Recent Developments in the Photocatalytic Treatment of Cyanide Wastewater: An Approach to Remediation and Recovery of Metals
Abstract
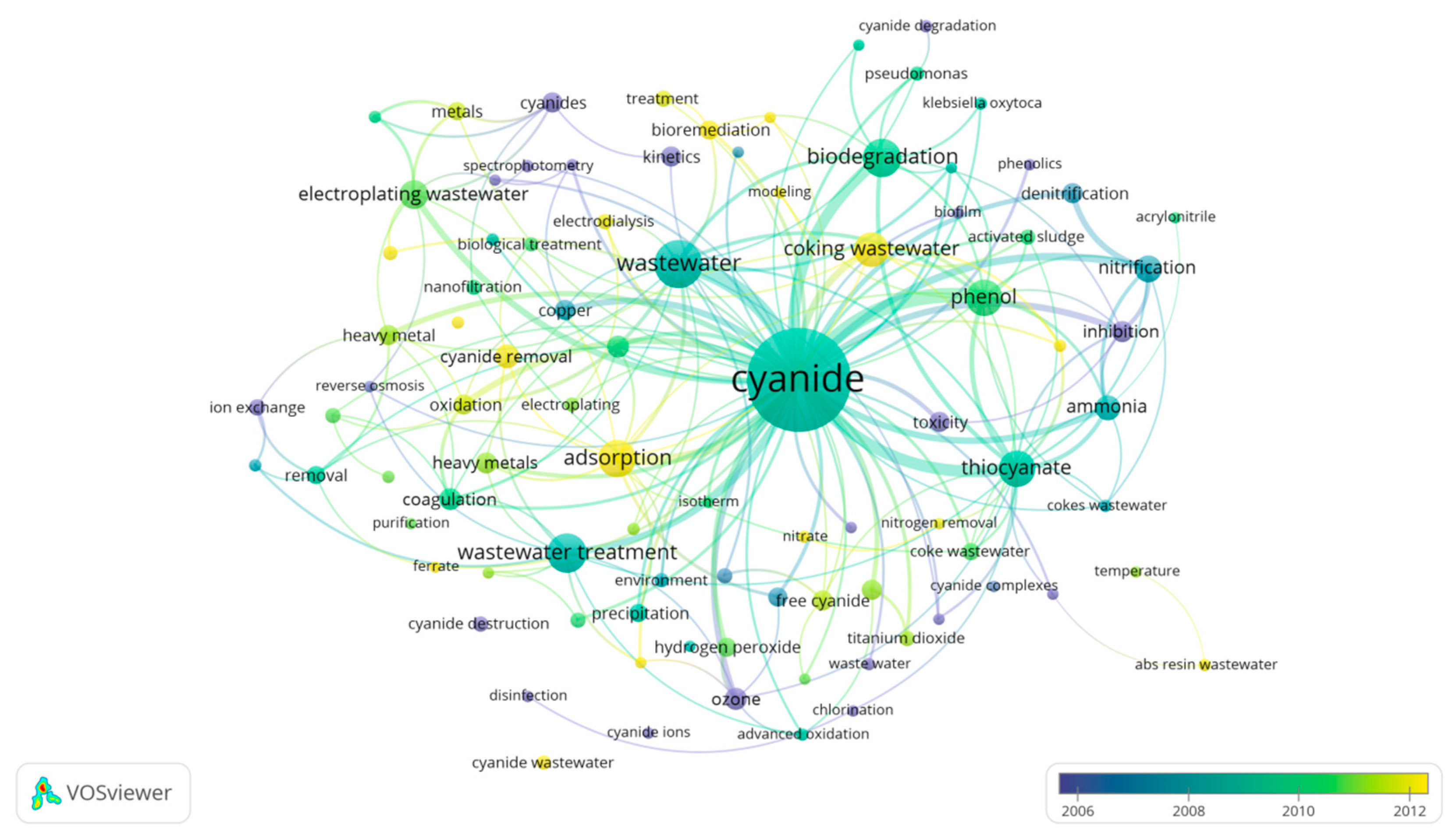
1. Introduction
2. Production and Characterization of Cyanide Wastewater
3. Existing Treatment Options
4. Photocatalytic Treatment Alternatives
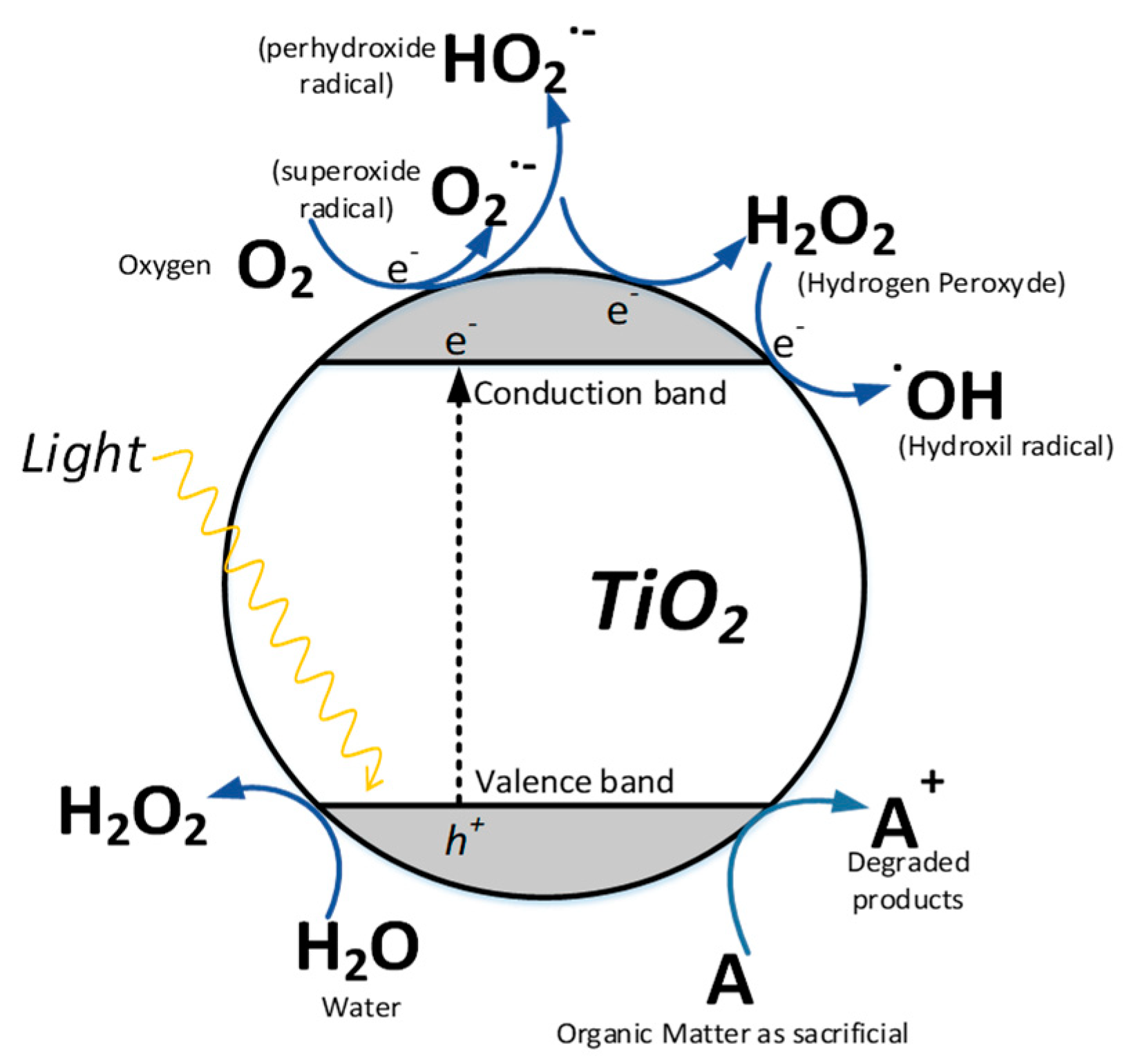
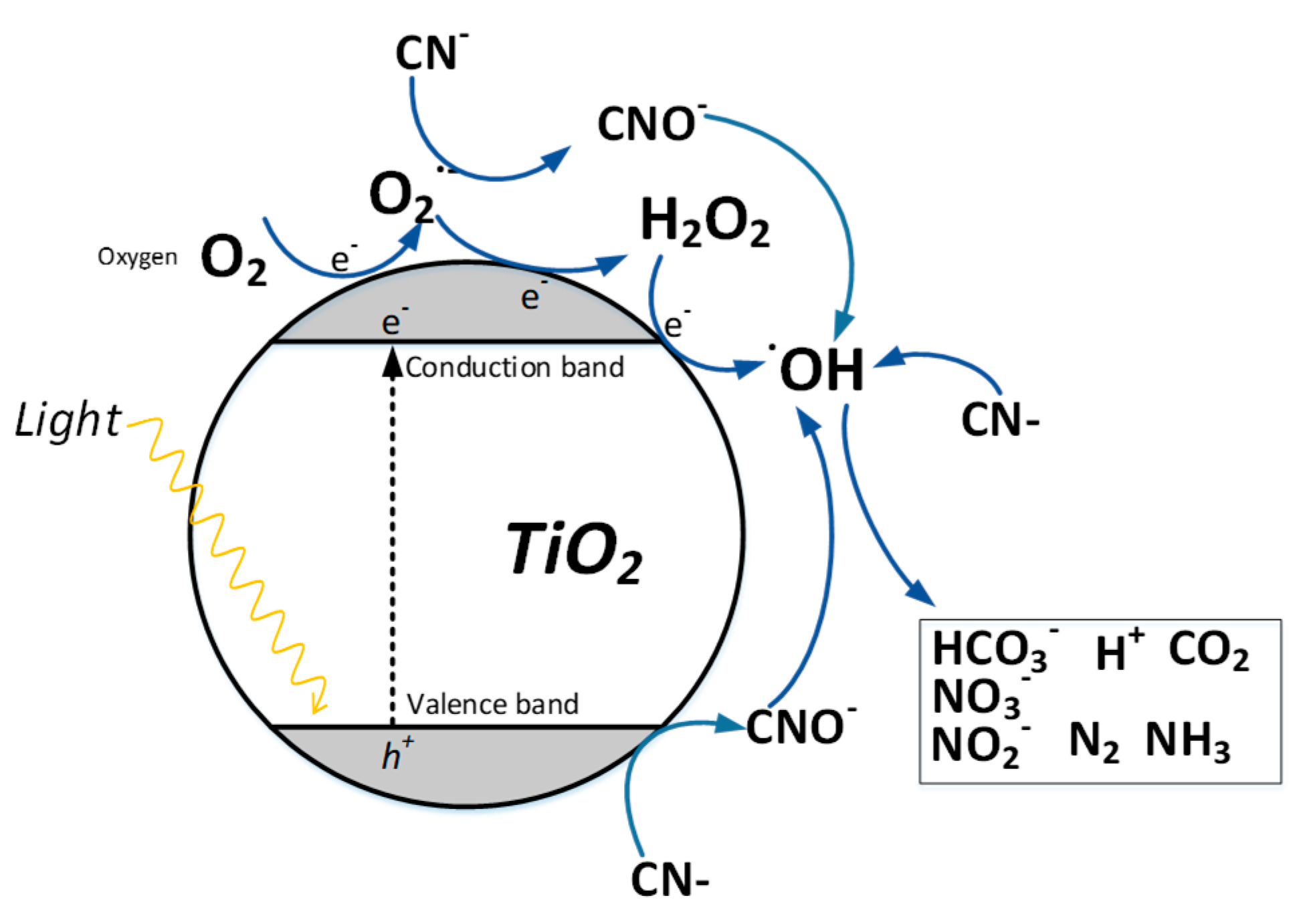
