Synthesis and Characterization of Natural Extracted Precursor Date Palm Fibre-Based Activated Carbon for Aluminum Removal by RSM Optimization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Activated Carbon
2.3. Experimental Design and Optimization of Synthesized PAC
2.4. Adsorption Study
2.4.1. Screening of Different Conditions Adsorbent
2.4.2. Optimization of Al3+ Adsorption
2.4.3. Adsorption Isotherm and Kinetic Studies
2.5. Characterization of Powder-Activated Carbon
3. Results and Discussion
3.1. Model Establishment and Analysis
3.2. Statistical Analysis and Modelling
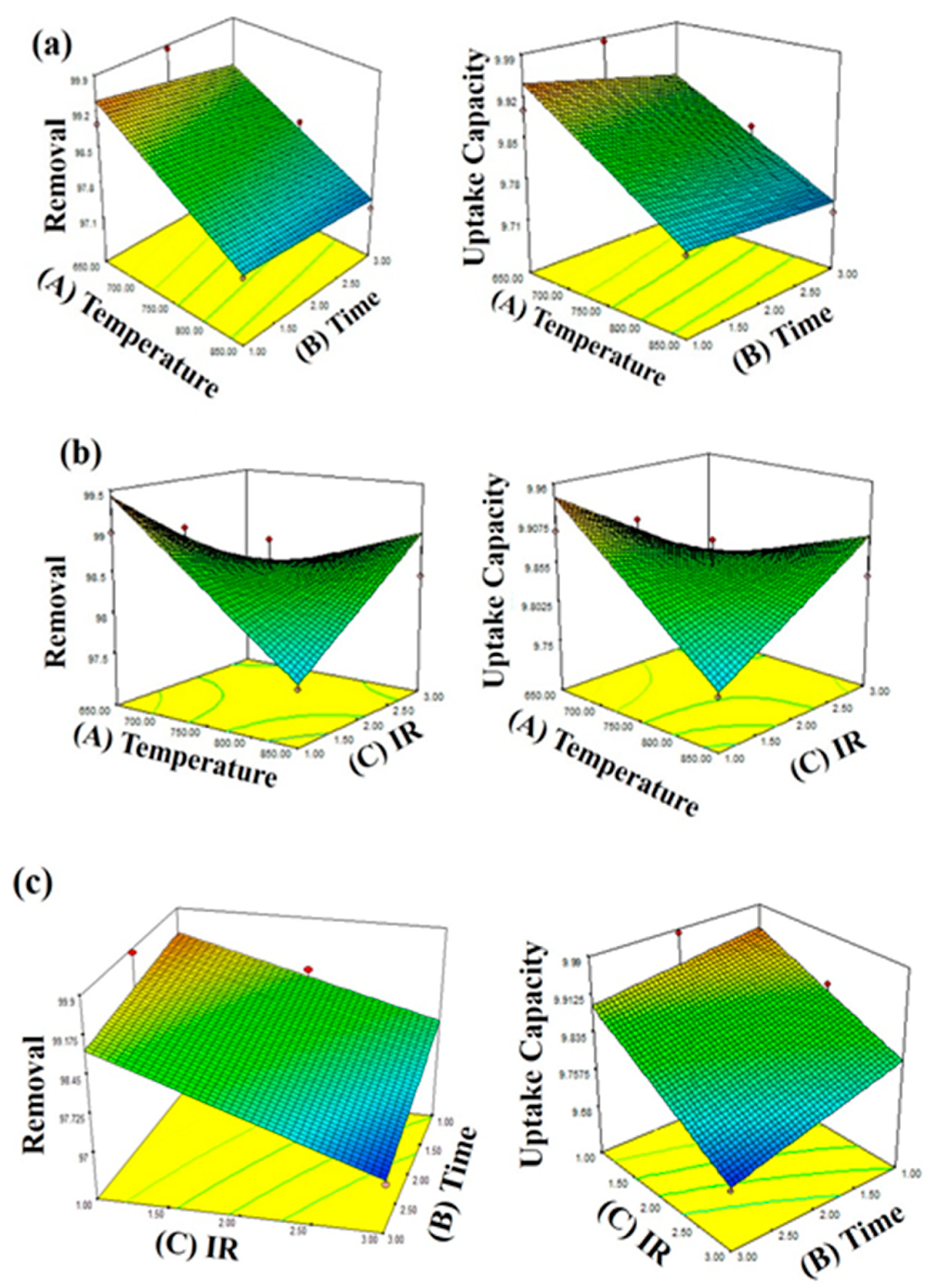
3.3. Effects of Activation Temperature, Activation Time, and Impregnation Ratio
3.4. Optimization Study of Synthesis bio-PAC
3.5. Analysis and Characterization
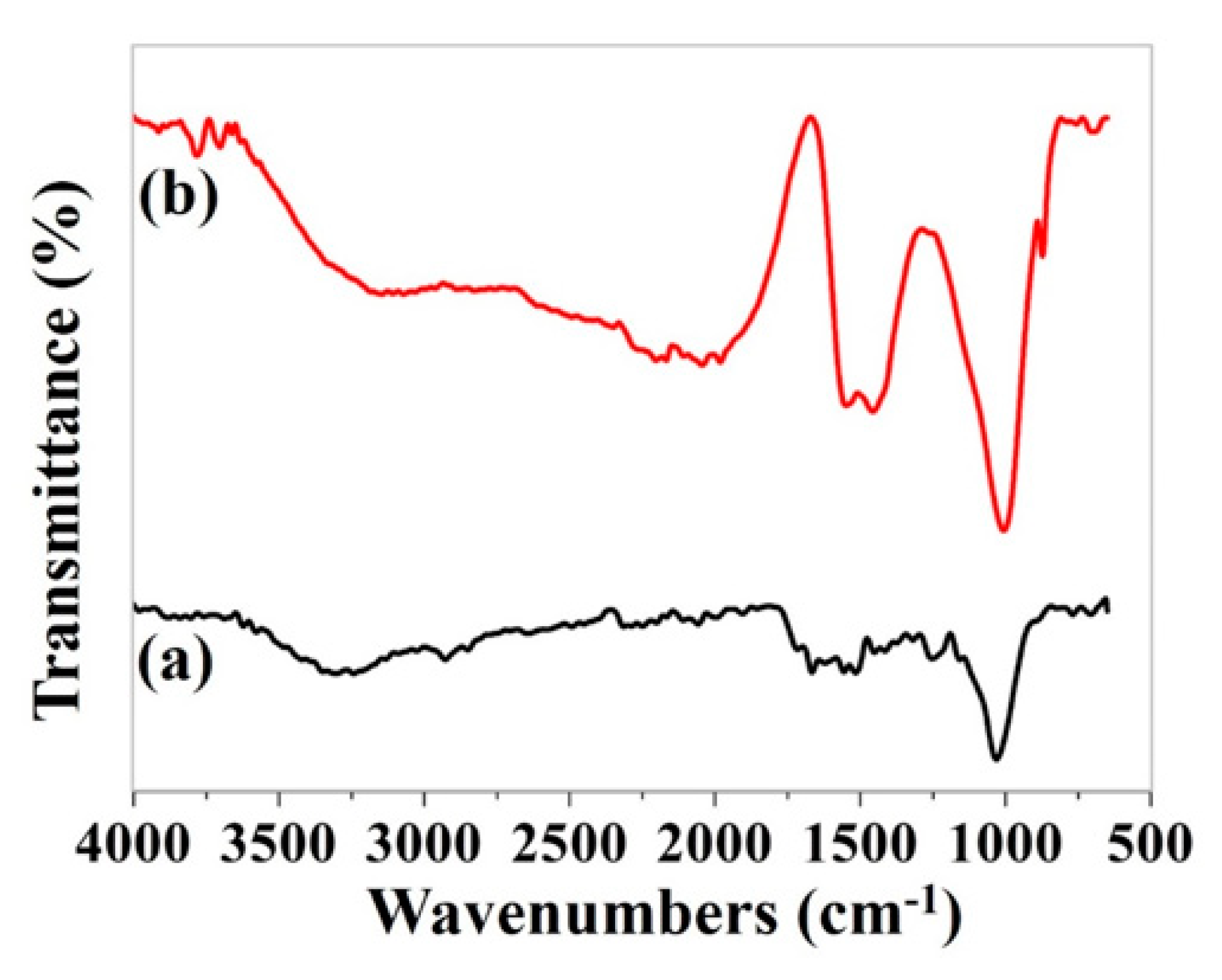
3.5.1. FTIR analysis
3.5.2. XRD Analysis
3.5.3. Thermogravimetric Analysis (TGA)
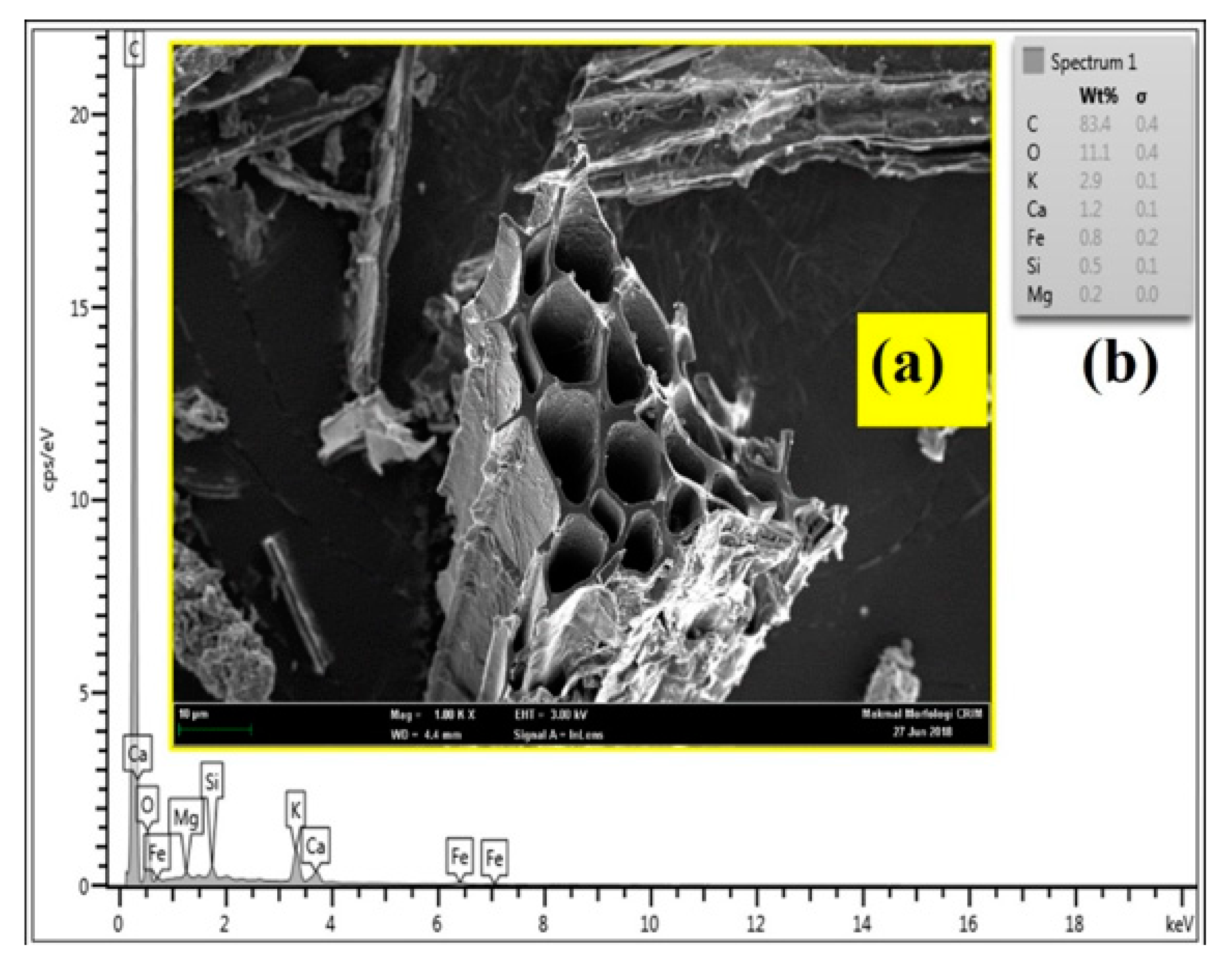
3.5.4. FESEM and EDX
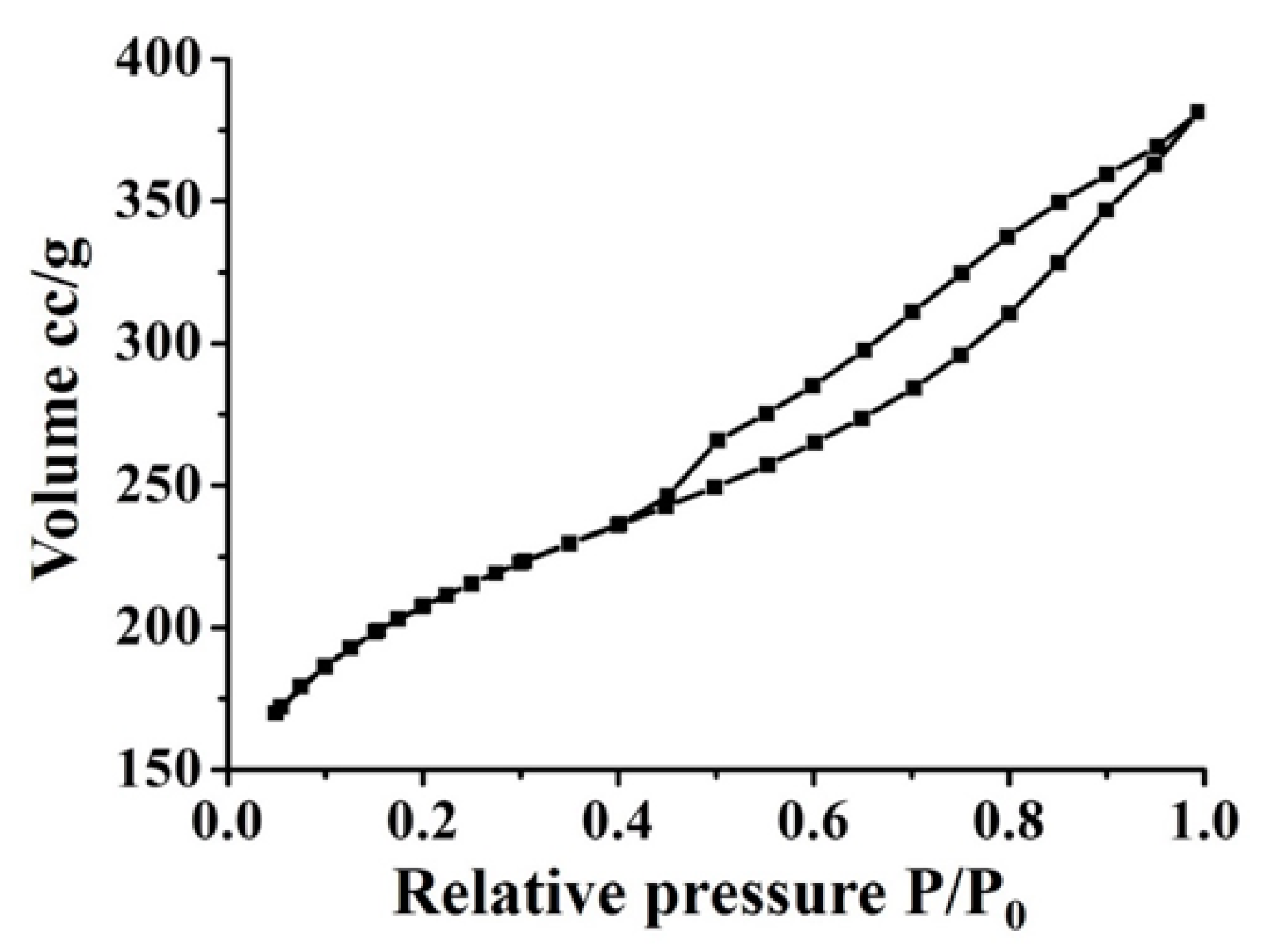
3.5.5. BET Analysis
3.5.6. Zeta Potential
3.6. Adsorption Study
3.6.1. Optimization and Analysis of Variance (ANOVA) on Adsorbent of Al3+
3.6.2. Effects of Optimization Variables on Adsorption of Al3+
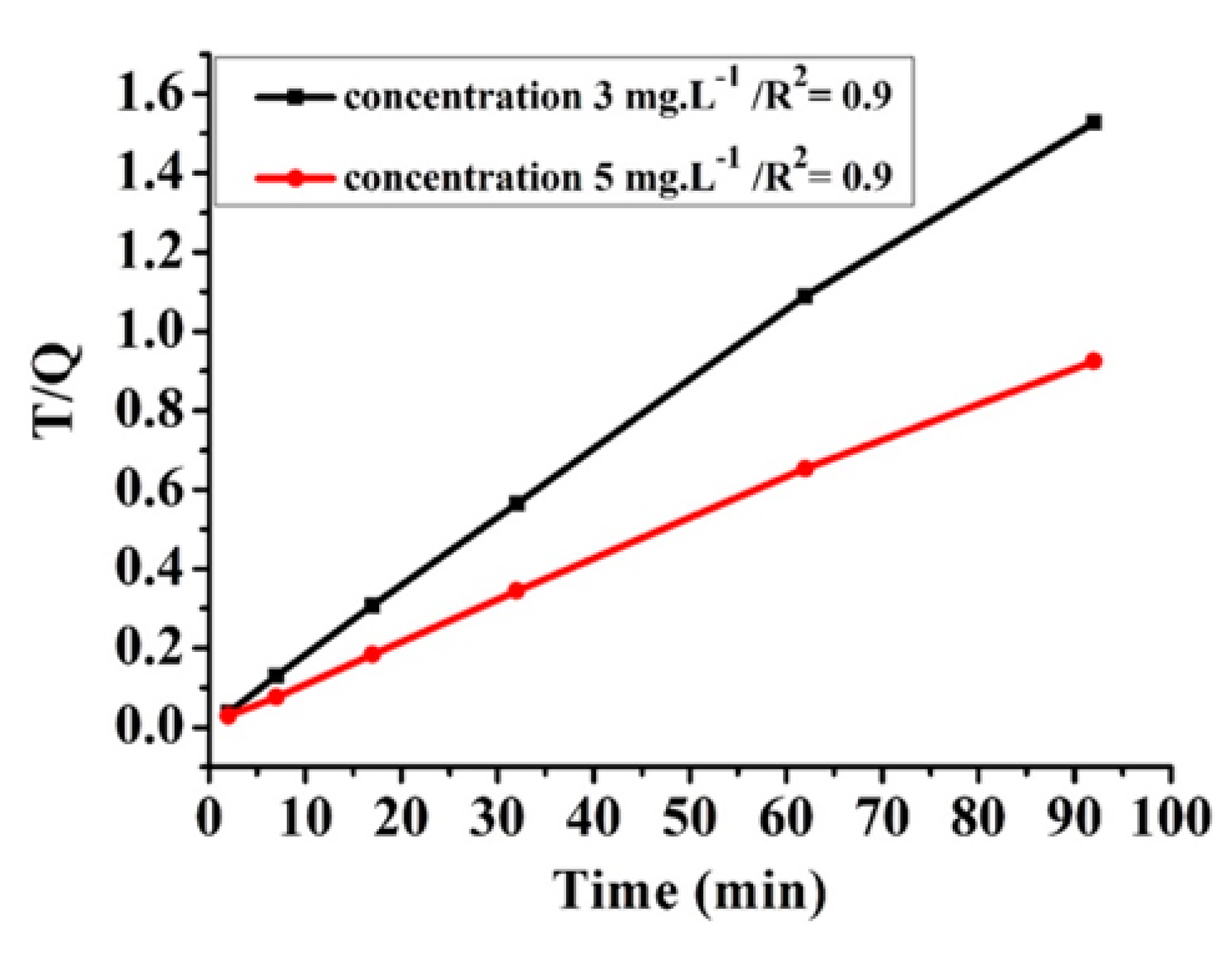
3.6.3. Kinetic Study
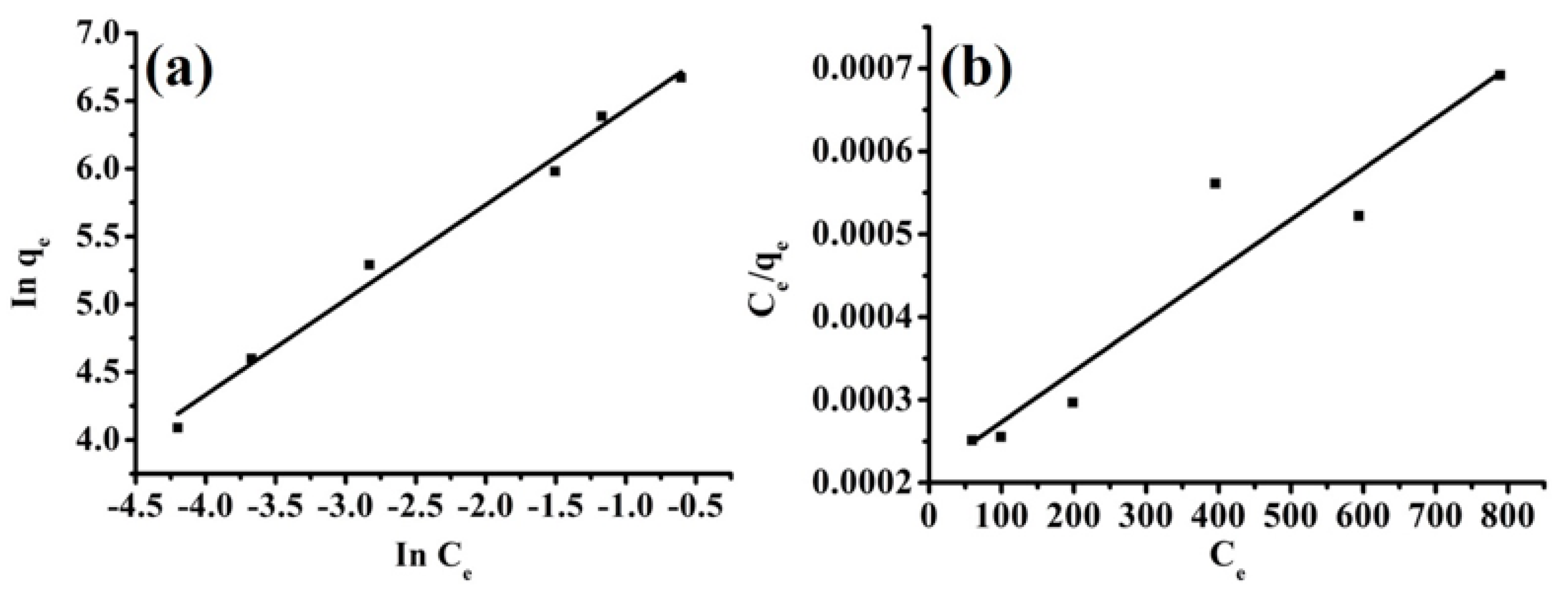
3.6.4. Isotherm Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balkhair, K.S.; Ashraf, M.A. Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi J. Biol. Sci. 2016, 23, S32–S44. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Hanfiah, M.M. Recent Advances in Assessment on Clear Water, Soil and Air; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Awual, M.R.; Yaita, T.; Taguchi, T.; Shiwaku, H.; Suzuki, S.; Okamoto, Y. Selective cesium removal from radioactive liquid waste by crown ether immobilized new class conjugate adsorbent. J. Hazard. Mater. 2014, 278, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Tassist, A.; Lounici, H.; Abdi, N.; Mameri, N. Equilibrium, kinetic and thermodynamic studies on aluminum biosorption by a mycelial biomass (Streptomyces rimosus). J. Hazard. Mater. 2010, 183, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Chauhan, M. Biosorption as a Novel Approach for Removing Aluminium from Water Treatment Plant Residual—A Review. In Water Quality Management; Springer: Singapore, 2018; pp. 93–99. [Google Scholar]
- Aly, Z.; Graulet, A.; Scales, N.; Hanley, T. Removal of aluminium from aqueous solutions using PAN-based adsorbents: Characterisation, kinetics, equilibrium and thermodynamic studies. Environ. Sci. Pollut. Res. 2014, 21, 3972–3986. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.A.; El-Naas, M.H.; Abdallah, S. Removal of aluminum from aqueous solutions by adsorption on date-pit and BDH activated carbons. J. Hazard. Mater. 2008, 158, 300–307. [Google Scholar] [CrossRef] [PubMed]
