natural rubber composites. Polym. Test. 2016, 54, 176–185. [Google Scholar] [CrossRef]
- Cao, X.V.; Ismail, H.; Rashid, A.A.; Takeichi, T.; Vo-Huu, T. Maleated natural rubber as a coupling agent for recycled high density polyethylene/natural rubber/kenaf powder biocomposites. Polym. Plast. Technol. Eng. 2012, 51. [Google Scholar] [CrossRef]
- Jiang, C.; He, H.; Yu, P.; Wang, D.K.; Zhou, L.; Jia, D.M. Plane-interface-induced lignin based nanosheets and its reinforcing effect on styrene-butadiene rubber. Express Polym. Lett. 2014, 8, 619–634. [Google Scholar] [CrossRef]
- Xiao, S.; Feng, J.; Zhu, J.; Wang, X.; Yi, C.; Su, S. Preparation and characterization of lignin-layered double hydroxide/styrene-butadiene rubber composites. J. Appl. Polym. Sci. 2013, 130, 1308–1312. [Google Scholar] [CrossRef]
- Rowell, R.M. Acetylation of wood. For. Prod. J. 2006, 56, 4–12. [Google Scholar]
- Rowell, R.M. Chemical modification of wood: A short review. Wood Mater. Sci. Eng. 2006, 1, 29–33. [Google Scholar] [CrossRef]
- Siochi, E.J.; Ward, T.C.; Haney, M.A.; Mahn, B. The absolute molecular weight distribution of hydroxypropylated lignins. Macromolecules 1990, 23, 1420–1429. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, Y.; Liu, D.; Petrus, L. Qualitative analysis of products formed during the acid catalyzed liquefaction of bagasse in ethylene glycol. Bioresour. Technol. 2007, 98, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; He, H.; Yao, X.; Yu, P.; Zhou, L.; Jia, D. The aggregation structure regulation of lignin by chemical modification and its effect on the property of lignin/styrene butadiene rubber composites. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Popa, V.I.; Căpraru, A.M.; Grama, S.; Măluţan, T. Nanoparticles based on modified lignins with biocide properties. Cellullose Chem. Technol. 2011, 45, 221–226. [Google Scholar]
- Castro, D.F.; Suarez, J.C.M.; Nunes, R.C.R.; Visconte, L.L.Y. Influence of mixing procedures and mica addition on properties of NR/BR vulcanizates. I. J. Appl. Polym. Sci. 2004, 94, 1574–1585. [Google Scholar] [CrossRef]
- Pongdong, W.; Nakason, C.; Kummerlӧwe, C.; Vennemann, N. Influence of filler from a renewable resource and silane coupling agent on the properties of epoxidized natural rubber vulcanizates. J. Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Benar, P.; Gonçalves, A.R.; Mandelli, D.; Schuchard, U. Eucalyptus organosolv lignins: Study of the hydroxymethylation and use in resols. Bioresour. Technol. 1999, 68, 11–16. [Google Scholar] [CrossRef]
- Malutan, T.; Raluca, N.; Valentin, I. Contribution to the study of hydroxymetylation reaction of alkali lignin. BioResources 2007, 3, 13–20. [Google Scholar]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stabil. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Boeriu, C.; Bravo, G.; Gosselink, D.; Richard, J.A.; van Dam, E.G. Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Tejado, A.; Pẽna, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I.I. Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef]
- Constant, S.; Basset, C.; Dumas, C.; Renzo, F.D.; Robitzer, M.; Barakat, A.; Quignard, F. Reactive organosolv lignin extraction from wheat straw: Influence of Lewis acid catalysts on structural and chemical properties of lignins. Ind. Crops Prod. 2015, 65, 180–189. [Google Scholar] [CrossRef]
- Thielemans, W.; Can, E.; Morye, S.S.; Wool, R.P. Novel applications of lignin in composite materials. J. Appl. Polym. Sci. 2002, 83, 323–331. [Google Scholar] [CrossRef]
- Košíková, B.; Gregorová, A.; Osvald, A.; Krajčovičová, J. Role of lignin filler in stabilization of natural rubber-based composites. J. Appl. Polym. Sci. 2007, 103, 1226–1231. [Google Scholar] [CrossRef]