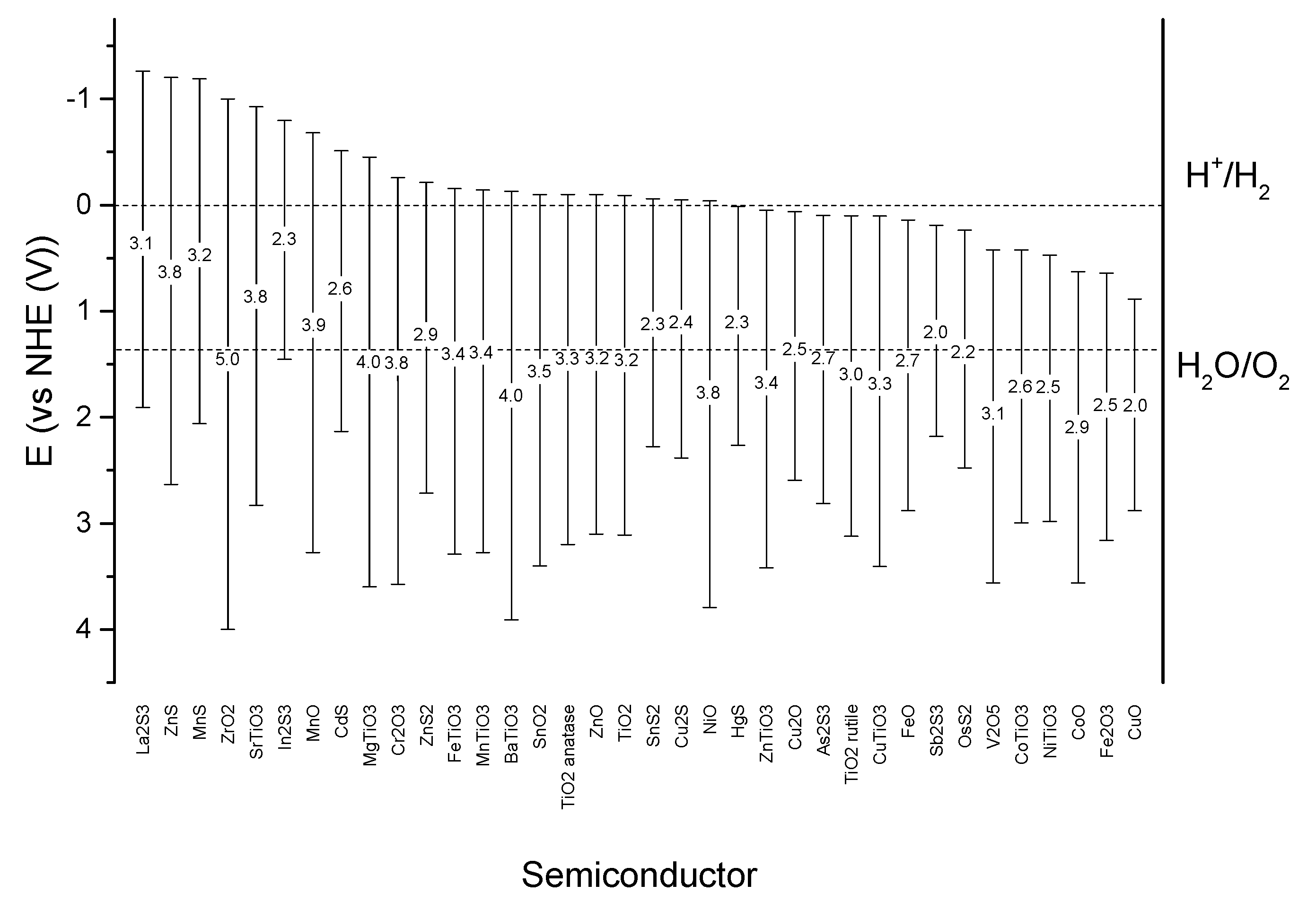
4.1. Classic Oxidative Photocatalysis
4.2. Photoreduction of Metals
4.3. Application of Traditional Photoreactors and LEDs in Mining Wastewater
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Gold Council Gold Demand Trends Q1. 2017. Available online: http://www.gold.org/research/gold-demand-trends (accessed on 1 June 2017).