- De Philippis, R.; Colica, G.; Micheletti, E. Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: Molecular basis and practical applicability of the biosorption process. Appl. Microbiol. Biotechnol. 2011, 92, 697. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Shenashen, M.A.; Shahat, A.; El-Safty, S.A. Ultra-trace recognition and removal of toxic chromium (VI) ions from water using visual mesocaptor. J. Hazard. Mater. 2013, 244, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Salehi, E.; Khadivi, M.A.; Moradian, R.; Astinchap, B. Novel polyethersulfone nanocomposite membrane prepared by PANI/Fe3O4 nanoparticles with enhanced performance for Cu (II) removal from water. J. Membr. Sci. 2012, 415, 250–259. [Google Scholar] [CrossRef]
- Shenashen, M.A.; El-Safty, S.A.; Elshehy, E.A. Monolithic scaffolds for highly selective ion sensing/removal of Co (II), Cu (II), and Cd (II) ions in water. Analyst 2014, 139, 6393–6405. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.K.; Kim, Y.M.; Woo, S.H.; Park, J.M. Removal of cadmium using acid-treated activated carbon in the presence of nonionic and/or anionic surfactants. Hydrometallurgy 2009, 99, 209–213. [Google Scholar] [CrossRef]
- Xiong, C.; Yao, C.; Wang, L.; Ke, J. Adsorption behavior of Cd (II) from aqueous solutions onto gel-type weak acid resin. Hydrometallurgy 2009, 98, 318–324. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.; Ang, W.; Chung, Y.; Oatley-Radcliffe, D.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, H. A New Method for the Process Division and Effect Evaluation of Coagulation Based on Particle Size Fractal Dimension. Processes 2018, 6, 237. [Google Scholar] [CrossRef]
- Loganathan, P.; Shim, W.G.; Sounthararajah, D.P.; Kalaruban, M.; Nur, T.; Vigneswaran, S. Modelling equilibrium adsorption of single, binary, and ternary combinations of Cu, Pb, and Zn onto granular activated carbon. Environ. Sci. Pollut. Res. 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.A.; Han, K.K.; Hanafiah, M.M. Removal of methylene blue from dye wastewater using river sand by adsorption. Nat. Environ. Pollut. Technol. 2015, 14, 89. [Google Scholar]
- Hanafiah, M.M.; Hashim, N.A.; Ahmed, S.; Ashraf, M.A. Removal of chromium from aqueous solutions using a palm kernel shell adsorbent. Desalin. Water Treat. 2018, 118, 172–180. [Google Scholar] [CrossRef]
- Emran, M.Y.; El-Safty, S.A.; Shenashen, M.A.; Minowa, T. A well-thought-out sensory protocol for screening of oxygen reactive species released from cancer cells. Sens. Actuators B Chem. 2019, 284, 456–467. [Google Scholar] [CrossRef]