- Guerrero, P.P.; Lisperguer, J.; Navarrete, J.; Rodrigue, D. Effect of modified Eucalyptus Nitens lignin on the morphology and thermo-mechanical properties of recycled polystyrene. BioResources 2014, 9, 6514–6526. [Google Scholar]
- Jagadale, S.C.; Chavan, R.P.; Rajkumar, K.; Shinde, D.N.; Patil, C.L. Lignin as plasticizer in nitrile rubber, it’s effect on properties. Int. J. Res. Eng. Appl. Sci. 2016, 6, 78–84. [Google Scholar]
- Jiang, C.; He, H.; Jiang, H.; Ma, L.; Jia, D.M. Nano-lignin filled natural rubber composites: Preparation and characterization. Express Polym. Lett. 2013, 7, 480–493. [Google Scholar] [CrossRef]
- Gu, J.; Chen, W.J.; Lin, L.; Luo, Y.F.; Jia, D.M. Effect of nanocrystalline cellulose of the curing characteristics and aging resistance properties of carbon black reinforced natural rubber. Chin. J. Polym. Sci. 2013, 31, 1382–1393. [Google Scholar] [CrossRef]
- Yu, P.; He, H.; Jiang, C.; Wang, D.; Jia, Y.; Zhou, L.; Jia, D.M. Reinforcing styrene butadiene rubber with lignin-novalac epoxy resin networks. Express Polym. Lett. 2015, 9, 36–48. [Google Scholar] [CrossRef]
- Tangudom, P.; Thongsang, S.; Sombatsompop, N. Cure and mechanical properties and abrasive wear behaviour of natural rubber, styrene–butadiene rubber and their blends reinforced with silica hybrid fillers. Mater. Des. 2014, 53, 856–864. [Google Scholar] [CrossRef]
- Maciejewska, M.; Walkiewicz, F.; Zaborski, M. Novel ionic liquids as accelerators for the sulfur vulcanization of butadiene-styrene elastomer composites. Ind. Eng. Chem. Res. 2013, 52, 8410–8415. [Google Scholar] [CrossRef]
- Rattanasom, N.; Prasertsri, S.; Ruangritnumchai, T. Comparison of the mechanical properties at similar hardness level of natural rubber filled with various reinforcing—Fillers. Polym. Test. 2009, 28, 8–12. [Google Scholar] [CrossRef]
- Mostafa, A.; Abouel-Kasem, A.; Bayoumi, M.R.; El-Sebaie, M.G. Effect of carbon black loading on the swelling and compression set behavior of SBR and NBR rubber compounds. Mater. Des. 2009, 30, 1561–1568. [Google Scholar] [CrossRef]
- Goldberg, A.; Lesuer, D.R.; Patt, J. Fracture morphologies of carbon-black loaded SBR subjected to low-cycle, high-stress fatigue. Rubber Chem. Technol. 1989, 62, 272–287. [Google Scholar] [CrossRef]
- Furtado, C.R.G.; Leblanc, J.L.; Nunes, R.C.R. Fatigue resistance of mica-carbon black-styrene butadiene rubber (SBR) compounds. Eur. Polym. J. 1999, 35, 1319–1325. [Google Scholar] [CrossRef]
- Bhowmick, A.K.; Nando, G.B.; Basu, S.; De, S.K. Scanning electron microscopy studies of fractured natural rubber surfaces. Rubber Chem. Technol. 1979, 53, 327–334. [Google Scholar] [CrossRef]
- Mars, W.V.; Fatemi, A. Factors that affect the fatigue life of rubber: A literature survey. Rubber Chem. Technol. 2004, 77, 391–412. [Google Scholar] [CrossRef]
- Cataldo, F. On the ozone protection of polymers having non-conjugated unsaturation. Polym. Degrad. Stab. 2001, 72, 287–296. [Google Scholar] [CrossRef]
- Rattanasom, N.; Thammasiripong, U.; Suchiva, K. Mechanical properties of deproteinized natural rubber in comparison with synthetic cis-1,4 polyisoprene vulcanizates: Gum and black-filled vulcanizates. J. Appl. Polym. Sci. 2005, 97, 1139–1144. [Google Scholar] [CrossRef]
- Hao, P.T.; Ismail, H.; Hashim, A.S. Study of two types of styrene butadiene rubber in tire tread compounds. Polym. Test. 2001, 20, 539–544. [Google Scholar] [CrossRef]
- Gusev, A.A. Micromechanical mechanism of reinforcement and losses in filled rubbers. Macromolecules 2006, 39, 5960–5962. [Google Scholar] [CrossRef]
- Kar, K.K.; Bhowmick, A.K. Hysteresis loss in filled rubber vulcanzates and its relationship with heat generation. J. Appl. Polym. Sci. 1997, 64, 1541–1555. [Google Scholar] [CrossRef]
- Kakroodi, A.R.; Sain, M. Lignin in reinforced rubber composites. In Lignin in Polymer Composites; Faruk, O., Sain, M., Eds.; Matthew Deans: Oxford, UK, 2016; pp. 195–206. [Google Scholar]