- Sparrow, G.J.; Woodcock, J.T. Cyanide and Other Lixiviant Leaching Systems for Gold with Some Practical Applications. Miner. Process. Extr. Metall. Rev. 1995, 14, 193–247. [Google Scholar] [CrossRef]
- Li, J.; Miller, J.D. A review of gold leaching in acid thiourea solutions. Miner. Process. Extr. Metall. Rev. 2006, 27, 177–214. [Google Scholar] [CrossRef]
- Konyratbekova, S.S.; Baikonurova, A.; Akcil, A. Non-cyanide Leaching Processes in Gold Hydrometallurgy and Iodine-Iodide Applications: A Review. Miner. Process. Extr. Metall. Rev. 2015, 36, 198–212. [Google Scholar] [CrossRef]
- Haque, K.E. Gold Leaching from Refractory Ores—Literature Survey. Miner. Process. Extr. Metall. Rev. 1987, 2, 235–253. [Google Scholar] [CrossRef]
- Brent Hiskey, J.; Atluri, V.P. Dissolution Chemistry of Gold and Silver in Different Lixiviants. Miner. Process. Extr. Metall. Rev. 1988, 4, 95–134. [Google Scholar] [CrossRef]
- Mpinga, C.N.; Bradshaw, S.M.; Akdogan, G.; Snyders, C.A.; Eksteen, J.J. Evaluation of the Merrill-Crowe process for the simultaneous removal of platinum, palladium and gold from cyanide leach solutions. Hydrometallurgy 2014, 142, 36–46. [Google Scholar] [CrossRef]
- Norgate, T.; Haque, N. Using life cycle assessment to evaluate some environmental impacts of gold production. J. Clean. Prod. 2012, 29–30, 53–63. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Saccomanni, A.; Selli, E. Cr(VI) photocatalytic reduction: Effects of simultaneous organics oxidation and of gold nanoparticles photodeposition on TiO2. J. Hazard. Mater. 2012, 211–212, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.V. Toxicidad del cianuro. Investigación bibliográfica de sus efectos en animales y en el hombre. An. Fac. med. 2010, 71, 54–61. [Google Scholar]
- López-Muñoz, M.-J.; Aguado, J.; van Grieken, R.; Marugán, J. Simultaneous photocatalytic reduction of silver and oxidation of cyanide from dicyanoargentate solutions. Appl. Catal. B Environ. 2009, 86, 53–62. [Google Scholar] [CrossRef]
- Little, E.E.; Calfee, R.D.; Theodorakos, P.; Brown, Z.A.; Johnson, C.A. Toxicity of cobalt-complexed cyanide to Oncorhynchus mykiss, Daphnia magna, and Ceriodaphnia dubia. Potentiation by ultraviolet radiation and attenuation by dissolved organic carbon and adaptive UV tolerance. Environ. Sci. Pollut. Res. Int. 2007, 14, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, M.; Hagelstein, K.; Mudder, T. El manejo del cianuro en la extracción de oro; ICME―Consejo Internacional de Metales y Medio Ambiente: Canada, 2001; ISBN 1-895720-35-4. [Google Scholar]
- Chi, G.; Fuerstenau, M.C.; Marsden, J.O. Study of Merrill-Crowe processing. Part I: S, olubility of zinc in alkaline cyanide solution. Int. J. Miner. Process 1997, 49, 171–183. [Google Scholar] [CrossRef]
- Gaya, U.I. Heterogeneous Photocatalysis Using Inorganic Semiconductor Solids; Springer Science & Business Media: Dordrecht, The Netherlands, 2014; ISBN 9789400777750. [Google Scholar]
- Domènech, X.; Jardim, W.F.; Litter, M.I. Procesos avanzados de oxidación para la eliminación de contaminantes. In Eliminación de Contaminantes por Fotocatálisis Heterogénea; Blesa, M.A., Ed.; CYTED: La Plata, Argentina, 2001; ISBN 987-43-3809-1. [Google Scholar]
- Malloch, K.R.; Craw, D. Comparison of contrasting gold mine processing residues in a temperate rain forest, New Zealand. Appl. Geochem. 2017, 84, 61–75. [Google Scholar] [CrossRef]
- Dobrosz-Gómez, I.; Ramos García, B.D.; GilPavas, E.; Gómez García, M.Á. Kinetic study on HCN volatilization in gold leaching tailing ponds. Miner. Eng. 2017, 110, 185–194. [Google Scholar] [CrossRef]
- Adams, M.D. Impact of recycling cyanide and its reaction products on upstream unit operations. Miner. Eng. 2013, 53, 241–255. [Google Scholar] [CrossRef]
- Johnson, C.A. The fate of cyanide in leach wastes at gold mines: An environmental perspective. Appl. Geochem. 2015, 57, 194–205. [Google Scholar] [CrossRef]
- Kim, S.-O.S.H.; Lee, S.W.; Lee, G.M.; Lee, B.-T.; Yun, S.-T.; Kim, S.-O.S.H. Monitoring of TiO2-catalytic UV-LED photo-oxidation of cyanide contained in mine wastewater and leachate. Chemosphere 2016, 143, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Constructed wetlands for treatment of industrial wastewaters: A review. Ecol. Eng. 2014, 73, 724–751. [Google Scholar] [CrossRef]
- Dai, X.; Simons, A.; Breuer, P. A review of copper cyanide recovery technologies for the cyanidation of copper containing gold ores. Miner. Eng. 2012, 25, 1–13. [Google Scholar] [CrossRef]
- Gupta, N.; Balomajumder, C.; Agarwal, V.K.K. Enzymatic mechanism and biochemistry for cyanide degradation: A review. J. Hazard. Mater. 2010, 176, 1–13. [Google Scholar] [CrossRef]
- Luque-Almagro, V.M.; Moreno-Vivián, C.; Roldán, M.D. Biodegradation of cyanide wastes from mining and jewellery industries. Curr. Opin. Biotechnol. 2016, 38, 9–13. [Google Scholar] [CrossRef]
- Mekuto, L.; Ntwampe, S.K.O.; Akcil, A. An integrated biological approach for treatment of cyanidation wastewater. Sci. Total Environ. 2016, 571, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.R.; Gaur, A.; Balomajumder, C. Cyanide in industrial wastewaters and its removal: A review on biotreatment. J. Hazard. Mater. 2009, 163, 1–11. [Google Scholar] [CrossRef]
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper removal from industrial wastewater: A comprehensive review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Gómez-Luna, E.; Navas, D.F.; Aponte-Mayor, G.; Betancourt-Buitrago, L.A. Literature review methodology for scientific and information management, through its structuring and systematization. Dyna 2014, 81, 158–163. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J. Solar Detoxification; Ilustrada; UNESCO-United Nations Educational, Scientific and Cultural Organization: Paris, Francia, 2003; ISBN 9789231039164. [Google Scholar]
- Wen, J.; Li, X.; Liu, W.; Fang, Y.; Xie, J.; Xu, Y. Photocatalysis fundamentals and surface modification of TiO2 nanomaterials. Chinese J. Catal. 2015, 36, 2049–2070. [Google Scholar] [CrossRef]
- Pichat, P. Photocatalysis and Water Purification; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; ISBN 9783527645404. [Google Scholar]
- Pollema, C.H.; Hendrix, J.L.; Milosavljević, E.B.; Solujić, L.; Nelson, J.H. Photocatalytic oxidation of cyanide to nitrate at TiO2 particles. J. Photochem. Photobiol. A Chem. 1992, 66, 235–244. [Google Scholar] [CrossRef]
- Augugliaro, V.; Gálvez, J.B.; Vázquez, J.C.; López, E.G.; Loddo, V.; Muñoz, M.J.L.; Rodrĺguez, S.M.; Marcĺ, G.; Palmisano, L.; Schiavello, M.; et al. Photocatalytic oxidation of cyanide in aqueous TiO2 suspensions irradiated by sunlight in mild and strong oxidant conditions. Catal. Today 1999, 54, 245–253. [Google Scholar] [CrossRef]
- Karunakaran, C.; Gomathisankar, P.; Manikandan, G. Preparation and characterization of antimicrobial Ce-doped ZnO nanoparticles for photocatalytic detoxification of cyanide. Mater. Chem. Phys. 2010, 123, 585–594. [Google Scholar] [CrossRef]
- Karunakaran, C.; Rajeswari, V.; Gomathisankar, P. Antibacterial and photocatalytic activities of sonochemically prepared ZnO and Ag–ZnO. J. Alloys Compd. 2010, 508, 587–591. [Google Scholar] [CrossRef]
- Karunakaran, C.; Abiramasundari, G.; Gomathisankar, P.; Manikandan, G.; Anandi, V. Preparation and characterization of ZnO–TiO2 nanocomposite for photocatalytic disinfection of bacteria and detoxification of cyanide under visible light. Mater. Res. Bull. 2011, 46, 1586–1592. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Baeissa, E.S. Preparation and characterisation of Pd–TiO2–hydroxyapatite nanoparticles for the photocatalytic degradation of cyanide under visible light. Appl. Catal. A Gen. 2013, 464–465, 218–224. [Google Scholar] [CrossRef]
- Kadi, M.W.; Mohamed, R.M. Enhanced Photocatalytic Activity of ZrO2-SiO2 Nanoparticles by Platinum Doping. Int. J. Photoenergy 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Aazam, E.S.S. Environmental remediation of cyanide solutions by photocatalytic oxidation using Au/CdS nanoparticles. J. Ind. Eng. Chem. 2014, 20, 2870–2875. [Google Scholar] [CrossRef]
- Baeissa, E.S. Photocatalytic removal of cyanide by cobalt metal doped on TiO2-SiO2 nanoparticles by photo-assisted deposition and impregnation methods. J. Ind. Eng. Chem. 2014, 20, 3761–3766. [Google Scholar] [CrossRef]
- Salinas-Guzmán, R.R.; Guzmán-Mar, J.L.; Hinojosa-Reyes, L.; Peralta-Hernández, J.M.; Hernández-Ramírez, A. Enhancement of cyanide photocatalytic degradation using sol–gel ZnO sensitized with cobalt phthalocyanine. J. Sol-Gel Sci. Technol. 2010, 54, 1–7. [Google Scholar] [CrossRef]