- Sajjad, M.; Khan, S.; Ali Baig, S.; Munir, S.; Naz, A.; Ahmad, S.S.; Khan, A. Removal of potentially toxic elements from aqueous solutions and industrial wastewater using activated carbon. Water Sci. Technol. 2017, 75, 2571–2579. [Google Scholar] [CrossRef]
- Teow, Y.H.; Kam, L.M.; Mohammad, A.W. Synthesis of cellulose hydrogel for copper (II) ions adsorption. J. Environ. Chem. Eng. 2018, 6, 4588–4597. [Google Scholar] [CrossRef]
- Awual, M.R.; Shenashen, M.; Jyo, A.; Shiwaku, H.; Yaita, T. Preparing of novel fibrous ligand exchange adsorbent for rapid column-mode trace phosphate removal from water. J. Ind. Eng. Chem. 2014, 20, 2840–2847. [Google Scholar] [CrossRef]
- Alidokht, L.; Khataee, A.; Reyhanitabar, A.; Oustan, S. Reductive removal of Cr (VI) by starch-stabilized Fe0 nanoparticles in aqueous solution. Desalination 2011, 270, 105–110. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J.; Zhong, A.; Jin, Y. Removal capacity and adsorption mechanism of heat-treated palygorskite clay for methylene blue. Chem. Eng. J. 2011, 174, 143–150. [Google Scholar] [CrossRef]
- Tomko, J.; Backor, M.; Stofko, M. Biosorption of heavy metals by dry fungi biomass. Acta Metall. Slovaca 2006, 12, 447–451. [Google Scholar]
- Ali, M.A.; Al-Hattab, T.A.; Al-Hydary, I.A. Extraction of date palm seed oil (phoenix dactylifera) by soxhlet apparatus. Int. J. Adv. Eng. Technol. 2015, 8, 261. [Google Scholar]
- Charola, S.; Patel, H.; Chandna, S.; Maiti, S. Optimization to prepare porous carbon from mustard husk using response surface methodology adopted with central composite design. J. Clean. Prod. 2019, 223, 969–979. [Google Scholar] [CrossRef]
- Aljumaily, M.M.; Alsaadi, M.A.; Das, R.; Hamid, S.B.A.; Hashim, N.A.; AlOmar, M.K.; Alayan, H.M.; Novikov, M.; Alsalhy, Q.F.; Hashim, M.A. Optimization of the Synthesis of Superhydrophobic Carbon Nanomaterials by Chemical Vapor Deposition. Sci. Rep. 2018, 8, 2778. [Google Scholar] [CrossRef] [PubMed]
- Azargohar, R.; Dalai, A. Production of activated carbon from Luscar char: Experimental and modeling studies. Microporous Mesoporous Mater. 2005, 85, 219–225. [Google Scholar] [CrossRef]
- Tan, I.; Ahmad, A.; Hameed, B. Optimization of preparation conditions for activated carbons from coconut husk using response surface methodology. Chem. Eng. J. 2008, 137, 462–470. [Google Scholar] [CrossRef]
- Uribe, E.; Pasten, A.; Lemus-Mondaca, R.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Ortiz, J.; Di Scala, K. Comparison of Chemical Composition, Bioactive Compounds and Antioxidant Activity of Three Olive-Waste Cakes. J. Food Biochem. 2015, 39, 189–198. [Google Scholar] [CrossRef]