- Liu, S.; Cheng, X. Applications of lignin as antioxidant in styrene butadiene rubber composite. AIP Conf. Proc. 2010, 1251, 344. [Google Scholar] [CrossRef]
- De Paoli, M.A.; Furlan, L.T. Lignin for reinforcing rubber. Quim. Nova 1983, 7, 121–122. [Google Scholar]
- Crabtree, J.; Kemp, A.R. Weathering of soft vulcanized rubber. Ind. Eng. Chem. 1946, 38, 278–295. [Google Scholar] [CrossRef]
- Muniandy, K.; Ismail, H.; Othman, N. Studies on natural weathering of rattan powder-filled natural rubber composites. BioResources 2012, 7, 3999–4011. [Google Scholar]
- Choi, S.S.; Han, D.H.; Ko, S.W.; Lee, H.S. Thermal aging behaviors of elemental sulfur-free polyisoprene vulcanizates. Korean Chem. Soc. 2005, 26, 1853–1855. [Google Scholar]
- Seidelt, S.; Müller-Hagedorn, M.; Bockhorn, H. Description of tire pyrolysis by thermal degradation behaviour of main components. J. Anal. Appl. Pyrolysis 2006, 75, 11–18. [Google Scholar] [CrossRef]
- Calvo, F.; Francisco, G.; Dobado, J.A.; Isac-Garcia, J.; Martin-Martínez, F.J. Lignin and Lignans as Renewable Raw Materials: Chemistry, Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Brazier, D.W.; Nickel, G.H. Thermoanalytical methods in vulcanizate analysis II. derivative thermogravimetric analysis. Rubber Chem. Technol. 1975, 48, 661–667. [Google Scholar] [CrossRef]




| Ingredients | Amount (phr) | |||||
|---|---|---|---|---|---|---|
| NR/BR/CB50 (without Lignin) | NR/BR/KL10 | NR/BR/HMKL-5 | NR/BR/HMKL-10 | NR/BR/HMKL-15 | NR/BR/HMKL-20 | |
| NR (SMR 10) 1 | 50 | 50 | 50 | 50 | 50 | 50 |
| BR (BR9000) 2 | 50 | 50 | 50 | 50 | 50 | 50 |
| Zinc Oxide (ZnO) | 5 | 5 | 5 | 5 | 5 | 5 |
| Stearic Acid | 2 | 2 | 2 | 2 | 2 | 2 |
| Carbon Black (N220) | 50 | 40 | 45 | 40 | 35 | 30 |
| Unmodified lignin 3 | - | 10 | - | - | - | - |
| HMKL 4 | - | - | 5 | 10 | 15 | 20 |
| TDAE 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| 6PPD 6 | 2 | 2 | 2 | 2 | 2 | 2 |
| TMQ 7 | 1 | 1 | 1 | 1 | 1 | 1 |
| Paraffin Wax | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| TBBS 8 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 |
| TMTD 9 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 |
| Sulfur | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Sample | Optimum Cure Time, t90 (min) | Scorch Time, tS2 (min) | Minimum Torque, ML (dN·m) | Maximum Torque, MH (dN·m) | Torque Difference, ∆M (MH–ML) (dN·m) | Crosslink Density, VC (×10−4 mol/m3) |
|---|---|---|---|---|---|---|
| NR/BR/CB50 (without lignin) | 4.57 | 1.76 | 1.45 | 13.12 | 11.67 | 6.5 ± 0.0 |
| NR/BR/KL10 | 6.23 | 2.27 | 1.09 | 9.41 | 8.32 | 5.2 ± 0.2 |
| NR/BR/HMKL5 | 3.54 | 1.89 | 1.33 | 11.78 | 10.45 | 10.3 ± 0.1 |
| NR/BR/HMKL10 | 3.97 | 1.99 | 1.10 | 10.41 | 9.31 | 10.9 ± 0.4 |
| NR/BR/HMKL15 | 4.82 | 2.22 | 1.08 | 9.88 | 8.80 | 6.8 ± 0.1 |
| NR/BR/HMKL20 | 5.49 | 2.36 | 0.91 | 9.03 | 8.12 | 5.0 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamad Aini, N.A.; Othman, N.; Hussin, M.H.; Sahakaro, K.; Hayeemasae, N. Hydroxymethylation-Modified Lignin and Its Effectiveness as a Filler in Rubber Composites. Processes 2019, 7, 315. https://doi.org/10.3390/pr7050315
Mohamad Aini NA, Othman N, Hussin MH, Sahakaro K, Hayeemasae N. Hydroxymethylation-Modified Lignin and Its Effectiveness as a Filler in Rubber Composites. Processes. 2019; 7(5):315. https://doi.org/10.3390/pr7050315
Chicago/Turabian StyleMohamad Aini, Nor Anizah, Nadras Othman, M. Hazwan Hussin, Kannika Sahakaro, and Nabil Hayeemasae. 2019. "Hydroxymethylation-Modified Lignin and Its Effectiveness as a Filler in Rubber Composites" Processes 7, no. 5: 315. https://doi.org/10.3390/pr7050315
APA StyleMohamad Aini, N. A., Othman, N., Hussin, M. H., Sahakaro, K., & Hayeemasae, N. (2019). Hydroxymethylation-Modified Lignin and Its Effectiveness as a Filler in Rubber Composites. Processes, 7(5), 315. https://doi.org/10.3390/pr7050315