- Van Grieken, R.; Aguado, J.; López-Muñoz, M.J.; Marugán, J. Synthesis of size-controlled silica-supported TiO2 photocatalysts. J. Photochem. Photobiol. A Chem. 2002, 148, 315–322. [Google Scholar] [CrossRef]
- Rader, W.S.; Solujic, L.; Milosavljevic, E.B.; Hendrix, J.L.; Nelson, J.H. Photocatalytic detoxification of cyanide and metal cyano-species from precious-metal mill effluents. Environ. Pollut. 1995, 90, 331–334. [Google Scholar] [CrossRef]
- Durán, A.; Monteagudo, J.M.; San Martín, I.; García-Peña, F.; Coca, P. Photocatalytic degradation of pollutants from Elcogas IGCC power station effluents. J. Hazard. Mater. 2007, 144, 132–139. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Durán, A.; Guerra, J.; García-Peña, F.; Coca, P. Solar TiO2-assisted photocatalytic degradation of IGCC power station effluents using a Fresnel lens. Chemosphere 2008, 71, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Addamo, M.; Augugliaro, V.; Coluccia, S.; Faga, M.; Garcia-Lopez, E.; Loddo, V.; Marci, G.; Martra, G.; Palmisano, L. Photocatalytic oxidation of acetonitrile in gas–solid and liquid–solid regimes. J. Catal. 2005, 235, 209–220. [Google Scholar] [CrossRef]
- Aguado, J.; van Grieken, R.; López-Muñoz, M.; Marugán, J. Removal of cyanides in wastewater by supported TiO2-based photocatalysts. Catal. Today 2002, 75, 95–102. [Google Scholar] [CrossRef]
- Osathaphan, K.; Chucherdwatanasak, B.; Rachdawong, P.; Sharma, V.K. Photocatalytic oxidation of cyanide in aqueous titanium dioxide suspensions: Effect of ethylenediaminetetraacetate. Sol. Energy 2008, 82, 1031–1036. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, S.G.; Lee, H.-I. Photocatalytic production of hydrogen from aqueous solution containing CN−as a hole scavenger. Appl. Catal. A Gen. 2001, 207, 173–181. [Google Scholar] [CrossRef]
- Hernández-Alonso, M.D.; Coronado, J.M.; Javier Maira, A.; Soria, J.; Loddo, V.; Augugliaro, V. Ozone enhanced activity of aqueous titanium dioxide suspensions for photocatalytic oxidation of free cyanide ions. Appl. Catal. B Environ. 2002, 39, 257–267. [Google Scholar] [CrossRef]
- Szpyrkowicz, L.; Ziliograndi, F.; Kaul, S.; Rigonistern, S. Electrochemical treatment of copper cyanide wastewaters using stainless steel electrodes. Water Sci. Technol. 1998, 38, 261–268. [Google Scholar] [CrossRef]
- Shinde, S.S.; Bhosale, C.H.; Rajpure, K.Y. Photocatalytic activity of sea water using TiO2 catalyst under solar light. J. Photochem. Photobiol. B Biol. 2011, 103, 111–117. [Google Scholar] [CrossRef]
- Parga, J.R.; Vázquez, V.; Casillas, H.M.; Valenzuela, J.L.; Vázquez, V.; Casillas, H.M.; Valenzuela, J.L. Cyanide Detoxification of Mining Wastewaters with TiO2 Nanoparticles and Its Recovery by Electrocoagulation. Chem. Eng. Technol. 2009, 32, 1901–1908. [Google Scholar] [CrossRef]
- Pedraza-Avella, J.A.; Acevedo-Peña, P.; Pedraza-Rosas, J.E. Photocatalytic oxidation of cyanide on TiO2: An electrochemical approach. Catal. Today 2008, 133–135, 611–618. [Google Scholar] [CrossRef]
- Durán, A.; Monteagudo, J.M.; San Martín, I.; Aguirre, M. Decontamination of industrial cyanide-containing water in a solar CPC pilot plant. Sol. Energy 2010, 84, 1193–1200. [Google Scholar] [CrossRef]
- Durán, A.; Monteagudo, J.M.; San Martín, I.; Sánchez-Romero, R. Photocatalytic treatment of IGCC power station effluents in a UV-pilot plant. J. Hazard. Mater. 2009, 167, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Mudliar, R.; Umare, S.S.; Ramteke, D.S.; Wate, S.R. Energy efficient--advanced oxidation process for treatment of cyanide containing automobile industry wastewater. J. Hazard. Mater. 2009, 164, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, M.D.; Coronado, J.M.; Soria, J.; Conesa, J.C.; Loddo, V.; Addamo, M.; Augugliaro, V. EPR and kinetic investigation of free cyanide oxidation by photocatalysis and ozonation. Res. Chem. Intermed. 2007, 33, 205–224. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Vidal, A.; Richter, C. Photocatalysis with solar energy at a pilot-plant scale: an overview. Appl. Catal. B Environ. 2002, 37, 1–15. [Google Scholar] [CrossRef]
- Chiang, K.; Amal, R.; Tran, T. Photocatalytic oxidation of cyanide: kinetic and mechanistic studies. J. Mol. Catal. A Chem. 2003, 193, 285–297. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, H.-I. Effect of surface hydroxyl groups of pure TiO2 and modified TiO2 on the photocatalytic oxidation of aqueous cyanide. Korean J. Chem. Eng. 2004, 21, 116–122. [Google Scholar] [CrossRef]
- Marugán, J.; van Grieken, R.; Cassano, A.E.; Alfano, O.M. Quantum efficiency of cyanide photooxidation with TiO2/SiO2 catalysts: Multivariate analysis by experimental design. Catal. Today 2007, 129, 143–151. [Google Scholar] [CrossRef]
- Marugan, J.; Vangrieken, R.; Cassano, A.; Alfano, O. Intrinsic kinetic modeling with explicit radiation absorption effects of the photocatalytic oxidation of cyanide with TiO2 and silica-supported TiO2 suspensions. Appl. Catal. B Environ. 2008, 85, 48–60. [Google Scholar] [CrossRef]
- Winkelmann, K.; Sharma, V.K.; Lin, Y.; Shreve, K.A.; Winkelmann, C.; Hoisington, L.J.; Yngard, R.A. Reduction of ferrate(VI) and oxidation of cyanate in a Fe(VI)–TiO2–UV–NCO− system. Chemosphere 2008, 72, 1694–1699. [Google Scholar] [CrossRef]
- Marugán, J.; van Grieken, R.; Cassano, A.E.; Alfano, O.M. Scaling-up of slurry reactors for the photocatalytic oxidation of cyanide with TiO2 and silica-supported TiO2 suspensions. Catal. Today 2009, 144, 87–93. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Mkhalid, I.A. Visible light photocatalytic degradation of cyanide using Au–TiO2/multi-walled carbon nanotube nanocomposites. J. Ind. Eng. Chem. 2015, 22, 390–395. [Google Scholar] [CrossRef]
- Pala, A.; Politi, R.R.; Kurşun, G.; Erol, M.; Bakal, F.; Öner, G.; Çelik, E. Photocatalytic degradation of cyanide in wastewater using new generated nano-thin film photocatalyst. Surf. Coatings Technol. 2015, 271, 207–216. [Google Scholar] [CrossRef]
- Baeissa, E.S. Synthesis and characterization of sulfur-titanium dioxide nanocomposites for photocatalytic oxidation of cyanide using visible light irradiation. Chinese J. Catal. 2015, 36, 698–704. [Google Scholar] [CrossRef]
- Barakat, M.A. Ag-Sm2O3 nanocomposite for environmental remediation of cyanide from aqueous solution. J. Taiwan Inst. Chem. Eng. 2016, 65, 134–139. [Google Scholar] [CrossRef]
- Kadi, M.W.; Hameed, A.; Mohamed, R.M.; Ismail, I.M.I.; Alangari, Y.; Cheng, H.-M. The effect of Pt nanoparticles distribution on the removal of cyanide by TiO2 coated Al-MCM-41 in blue light exposure. Arab. J. Chem. 2016. [Google Scholar] [CrossRef]
- Maya-Treviño, M.L.; Guzmán-Mar, J.L.; Hinojosa-Reyes, L.; Hernández-Ramírez, A. Synthesis and photocatalytic activity of ZnO-CuPc for methylene blue and potassium cyanide degradation. Mater. Sci. Semicond. Process. 2018, 77, 74–82. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Zhao, S.; Liu, Z.; Chang, H.; Zhao, X. Photocatalytic oxidation of free cyanide over graphitic carbon nitride nanosheets under visible light. Chem. Eng. J. 2019, 369, 553–562. [Google Scholar] [CrossRef]
- Núñez-Salas, R.E.; Hernández-Ramírez, A.; Hinojosa-Reyes, L.; Guzmán-Mar, J.L.; Villanueva-Rodríguez, M.; de Lourdes Maya-Treviño, M. Cyanide degradation in aqueous solution by heterogeneous photocatalysis using boron-doped zinc oxide. Catal. Today 2019, 328, 202–209. [Google Scholar] [CrossRef]
- Weinstein, J.A. Inorganic Photochemistry. In Applied Photochemistry; Springer: Dordrecht, The Netherlands, 2013; pp. 105–148. [Google Scholar]
- Kohtani, S.; Yoshioka, E.; Saito, K.; Kudo, A.; Miyabe, H. Photocatalytic hydrogenation of acetophenone derivatives and diaryl ketones on polycrystalline titanium dioxide. Catal. Commun. 2010, 11, 1049–1053. [Google Scholar] [CrossRef]
- Guzmán-Mar, J.L.; Villanueva-Rodríguez, M.; Hinojosa-Reyes, L. Application of Semiconductor Photocatalytic Materials for the Removal of Inorganic Compounds from Wastewater. In Photocatalytic Semiconductors; Hernández-Ramírez, A., Medina-Ramírez, I., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 229–254. ISBN 978-3-319-10998-5. [Google Scholar]
- Rodríguez, J.; Candal, R.J.; Solís, J.; Estrada, W.; Blesa, M.A. El fotocatalizador: síntesis, propiedades y limitaciones. In Microbiologia del agua. Conceptos Básicos; Argentina, 2005; Available online: http://www.psa.es/es/projects/solarsafewater/documents/curso/dia_14/9.%20Juan%20Rodriguez.pdf (accessed on 6 March 2019).