- Gottipati, R.; Mishra, S. Process optimization of adsorption of Cr (VI) on activated carbons prepared from plant precursors by a two-level full factorial design. Chem. Eng. J. 2010, 160, 99–107. [Google Scholar] [CrossRef]
- Vaez, M.; Zarringhalam Moghaddam, A.; Alijani, S. Optimization and modeling of photocatalytic degradation of azo dye using a response surface methodology (RSM) based on the central composite design with immobilized titania nanoparticles. Ind. Eng. Chem. Res. 2012, 51, 4199–4207. [Google Scholar] [CrossRef]
- Banerjee, S.; Sharma, G.C.; Chattopadhyaya, M.; Sharma, Y.C. Kinetic and equilibrium modeling for the adsorptive removal of methylene blue from aqueous solutions on of activated fly ash (AFSH). J. Environ. Chem. Eng. 2014, 2, 1870–1880. [Google Scholar] [CrossRef]
- Chen, X.W.; Su, D.S.; Schlögl, R. Immobilization of CNFs on the surface and inside of the modified activated carbon. Phys. Status Solidi (b) 2006, 243, 3533–3536. [Google Scholar] [CrossRef]
- Alayan, H.M.; Alsaadi, M.A.; AlOmar, M.K.; Hashim, M.A. Growth and optimization of carbon nanotubes in powder activated carbon for an efficient removal of methylene blue from aqueous solution. Environ. Technol. 2018, 1–16. [Google Scholar] [CrossRef]
- Yahaya, N.; Pakir, M.; Latiff, M.; Abustan, I.; Bello, O.; Ahmad, M. Process optimisation for Zn (II) removal by activated carbon prepared from rice husk using chemical activation. Int. J. Eng. Technol. 2010, 10, 132–136. [Google Scholar]
- Tan, I.; Ahmad, A.; Hameed, B. Preparation of activated carbon from coconut husk: Optimization study on removal of 2, 4, 6-trichlorophenol using response surface methodology. J. Hazard. Mater. 2008, 153, 709–717. [Google Scholar] [CrossRef]
- Sarici-Özdemir, Ç.; Önal, Y. Study to observe the applicability of the adsorption isotherms used for the adsorption of medicine organics onto activated carbon. Part. Sci. Technol. 2018, 36, 254–261. [Google Scholar] [CrossRef]
- Theydan, S.K.; Ahmed, M.J. Optimization of preparation conditions for activated carbons from date stones using response surface methodology. Powder Technol. 2012, 224, 101–108. [Google Scholar] [CrossRef]
- Sayğılı, H.; Güzel, F. High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: Process optimization, characterization and dyes adsorption. J. Clean. Prod. 2016, 113, 995–1004. [Google Scholar] [CrossRef]
- Sulaiman, N.S.; Hashim, R.; Amini, M.H.M.; Danish, M.; Sulaiman, O. Optimization of activated carbon preparation from cassava stem using response surface methodology on surface area and yield. J. Clean. Prod. 2018, 198, 1422–1430. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, Y.; Yue, X.; Wang, L.; Zhu, H.; Wang, C. From environmental pollutant to activated carbons for high-performance supercapacitors. Electrochim. Acta 2016, 201, 96–105. [Google Scholar] [CrossRef]