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Parga, J.R.; Shukla, S.S.; Carrillo-Pedroza, F.R. Destruction of cyanide waste solutions using chlorine dioxide, ozone and titania sol. Waste Manag. 2003, 23, 183–191. [Google Scholar] [CrossRef]
- Bozzi, A.; Guasaquillo, I.; Kiwi, J. Accelerated removal of cyanides from industrial effluents by supported TiO2 photo-catalysts. Appl. Catal. B Environ. 2004, 51, 203–211. [Google Scholar] [CrossRef]
- Barakat, M. Removal of toxic cyanide and Cu(II) Ions from water by illuminated TiO2 catalyst. Appl. Catal. B Environ. 2004, 53, 13–20. [Google Scholar] [CrossRef]
- Van Grieken, R.; Aguado, J.; López-Muñoz, M.-J.; Marugán, J. Photocatalytic gold recovery from spent cyanide plating bath solutions. Gold Bull. 2005, 38, 180–187. [Google Scholar] [CrossRef]
- Van Grieken, R.; Aguado, J.; López-Muñoz, M.-J.; Marugán, J. Photocatalytic degradation of iron–cyanocomplexes by TiO2 based catalysts. Appl. Catal. B Environ. 2005, 55, 201–211. [Google Scholar] [CrossRef]
- López-Muñoz, M.-J.; Van Grieken, R.; Aguado, J.; Marugán, J. Role of the support on the activity of silica-supported TiO2 photocatalysts: Structure of the TiO2/SBA-15 photocatalysts. Catal. Today 2005, 101, 307–314. [Google Scholar] [CrossRef]
- Osathaphan, K.; Ruengruehan, K.; Yngard, R.A.; Sharma, V.K. Photocatalytic Degradation of Ni(II)-Cyano and Co(III)-Cyano Complexes. Water Air Soil Pollut. 2013, 224, 1647. [Google Scholar] [CrossRef]
- Harraz, F.A.; Abdel-Salam, O.E.; Mostafa, A.A.; Mohamed, R.M.; Hanafy, M. Rapid synthesis of titania–silica nanoparticles photocatalyst by a modified sol–gel method for cyanide degradation and heavy metals removal. J. Alloys Compd. 2013, 551, 1–7. [Google Scholar] [CrossRef]
- Betancourt-Buitrago, L.A.; Ossa-Echeverry, O.E.; Rodriguez-Vallejo, J.C.; Barraza, J.M.; Marriaga, N.; Machuca-Martínez, F. Anoxic photocatalytic treatment of synthetic mining wastewater using TiO2 and scavengers for complexed cyanide recovery. Photochem. Photobiol. Sci. 2018, 18, 853–862. [Google Scholar] [CrossRef]
- Devia-Orjuela, J.S.; Betancourt-Buitrago, L.A.; Machuca-martinez, F. CFD modeling of a UV-A LED baffled flat-plate photoreactor for environment applications: a mining wastewater case. Environ. Sci. Pollut. Res. 2019, 26, 4510–4520. [Google Scholar] [CrossRef]
- Vilhunen, S.; Sillanpää, M. Recent developments in photochemical and chemical AOPs in water treatment: A mini-review. Rev. Environ. Sci. Biotechnol. 2010, 9, 323–330. [Google Scholar] [CrossRef]
- Petrik, N.G.; Kimmel, G.A. Probing the photochemistry of chemisorbed oxygen on TiO2 (110) with Kr and other co-adsorbates. Phys. Chem. Chem. Phys. 2014, 16, 2338–2346. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Hong, F.; He, Z.; Cai, Q.; Chen, J. AgIO3-modified AgI/TiO2 composites for photocatalytic degradation of p-chlorophenol under visible light irradiation. J. Colloid Interface Sci. 2012, 378, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, J.; Kamiya, Y. Combining the Photocatalyst Pt/TiO2 and the Nonphotocatalyst SnPd/Al2O3 for Effective Photocatalytic Purification of Groundwater Polluted with Nitrate. ACS Catal. 2014, 4, 2207–2215. [Google Scholar] [CrossRef]
- Chen, G.; Sun, M.; Wei, Q.; Ma, Z.; Du, B. Efficient photocatalytic reduction of aqueous Cr(VI) over CaSb2O5(OH)2 nanocrystals under UV light illumination. Appl. Catal. B Environ. 2012, 125, 282–287. [Google Scholar] [CrossRef]
- Zhang, L.S.; Wong, K.H.; Yip, H.Y.; Hu, C.; Yu, J.C.; Chan, C.Y.; Wong, P.K. Effective photocatalytic disinfection of E. coli K-12 using AgBr-Ag-Bi2WO6 nanojunction system irradiated by visible light: The role of diffusing hydroxyl radicals. Environ. Sci. Technol. 2010, 44, 1392–1398. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, N.; Tang, Z.R.; Xu, Y.J. Synthesis of one-dimensional CdS@TiO2 core-shell nanocomposites photocatalyst for selective redox: The dual role of TiO2 shell. ACS Appl. Mater. Interfaces 2012, 4, 6378–6385. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.; Lin, H.; Chen, S.; Fu, X. In situ preparation of novel p–n junction photocatalyst BiOI/(BiO)2CO3 with enhanced visible light photocatalytic activity. J. Hazard. Mater. 2012, 239–240, 316–324. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, S.; Hong, Z.; Li, X.; Xu, G.; Lin, Y.; Chen, G. Enhanced photoelectrochemical activity of a hierarchical-ordered TiO2 mesocrystal and its sensing application on a carbon nanohorn support scaffold. Anal. Chem. 2014, 86, 6418–6424. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Kontos, A.G.; Moustakas, N.G.; Faria, J.L.; Doña-Rodríguez, J.M.; Falaras, P.; Silva, A.M.T. TiO2, surface modified TiO2 and graphene oxide-TiO2 photocatalysts for degradation of water pollutants under near-UV/Vis and visible light. Chem. Eng. J. 2013, 224, 17–23. [Google Scholar] [CrossRef]
- Yin, M.; Li, Z.; Kou, J.; Zou, Z. Mechanism investigation of visible light-induced degradation in a heterogeneous TiO2/eosin Y/rhodamine B system. Environ. Sci. Technol. 2009, 43, 8361–8366. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, S.; Wang, K.; Lou, L. Role of primary active species and TiO2 surface characteristic in UV-illuminated photodegradation of Acid Orange 7. J. Photochem. Photobiol. A Chem. 2005, 172, 47–54. [Google Scholar] [CrossRef]
- Chen, M.; Chu, W. Degradation of antibiotic norfloxacin in aqueous solution by visible-light-mediated C-TiO2 photocatalysis. J. Hazard. Mater. 2012, 219–220, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Pandikumar, A.; Ramaraj, R. Titanium dioxide-gold nanocomposite materials embedded in silicate sol-gel film catalyst for simultaneous photodegradation of hexavalent chromium and methylene blue. J. Hazard. Mater. 2012, 203–204, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Krall, A.; Zhao, H.; Zhang, Q.; Li, Y. Ultrasonic spray pyrolysis synthesis of Ag/TiO2 nanocomposite photocatalysts for simultaneous H2 production and CO2 reduction. Int. J. Hydrogen Energy 2012, 37, 9967–9976. [Google Scholar] [CrossRef]
- Sheng, F.; Zhu, X.; Wang, W.; Bai, H.; Liu, J.; Wang, P.; Zhang, R.; Han, L.; Mu, J. Synthesis of novel polyoxometalate K6ZrW11O39Sn12H2O and photocatalytic degradation aqueous azo dye solutions with solar irradiation. J. Mol. Catal. A Chem. 2014, 393, 232–239. [Google Scholar] [CrossRef]
- Tian, L.; Ye, L.; Liu, J.; Zan, L. Solvothermal synthesis of CNTs–WO3 hybrid nanostructures with high photocatalytic activity under visible light. Catal. Commun. 2012, 17, 99–103. [Google Scholar] [CrossRef]
- Villa, K.; Murcia-López, S.; Andreu, T.; Morante, J.R. Mesoporous WO3 photocatalyst for the partial oxidation of methane to methanol using electron scavengers. Appl. Catal. B Environ. 2015, 163, 150–155. [Google Scholar] [CrossRef]
- Kowalska, E.; Rau, S. Photoreactors for Wastewater Treatment: A Review. Recent Patents Eng. 2010, 4, 242–266. [Google Scholar] [CrossRef]
- Yeh, N.; Yeh, P.; Shih, N.; Byadgi, O.; Cheng, T.C. Applications of light-emitting diodes in researches conducted in aquatic environment. Renew. Sustain. Energy Rev. 2014, 32, 611–618. [Google Scholar] [CrossRef]
- Jo, W.; Tayade, R.J. New Generation Energy-Efficient Light Source for Photocatalysis: LEDs for Environmental Applications. Ind. Eng. Chem. Res. 2014, 53, 2073–2084. [Google Scholar] [CrossRef]
- Izadifard, M.; Achari, G.; Langford, C. Application of Photocatalysts and LED Light Sources in Drinking Water Treatment. Catalysts 2013, 3, 726–743. [Google Scholar] [CrossRef]
- Chatterley, C. UV-LED irradiation technology for point-of-use water disinfection in developing communities. Master’s Thesis, University of Colorado, Boulder, CO, USA, 2009. [Google Scholar]
- Vilhunen, S.; Puton, J.; Virkutyte, J.; Sillanpää, M. Efficiency of hydroxyl radical formation and phenol decomposition using UV light emitting diodes and H2O2. Environ. Technol. 2011, 32, 865–872. [Google Scholar] [CrossRef]
- Jamali, A.; Vanraes, R.; Hanselaer, P.; Van Gerven, T. A batch LED reactor for the photocatalytic degradation of phenol. Chem. Eng. Process. Process Intensif. 2013, 71, 43–50. [Google Scholar] [CrossRef]
- Malkhasian, A.Y.S.; Izadifard, M.; Achari, G.; Langford, C.H. Photocatalytic degradation of agricultural antibiotics using a UV-LED light source. J. Environ. Sci. Heal. Part B Pestic. Food Contam. Agric. Wastes 2014, 49, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi Kakroudi, M.; Kazemi, F.; Kaboudin, B. Highly efficient photodeoximation under green and blue LEDs catalyzed by mesoporous CN codoped nano TiO2. J. Mol. Catal. A Chem. 2014, 392, 112–119. [Google Scholar] [CrossRef]
- Liu, X.; Pan, L.; Lv, T.; Sun, Z. CdS sensitized TiO2 film for photocatalytic reduction of Cr(VI) by microwave-assisted chemical bath deposition method. J. Alloys Compd. 2014, 583, 390–395. [Google Scholar] [CrossRef]
- Tokode, O.; Prabhu, R.; Lawton, L. a.; Robertson, P.K.J. Mathematical modelling of quantum yield enhancements of methyl orange photooxidation in aqueous TiO2 suspensions under controlled periodic UV LED illumination. Appl. Catal. B Environ. 2014, 156–157, 398–403. [Google Scholar] [CrossRef]
- Zand, Z.; Kazemi, F.; Hosseini, S. Development of chemoselective photoreduction of nitro compounds under solar light and blue LED irradiation. Tetrahedron Lett. 2014, 55, 338–341. [Google Scholar] [CrossRef]
- Eskandari, P.; Kazemi, F.; Zand, Z. Photocatalytic reduction of aromatic nitro compounds using CdS nanostructure under blue LED irradiation. J. Photochem. Photobiol. A Chem. 2014, 274, 7–12. [Google Scholar] [CrossRef]
- Jenny, R.M.; Simmons, O.D.; Shatalov, M.; Ducoste, J.J. Modeling a continuous flow ultraviolet Light Emitting Diode reactor using computational fluid dynamics. Chem. Eng. Sci. 2014, 116, 524–535. [Google Scholar] [CrossRef]
- Tokode, O.; Prabhu, R.; Lawton, L.A.; Robertson, P.K.J. The effect of pH on the photonic efficiency of the destruction of methyl orange under controlled periodic illumination with UV-LED sources. Chem. Eng. J. 2014, 246, 337–342. [Google Scholar] [CrossRef]
- Doss, N.; Bernhardt, P.; Romero, T.; Masson, R.; Keller, V.; Keller, N. Photocatalytic degradation of butanone (methylethylketone) in a small-size TiO2/β-SiC alveolar foam LED reactor. Appl. Catal. B Environ. 2014, 154–155, 301–308. [Google Scholar] [CrossRef]
- Dai, K.; Lu, L.; Liang, C.; Dai, J.; Zhu, G.; Liu, Z.; Liu, Q.; Zhang, Y. Graphene oxide modified ZnO nanorods hybrid with high reusable photocatalytic activity under UV-LED irradiation. Mater. Chem. Phys. 2014, 143, 1410–1416. [Google Scholar]
- Hossaini, H.; Moussavi, G.; Farrokhi, M. The investigation of the LED-activated FeFNS-TiO2 nanocatalyst for photocatalytic degradation and mineralization of organophosphate pesticides in water. Water Res. 2014, 59, 130–144. [Google Scholar] [CrossRef]
- Marugán, J.; van Grieken, R.; Pablos, C.; Satuf, M.L.; Cassano, A.E.; Alfano, O.M. Kinetic modelling of Escherichia coli inactivation in a photocatalytic wall reactor. Catal. Today 2015, 240, 9–15. [Google Scholar] [CrossRef]
- Rasoulifard, M.H.; Fazli, M.; Eskandarian, M.R. Performance of the light-emitting-diodes in a continuous photoreactor for degradation of Direct Red 23 using UV-LED/S2O82− process. J. Ind. Eng. Chem. 2015, 24, 121–126. [Google Scholar] [CrossRef]
- Levchuk, I.; Rueda-Márquez, J.J.; Suihkonen, S.; Manzano, M. a.; Sillanpää, M. Application of UVA-LED based photocatalysis for plywood mill wastewater treatment. Sep. Purif. Technol. 2015, 143, 1–5. [Google Scholar] [CrossRef]
- Betancourt-Buitrago, L.A.; Vásquez, C.; Veitia, L.; Ossa-Echeverry, O.; Rodriguez-Vallejo, J.; Barraza-Burgos, J.; Marriaga-Cabrales, N.; Machuca-Martínez, F. An approach to utilize the artificial high power LED UV-A radiation in photoreactors for the degradation of methylene blue. Photochem. Photobiol. Sci. 2017, 16, 79–85. [Google Scholar] [CrossRef]
| Group | Species | Toxicity [20] | Stability Constant (Log Kn) |
|---|---|---|---|
| Free cyanide | CN− | High | n.a. |
| HCN(g) | 9.2 | ||
| Simpler compounds: Easily soluble | NaCN, KCN, Ca(CN)2, Hg(CN)2, Zn(CN)2, CuCN, Ni(CN)2, AgCN | High | n.d. |
| Weak complex (WAD—Weak Acid Dissociable) | Intermediate | 17.9 | |
| n.d. | |||
| 19.6 | |||
| 20.5 | |||
| 30.2 | |||
| 21.6 | |||
| n.a. | |||
| n.a. | |||
| Strong complexes (SAD—Strong Acid Dissociable) | 35.4 | ||
| Low | 43.6 | ||
| 38.3 | |||
| High | 64.0 | ||
| Unstable inorganic | SCN−, CNO− | High | n.d. |
| Aliphatic organic | Acetonitrile, acrylonitrile, adiponitrile, propionitrile | Intermediate | n.d. |
| Year | Substance [C0] | Source of Light | Wavelength | Type of Reactor | Degradation/Reaction Time | Catalyst | Main Findings |
|---|---|---|---|---|---|---|---|
| 1992 | KCN [100 ppm] | 14 W UV Hg Low Pressure | 360 nm | Compact Square batch reactor | 100%/60 min | TiO2 P25 | Achieve total degradation to nitrates and cyanates. They find the CO2 in air bubbling as harmful for the photocatalytic mechanism [33] |
| 1999 | NaCN, NaCNO [3.85 mM] | Solar light | Solar spectrum | CPC pilot scale | 100%/4.1 Einstein accumulated | TiO2 P25 | Total degradation with solar light, but kinetics is only related to accumulated energy [34] |
| 2001 | NaCN [666 ppm CN−] | 450 W, 700 W Hg high-pressure lamp | UV-A | Laboratory Batch | 1.5 mmol/h H2 produced at 70 °C and 700 W | NiO/TiO2 | The process produced hydrogen and cyanate from cyanide as a photocatalytic strategy of remediation [50] |
| 2002 | Free Cyanide, phenol, atrazine, EPTC, dichloroacetic acid, and Cr(VI) among others. | Solar | Solar spectrum | Pilot-scale PSA–Solar platform of Almeria | 100%/N.D. | photo Fenton and photocatalysis applications. | Several experiments applied at a solar pilot plant in Almeria with successful results [60] |
| 2002 | KCN [100 ppm] | 150W Hg medium pressure lamp | >300 nm | Batch cylindrical | 47%/2 h TiO2/SBA-15 | Supported TiO2 on SBA-15 and MCM-41 | Achieved geometry optimization using the support SBA. However, degradation resulted low [43] |
| 2002 | NaCN [100 ppm CN−] | 150W Hg Medium pressure | n.a. | Batch cylindrical | 50%/350 min | TiO2 Sol-gel method on four different support | Achieved a low degradation of free cyanide exploring a novel geometry configuration on the TiO2 distribution [48] |
| 2003 | NaCN [3.85 mM] | n.a. | n.a. | n.a. | 100%/420 min | TiO2 P25 | Although total degradation was achieved, authors argue the photonic efficiency is very low and radical recombination occurred. They propose a very detail degradation kinetic mechanism [61] |
| 2004 | KCN [50 ppm] | 450 W High-pressure Hg lamp | >300 nm | binaural pyrex batch with intern lamp | n.a. | TPA/TiO2, Cs-TPA/TiO2 | They determine the interaction of CN− with holes and electrons photogenerated. The Cs resulted in photocatalytic inhibition [62]. |
| 2005 | CH3CN (gas and liquid) [24 mM] | 500 W Hg medium pressure lamp | 365 nm | Annular photoreactor steady state for liquid and gas phase | 21%/4 g gas phase 35%/5 g liquid phase | TiO2 anatase for gas, and TiO2 P25 for the liquid phase | Photocatalytic activity was low, and free cyanide ions remain in solution [47] |