- Moralı, U.; Demiral, H.; Şensöz, S. Optimization of activated carbon production from sunflower seed extracted meal: Taguchi design of experiment approach and analysis of variance. J. Clean. Prod. 2018, 189, 602–611. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, H.L.; Zattera, A.J. Native cellulose: Structure, characterization and thermal properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef]
- Zubrik, A.; Matik, M.; Hredzák, S.; Lovás, M.; Danková, Z.; Kováčová, M.; Briančin, J. Preparation of chemically activated carbon from waste biomass by single-stage and two-stage pyrolysis. J. Clean. Prod. 2017, 143, 643–653. [Google Scholar] [CrossRef]
- Krahnstöver, T.; Plattner, J.; Wintgens, T. Quantitative detection of powdered activated carbon in wastewater treatment plant effluent by thermogravimetric analysis (TGA). Water Res. 2016, 101, 510–518. [Google Scholar] [CrossRef]
- Danish, M.; Hashim, R.; Ibrahim, M.M.; Sulaiman, O. Optimization study for preparation of activated carbon from Acacia mangium wood using phosphoric acid. Wood Sci. Technol. 2014, 48, 1069–1083. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati, S.; Hadibarata, T.; Kueh, A.B.H.; Salim, M.R.; Zaini, M.A.A. Silver Nanoparticles in the Water Environment in Malaysia: Inspection, characterization, removal, modeling, and future perspective. Sci. Rep. 2018, 8, 986. [Google Scholar] [CrossRef] [PubMed]
- Ab Ghani, Z.; Yusoff, M.S.; Zaman, N.Q.; Zamri, M.F.M.A.; Andas, J. Optimization of preparation conditions for activated carbon from banana pseudo-stem using response surface methodology on removal of color and COD from landfill leachate. Waste Manag. 2017, 62, 177–187. [Google Scholar] [CrossRef]
- Bohli, T.; Ouederni, A. Improvement of oxygen-containing functional groups on olive stones activated carbon by ozone and nitric acid for heavy metals removal from aqueous phase. Environ. Sci. Pollut. Res. 2016, 23, 15852–15861. [Google Scholar] [CrossRef]
- Ben-Ali, S.; Jaouali, I.; Souissi-Najar, S.; Ouederni, A. Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J. Clean. Prod. 2017, 142, 3809–3821. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shenashen, M. Optical mesosensor for capturing of Fe (III) and Hg (II) ions from water and physiological fluids. Sens. Actuators B Chem. 2013, 183, 58–70. [Google Scholar] [CrossRef]
- Danish, M.; Hashim, R.; Ibrahim, M.M.; Sulaiman, O. Response surface methodology approach for methyl orange dye removal using optimized Acacia mangium wood activated carbon. Wood Sci. Technol. 2014, 48, 1085–1105. [Google Scholar] [CrossRef]
- El-Sewify, I.M.; Shenashen, M.A.; Shahat, A.; Yamaguchi, H.; Selim, M.M.; Khalil, M.M.; El-Safty, S.A. Dual colorimetric and fluorometric monitoring of Bi3+ ions in water using supermicroporous Zr-MOFs chemosensors. J. Luminescence 2018, 198, 438–448. [Google Scholar] [CrossRef]
- Hu, X.-J.; Liu, Y.-G.; Wang, H.; Zeng, G.-M.; Hu, X.; Guo, Y.-M.; Li, T.-T.; Chen, A.-W.; Jiang, L.-H.; Guo, F.-Y. Adsorption of copper by magnetic graphene oxide-supported β-cyclodextrin: Effects of pH, ionic strength, background electrolytes, and citric acid. Chem. Eng. Res. Des. 2015, 93, 675–683. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Alsaadi, M.A.; Hayyan, M.; Akib, S.; Hashim, M.A. Functionalization of CNTs surface with phosphonuim based deep eutectic solvents for arsenic removal from water. Appl. Surf. Sci. 2016, 389, 216–226. [Google Scholar] [CrossRef]
- Awual, M.R.; Miyazaki, Y.; Taguchi, T.; Shiwaku, H.; Yaita, T. Encapsulation of cesium from contaminated water with highly selective facial organic–inorganic mesoporous hybrid adsorbent. Chem. Eng. J. 2016, 291, 128–137. [Google Scholar] [CrossRef]
- Ifelebuegu, A.; Salauh, H.; Zhang, Y.; Lynch, D. Adsorptive Properties of Poly (1-methylpyrrol-2-ylsquaraine) Particles for the Removal of Endocrine-Disrupting Chemicals from Aqueous Solutions: Batch and Fixed-Bed Column Studies. Processes 2018, 6, 155. [Google Scholar] [CrossRef]
- Fiyadh, S.S.; AlSaadi, M.A.; AlOmar, M.K.; Fayaed, S.S.; Mjalli, F.S.; El-Shafie, A. BTPC-Based DES-Functionalized CNTs for A s 3+ Removal from Water: NARX Neural Network Approach. J. Environ. Eng. 2018, 144, 04018070. [Google Scholar] [CrossRef]
- Xu, H.; Qin, Q.; Zhang, C.; Ning, K.; Zhao, R.; Wang, P.; Deng, J.; Huang, G. Adsorption of Organic Constituents from Reverse Osmosis Concentrate in Coal Chemical Industry by Coking Coal. Processes 2019, 7, 44. [Google Scholar] [CrossRef]





| Factor | Name | Units | Low Actual | High Actual | Low Coded | High Coded | Mean | Std. Dev. |
|---|---|---|---|---|---|---|---|---|
| A | Activation Temperature | °C | 650.00 | 850.00 | −1.00 | 1.00 | 753.12 | 83.79 |
| B | Activation Time | h | 1.00 | 3.00 | −1.00 | 1.00 | 2.00 | 0.79 |
| C | Impregnation Ratio | 1.00 | 3.00 | −1.00 | 1.00 | 1.81 | 0.72 |
| Run | Parameters | Responses | |||
|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | KOH Ratio | Removal (%) | Capacity (mg·g−1) | |
| 1 | 850.00 | 3.00 | 1.00 | 97.18 | 9.71 |
| 2 | 650.00 | 3.00 | 3.00 | 97 | 9.7 |
| 3 | 750.00 | 1.00 | 2.00 | 98.85 | 9.88 |
| 4 | 750.00 | 2.00 | 3.00 | 98.65 | 9.86 |
| 5 | 750.00 | 2.00 | 2.00 | 97.98 | 9.79 |
| 6 | 750.00 | 2.00 | 1.00 | 98.2 | 9.82 |
| 7 | 850.00 | 3.00 | 2.00 | 97.6 | 9.76 |