| 2007 | NH3, HCOOH, CN− from Electric Power Plant wastewater [10 ppm CN−, 1700 HCOOH, 150 ppm NH3] | 150 W Hg lamp | 190–280 nm | Batch cylindrical | 100% CN 90% NH3 100% HCOOH/10 min | TiO2 P25 + H2O2 | Requires addition of H2O2 to enhance photocatalytic degradation [45] |
| 2007 | KCN [45 ppm CN−] | 400 W Hg UV Lamp | >300 nm | Recirculating cylindrical photoreactor | 5%/100 min | Sol-gel TiO2/SiO2 | Apply an optimization methodology to optimize the photonic efficiency of the photoreactor. However, a very low photodegradation was evidenced [63] |
| 2007 | KCN [40 ppm CN−] | 400 W Hg medium pressure lamp | >300 nm | Cylindrical with reflector | 95%/60 min | Three photocatalysts were evaluated: TiO2 P25, DBH TiO2, nanometric TiO2. | Evaluated the photocatalytic degradation with three photocatalysts and with the addition of O3. A good degradation was achieved but the addition of O3 instead O2 resulted in photocatalytic inhibition [59] |
| 2008 | KCN [3.85 nM] | 80 W and 36 W Low-pressure lamp | UV-A | Cylindrical photoreactor | n.a. | TiO2 P25, TiO2/SiO2 | The authors proposed an intrinsic kinetic model of cyanide degradation with an accurate fitting of experimental data. The study was more kinetic than a photocatalytic evaluation [64] |
| 2008 | NaCN, gasification plant wastewater [10 ppm CN−] | Solar light | 200 W/cm2 of solar spectrum concentrated with a Fresnel Lens | Cylindrical photoreactor | 100%/90 min | TiO2 P25. | The evaluated the effect of solar light using a Fresnel lens to concentrate energy. They required the addition of H2O2 to achieve total mineralization of free cyanide [46] |
| 2008 | KCNO, Fe+4 [1 mM CNO−] [1 mM Fe+4] | n.a. | UV-A | Borosilicate glass cylindrical | 80% cyanate degradation/120 min | TIO2 P25 | The process reduced ferrate(VI) and oxidated cyanate in a Fe(VI)-TiO2-UV-NCO− system. However, the role of the TiO2 in the degradation was not specified. The possible reduction-oxidation mechanism for Fe+4 reduction was not clarified [65] |
| 2008 | KCN [100 ppm CN−] | 15 W Hg low-pressure lamp | UV-A | Cylindrical batch | 100%/350 min | TiO2 P25 | The degradation was done using 10.5 mM EDTA as a hole scavenger. Addition of EDTA evidenced an increase in the cyanide oxidation to CNO− [49] |
| 2009 | KCN [30 ppm CN−] | 400 W and 36 W Blacklight lamp | 365 nm | Annular reactor | 100%/120 min | TiO2/SiO2 | They evaluate and compared the scaling-up process from laboratory to pilot plant, using supported TiO2. Total elimination of cyanide was achieved in both systems. Propose a scaling up methodology for photoreactors [66] |
| 2009 | NaCN [400 ppm] | 450 W Halide lamp | UV-A | Cylindrical glass batch | 90%/30 min | TiO2 nanoparticles coupled with an electrocoagulation recovery | It is proposed a recovery technique using electrocoagulation after a typical photocatalytic cyanide degradation. A study of TiO2 reuse was also performed [54] |
| 2010 | KCN [250 ppm] | 8 W Hg lamp | 365 nm | Batch cylindrical | 40%/100 min | Ce-ZnO sonochemical impregnation | Doping relations of 2% Ce-ZnO calcined at 500 °C. This photocatalyst works better in the visible region. There is an excess of light applied to the system, which could mix the photocatalysis with the photolysis effect on CN− degradation [35] |
| 2010 | KCN [11 mM CN–] | 8 W Hg Lamp | 365 nm | Batch cylindrical Reactor | 86%/90 min | Ag-ZnO sonochemical impregnation synthesis | Ag-ZnO was found to be three times better than ZnO pure [36] |
| 2011 | KCN [10 mM] | 150 W halide and 8 W Hg UV lamp | 365 nm UV | Annular batch reactor | 16%/150 min | ZnO-TiO2 | Photocatalytic activity was demonstrated but with important radiant field losses in the photoreactor [37] |
| 2013 | KCN [100 ppm] | 150 W fluorescent lamp | 450 nm | Batch cylindrical reactor | 98%/60 min | Pt-TiO2- hydroxyapatite. Prepared by Sonic method. | Hydroxyapatite enhanced the photocatalytic behavior of bare suspended TiO2 [38] |
| 2013 | KCN [100 ppm] | 150 W fluorescent lamp | 450 nm | Batch annular reactor | 100%/20 min | Pt/ZrO2-SiO2 prepared by a photo-assisted deposition method. | Evaluated the effect of catalyst load on the reactor [39] |
| 2014 | KCN [100 ppm] | 150 W fluorescent blue lamp | 450 nm | Batch cylindrical reactor | 100%/360 min 96%/240 min | Co-TiO2-SiO2 prepared by a photo-assisted method and impregnation. | Obtained the best catalyst load obtained at 0.08 g/L and a decreased in the TiO2 band-gap with the total elimination of CN− [41] |
| 2015 | NaCN [30 ppm] | UV-LED | Not specified. UV-A UV-B UV-C | Submerged cylindrical LED photoreactor | 100%/>600 min | TiO2 P25 | Demonstrated the possibility of using LED as a source of UV light in a photocatalytic treatment. The most efficient was UV-C, due to photolytic effect [21] |
| 2015 | KCN [100 ppm] | 500 W Xe bulb lamp | >420nm | Pyrex reaction cell | 100%/60 min | MWCNT/Au-TiO2 | They found carbon nanotubes beneficial for photocatalytic degradation in the presence of oxygen and visible light [67] |
| 2015 | KCN [100 ppm] | 700 W Xenon lamp | n.a. | Pyrex reaction cell | 100%/5 h | CeO2/KLTO | CeO2/KLTO enhanced the photocatalytic activity compared to a photolytic effect at 750W/m2 [68] |
| 2015 | KCN [100 ppm] | 150 W Blue fluorescent lamp | >400 nm | Horizontal cylinder annular batch reactor | 100%/30 min | S-TiO2 | Photocatalytic activity resulted enhanced with the addition of S, being 0.3 wt %S-TiO2 the most efficient with visible light [69] |
| 2016 | KCN [150 ppm] | 150 W Blue fluorescent lamp | >400 nm | Pyrex cell reactor | 100%/60 min | Ag-Sm2O3 | The Ag was beneficial for the photocatalytic activity by 90% more than bare Sm2O3. The catalyst is useful up to 5 times cycles [70] |
| 2016 | CN− [100 ppm] | 150 W Blue fluorescent lamp | n.a. | Pyrex glass cylindrical | 100%/70 min | Pt/Ti-Al-MCM-41 | The Pt addition to Ti-Al-MCM41 resulted in 10 times more efficient the suspended TiO2 photocatalytic activity [71] |
| 2018 | KCN [30 ppm] | 25 W metal halide lamp, and UV Lamp | 365 nm and 400–700 nm | Pyrex glass cylindrical | 100%/350 min | ZnO-CuPc | 0.5wt%Zn-CuPc enhanced cyanide degradation. However, the TiO2 P25 still showed faster kinetic of degradation [72] |
| 2019 | NaCN [0.18 mM CN−] | 300 W Xe lamp | >400 nm | Quartz batch reactor | 90%/150 min | g-C3N4−Nanosheets | Nanotubes exhibited good photocatalytic activity, but it was the dissolved oxygen played the most important role in the oxidation of cyanide in visible light [73] |
| 2019 | KCN [10 ppm] | Xe lamp | 400–800 nm | Pyrex glass cylindrical | 89%/120 min | B-ZnO | B-ZnO enhanced photocatalytic activity compared to bare ZnO with visible light at low cyanide concentrations [74] |
| Year | Matrix | Light Source | Wavelength | Type of Photoreactor | Removal/Reaction Time | Catalyst | Main Finding |
|---|---|---|---|---|---|---|---|
| 1995 | Real mining wastewater Cu(CN)32– [22 mM] Zn(CN)42– [300 mM] Fe(CN)64– [5.2 mM] Fe [29 mM] Hg [11 mM] As [16 mM] | Solar light | Solar spectrum | Dish PVC | 99% metal removals/17 days | TiO2 P25 | All metal was removed with the formation of metal-hydroxides and nitrate [44] |
| 2002 | Fe(CN)63− [0.64 mM] | 150W Hg high pressure lamp | >300 nm | Pyrex batch photoreactor | 50%/350 min for SBA-15/TiO2 | TiO2 MCA-41, SBA-15 | The photocatalytic activity was evaluated using two different support for TiO2. The porous SBA-15 resulted in better degradation of Fe(CN)63− but also for the free cyanide mineralization [48]. |
| 2003 | Fe(CN)63− [1 mM] | 4W Hg low mercury lamp and solar light | >300 nm | Cylindrical batch | 100%/1.5 h solar radiation, 77%/6 h UV Lamp | TiO2 sol-gel | TiO2 resulted in a better way to destroy Fe(CN)63−, however resulting wastewater was rich in cyanate and incomplete oxidation was observed. Solar light exhibited better degradation rates [80]. |