| 8 | 750.00 | 3.00 | 1.00 | 98.3 | 9.83 |
| 9 | 850.00 | 1.00 | 3.00 | 98.34 | 9.83 |
| 10 | 650.00 | 1.00 | 1.00 | 98.98 | 9.89 |
| 11 | 850.00 | 1.00 | 1.00 | 97.5 | 9.75 |
| 12 | 750.00 | 3.00 | 2.00 | 97.65 | 9.76 |
| 13 | 650.00 | 2.00 | 2.00 | 98 | 9.8 |
| 14 | 650.00 | 2.00 | 1.00 | 99.82 | 9.98 |
| 15 | 650.00 | 1.00 | 2.00 | 98.88 | 9.88 |
| 16 | 900.00 | 2.00 | 2.00 | 98.5 | 9.85 |
| Factors | Response | ||||
|---|---|---|---|---|---|
| Dose | pH | Contact Time (min) | Removal (%) | Uptake Capacity (mg·g−1) | |
| 1 | 5.00 | 11.00 | 10.00 | 76.48 | 76.48 |
| 2 | 20.00 | 3.00 | 120.00 | 58.32 | 14.58 |
| 3 | 12.50 | 7.00 | 65.00 | 98.64 | 39.45 |
| 4 | 12.50 | 7.00 | 65.00 | 98.64 | 39.45 |
| 5 | 12.50 | 7.00 | 10.00 | 97.74 | 39.09 |
| 6 | 5.00 | 7.00 | 65.00 | 98.48 | 98.48 |
| 7 | 20.00 | 11.00 | 120.00 | 89.84 | 22.46 |
| 8 | 12.50 | 7.00 | 120.00 | 99.16 | 39.66 |
| 9 | 12.50 | 3.00 | 65.00 | 59.52 | 23.80 |
| 10 | 20.00 | 3.00 | 10.00 | 56.92 | 14.23 |
| 11 | 12.50 | 7.00 | 65.00 | 98.64 | 39.45 |
| 12 | 20.00 | 7.00 | 65.00 | 99.98 | 24.99 |
| 13 | 12.50 | 11.00 | 65.00 | 89.58 | 35.83 |
| 14 | 5.00 | 3.00 | 10.00 | 53.9 | 53.9 |
| 15 | 5.00 | 3.00 | 120.00 | 54.08 | 54.08 |
| 16 | 20.00 | 11.00 | 10.00 | 60.74 | 15.18 |
| 17 | 5.00 | 11.00 | 120.00 | 95.98 | 95.98 |
| Source | Sum of Squares | DF | Mean Square | F Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 6.04 | 6 | 1.01 | 4.28 | 0.0258 |
| A-Activation Temperature | 0.28 | 1 | 0.28 | 1.18 | 0.3054 |
| B-Activation Time | 0.61 | 1 | 0.61 | 2.61 | 0.1408 |
| C-Impregnation Ratio | 0.13 | 1 | 0.13 | 0.53 | 0.4833 |
| AB | 0.03 | 1 | 0.03 | 0.13 | 0.7224 |
| AC | 1.75 | 1 | 1.75 | 7.42 | 0.0235 |
| BC | 0.06 | 1 | 0.06 | 0.26 | 0.6250 |
| Source | Sum of Squares | DF | Mean Square | F Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 0.060 | 6 | 0.010 | 4.28 | 0.0258 |
| A-Activation Temperature | 2.780 × 10−3 | 1 | 2.780 × 10−3 | 1.18 | 0.3054 |
| B-Activation Time | 6.139 × 10−3 | 1 | 6.139 × 10−3 | 2.61 | 0.1408 |
| C-Impregnation Ratio | 1.258 × 10−3 | 1 | 1.258 × 10−3 | 0.53 | 0.4833 |
| AB | 3.163 × 10−4 | 1 | 3.163 × 10−4 | 0.13 | 0.7224 |
| AC | 0.017 | 1 | 0.017 | 7.42 | 0.0235 |
| BC | 6.026 × 10−4 | 1 | 6.026 × 10−4 | 0.26 | 0.6250 |
| Name | Goal | Lower Limit | Upper Limit | Importance |
|---|---|---|---|---|
| Activation Temperature (°C) | in range | 650 | 850 | 3 |
| Activation Time (h) | in range | 1 | 3 | 3 |
| IR | in range | 1 | 3 | 3 |
| Removal (%) | maximize | 97 | 99.82 | 5 |
| Uptake capacity (mg·g−1) | maximize | 9.7 | 9.982 | 5 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 5931.26 | 9 | 659.03 | 48.89 | <0.0001 |
| A-Dose | 17.21 | 1 | 17.21 | 1.28 | 0.2957 |
| B-pH | 1686.88 | 1 | 1686.88 | 125.13 | <0.0001 |
| C-Contact Time | 266.26 | 1 | 266.26 | 19.75 | 0.0030 |
| AB | 106.14 | 1 | 106.14 | 7.87 | 0.0263 |
| AC | 14.63 | 1 | 14.63 | 1.09 | 0.3321 |
| BC | 276.36 | 1 | 276.36 | 20.50 | 0.0027 |
| A2 | 11.14 | 1 | 11.14 | 0.83 | 0.3935 |
| B2 | 1912.79 | 1 | 1912.79 | 141.89 | <0.0001 |
| C2 | 21.30 | 1 | 21.30 | 1.58 | 0.2491 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 60.11 | 13 | 4.62 | 92.30 | 0.0016 |
| A-Dose | 0.011 | 1 | 0.01 | 0.22 | 0.6679 |
| B-pH | 4.52 | 1 | 4.52 | 90.20 | 0.0025 |
| C-Contact Time | 0.01 | 1 | 0.01 | 0.20 | 0.6841 |
| AB | 1.06 | 1 | 1.06 | 21.19 | 0.0193 |
| AC | 0.15 | 1 | 0.15 | 2.92 | 0.1859 |
| BC | 2.76 | 1 | 2.76 | 55.17 | 0.0050 |
| A2 | 0.11 | 1 | 0.11 | 2.22 | 0.2326 |
| B2 | 19.13 | 1 | 19.13 | 381.86 | 0.0003 |
| C2 | 0.21 | 1 | 0.21 | 4.25 | 0.1312 |
| ABC | 0.08 | 1 | 0.08 | 1.75 | 0.2774 |
| A2B | 0.10 | 1 | 0.10 | 2.08 | 0.2448 |
| A2C | 0.50 | 1 | 0.50 | 9.88 | 0.0515 |
| AB2 | 0.11 | 1 | 0.11 | 2.12 | 0.2412 |
| Pseudo-First-Order ln(qc − qt) vs time (t) | Pseudo-Second-Order (t/qcvs t) | Intraparticle (qc vs t0.5) | |||
|---|---|---|---|---|---|
| Dose mg | pH | C0 mg·L−1 | R2 | R2 | R2 |
| 3 | 9.86 | 3 | 0.749 | 0.9996 | 0.8898 |
| 5 | 9.86 | 5 | 0.4929 | 0.9989 | 0.5908 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O. Basheer, A.; M. Hanafiah, M.; Abdulhakim Alsaadi, M.; Al-Douri, Y.; Malek, M.A.; Mohammed Aljumaily, M.; Saadi Fiyadh, S. Synthesis and Characterization of Natural Extracted Precursor Date Palm Fibre-Based Activated Carbon for Aluminum Removal by RSM Optimization. Processes 2019, 7, 249. https://doi.org/10.3390/pr7050249
O. Basheer A, M. Hanafiah M, Abdulhakim Alsaadi M, Al-Douri Y, Malek MA, Mohammed Aljumaily M, Saadi Fiyadh S. Synthesis and Characterization of Natural Extracted Precursor Date Palm Fibre-Based Activated Carbon for Aluminum Removal by RSM Optimization. Processes. 2019; 7(5):249. https://doi.org/10.3390/pr7050249
Chicago/Turabian StyleO. Basheer, Alfarooq, Marlia M. Hanafiah, Mohammed Abdulhakim Alsaadi, Y. Al-Douri, M.A. Malek, Mustafa Mohammed Aljumaily, and Seef Saadi Fiyadh. 2019. "Synthesis and Characterization of Natural Extracted Precursor Date Palm Fibre-Based Activated Carbon for Aluminum Removal by RSM Optimization" Processes 7, no. 5: 249. https://doi.org/10.3390/pr7050249
APA StyleO. Basheer, A., M. Hanafiah, M., Abdulhakim Alsaadi, M., Al-Douri, Y., Malek, M. A., Mohammed Aljumaily, M., & Saadi Fiyadh, S. (2019). Synthesis and Characterization of Natural Extracted Precursor Date Palm Fibre-Based Activated Carbon for Aluminum Removal by RSM Optimization. Processes, 7(5), 249. https://doi.org/10.3390/pr7050249