| 2004 | CuCN [90 ppm CN–] | 400 and 700 W halide lamp medium pressure Hg | UV | Batch cylindrical reactor | 100%/180 min | TiO2 in Raschig rings support | Evaluated four different methods and the hydrothermal was the best [81]. |
| 2004 | NaCN, Cu(CN)32– [1 mM NaCN], [10 mM Cu(CN)32–] | 100 W high pressure Hg lamp | 228–420 nm | Batch annular reactor bench scale. | 100%/150 min | TiO2 P25 | The ratio Cu:CN influences photocatalytic degradation. A 10:1 ratio was the best for the process [82] |
| 2005 | AuCN2– [75 mg/L AuCN2−] | 150 W medium pressure Hg lamp | 365 nm | Beaker | 86%/240 min | TiO2/L | The recovery of free cyanide is made adding methanol as •OH acceptor. Thus, oxidation of CN– to CNO– is avoided. The cyano-complex AuCN2− is the electron acceptor and Au0 is deposited on the TiO2 particles [83] |
| 2005 | [Fe(CN)6]3– and [Fe(CN)6]4– [100 ppm CN– equivalent] | 150 W Hg medium pressure | >320 nm | Beaker | 70%/240 min | TiO2 P25, TiO2/SiO2 prepared by sol-gel and hydrothermal method. | The maximum degradation was about 70% of the cyano-complex. It requires additional treatment. Iron complexes contaminated the semiconductor [84] |
| 2005 | KCN, K3(Fe(CN)6), KAu(CN)2 [3.85 mM KCN; 0.64 mM K3(Fe(CN)6); 0.38 mM KAu(CN)2] | 150 W Hg medium pressure | 365 nm | Beaker | n.d. | TiO2/GrSiO2, TiO2/SBA-15. | Different methods of support were evaluated, 60% of TiO2/SBA-15 performed better for iron-complex degradation [85] |
| 2008 | CNO− [0.5 mM] Fe(IV) [1 mM] | Spectro line UV-A lamp | 365 nm | Beaker | 80%/120 min | TiO2 P25 Degussa | There is an enhancement in the cyanate degradation related to the presence of ferrate [65] |
| 2009 | Real Wastewater from Energy Plant | UVA UVC | 200–280; 320–400 nm | Pilot photoreactor | 100%/15 min | FeSO4, H2O2 | Although the study demonstrates the ability of a pilot plant for cyanide degradation, it only is evaluated the degradation of free cyanide and not of its complexes [57] |
| 2013 | KCN, Co(CN)63–, Ni(CN)42– [100 µM] | 15 W Hg low-pressure lamp. | n.d. | Cylindrical borosilicate reactor | Ni:90%/180 min, Co: 30%/180 min | TiO2 P25 suspension | Nickel removal was shown to be achievable by photocatalysis; however, cobalt removal is more challenging [86] |
| 2013 | KCN, Co, Pb, Cr [100 ppm CN–, Co, Pb, Cr] | Blacklight lamp and blue light | 365 nm | Annular photoreactor | 100%/180 min | TiO2/SiO2 sol-gel. | Synthesized photocatalyst could degrade free cyanide and dissolved Co, Pb, Cr. However, the evaluation of metal photo-removal was not done in the presence of cyanide [87] |
| 2018 | Fe(CN)63− [100 ppm] | 30 W UV-LED | 300–400 nm | Mini CPC UVLED photoreactor | 70%/20 min | TiO2 P25 | Using UV-LED at 30W/m2 in a mini CPC resulted better for recovery of cyanide instead remediation [88] |
| 2018 | Fe(CN)63− [100 ppm] | 5W UV-LED | 300–400 nm | UV baffled flat plate reactor | 60%/90 min | TiO2 P25 | Configuration resulted useful for light harvesting, but it is required more UV Power since the complex was not complete degraded [89] |
| Scavengers | Compound |
|---|---|
| Holes (h+) | Glucose [93]; formic acid, sulfuric acid [9,94]; sodium oxalate [95]; ammonium oxalate [96,97]; 4-methylimidozal [98]; EDTA [97,99,100]; KI [92,100,101]; NH4+ [102]; oxalic acid and methylene blue [103] |
| Hydroxyl radical (•OH) | t-butanol [92,96,99]; isopropyl alcohol [97]; methanol [100,104]; ethanol [101]; acetonitrile [101]; KBr [105]; terephthalic acid [106] |
| Electrons on the conduction band (e−) | Fe3+, Cu2+, Ag+ [106,107]; AgNO3 [96]; Cr6+ [95]; KIO3 [102], (S2O8)2− [92] |
| Superoxide radical (O2•−) | Benzoquinone [96,97] |
| Year | Description | Main Findings |
|---|---|---|
| 2013 | Phenol photodegradation using batch UV LED at 375 nm. | It is reported that UV LEDs at 800 mW are 100 times more efficient in comparison with 12 and 16 W fluorescent UV Lamps [114]. |
| 2013 | Drinking water potabilization using UV LED at 365 nm | Natural organic matter and emerging pollutants were removed from drinking water. It is concluded that the photoreactor design with this type of light is more critical than the catalyst load [111]. |
| 2014 | Used in the dyes photodegradation, organic matter of air and water. | Proved the capability of organic matter using this type of light, which is better in term of the photoreactor size, energy consumption [110]. |
| 2014 | Oxytetracycline and 17-α-etinil estradiol as agriculture antibiotic degradation using UV LED light. | Reached a 100% degradation of total organic carbon with cumulative energy of about 12.5 kJ/L [115]. |
| 2014 | Acetonitrile degradation in Green blue and red LED photoreactor with C-N TiO2. | Degradation of about 100% was achieved in 2 h using low power (3 W) LEDs [116]. |
| 2014 | Chromium photoreduction using CdS and TiO2 with white LED photoreactor. | The removal of chromium was about 93% in 240 min of reaction [117]. |
| 2014 | Methyl orange degradation modeling applying Controlled Periodic Illumination in a UV LED photoreactor. | It was found that Langmuir-Hinshelwood kinetics do not describe well the photoreactor operating at Controlled Periodic Illumination. Novel mathematic modeling is required for pulsed photoreactors [118]. |
| 2014 | Selective photocatalytic reduction of nitrobenzene carried out by UV LED light. | The transformation of nitrobenzene to aniline was achieved using ethanol as the electron donor with 100% of conversion [119]. |
| 2014 | Evaluation of nitro-aromatic compounds using CdS as the catalyst. | The photoreactor uses a visible LED to enhance photoreduction of amines using methanol and isopropanol as electron donors (hole scavenger). The conversion was about 90% with a selectivity of about 71% [120]. |
| 2014 | A CFD simulation experimental and validation of a UV LED photoreactor for Escherichia coli disinfection. | The CFD established the best amount of irradiation, flowrate and photoreactor dimension in which best photo absorption is achieved for E. coli disinfection [121]. |
| 2014 | Methyl orange degradation under Controlled Periodic Illumination with a UV LED. | The Controlled Periodic Illumination demonstrated being more critical in the photonic efficiency when the ON-OFF period is closer to the characteristic time of the reaction. Also proposes photo-reductive degradation instead of a photooxidation mechanism [122]. |
| 2014 | Methyl ketone degradation using UV-vis LED with supported TiO2 in alveolar foam. | The removal was 100% of methyl ketone in 600 min of reaction using 56 LEDs [123]. |
| 2014 | Photoreactor using graphene oxide ZnO for methylene blue degradation. | Degradation of 100% of Methylene blue is achieved in 150 min using UV-A LEDs. Graphene oxide resulted in photocatalytic degradation enhancement than Degussa P25 [124]. |
| 2014 | Evaluation of FeFNS-TiO2 activated by LED in pesticides mineralization. | Degradation of 90% achieved in 100 min of reaction [125] |
| 2015 | E. coli disinfection modeling in a LED photoreactor | Bacteria deactivation achieved in 120 min using TiO2 in an annular LED UV-A photoreactor [126]. |
| 2015 | Direct Red 23 degradation in a continuous UV LED photoreactor assisted with S2O82− | Complete oxidation of Direct Red 23 is done in homogeneous photocatalysis and 72 UV-LED units [127]. |
| 2015 | Uses a photoreactor with UV-A LED for phenol and plywood mill wastewater treatment. | Demonstrated a photocatalytic degradation of phenol about >90% in 13 min and total removal of tannic acid in plywood mill wastewater in 43 min [128]. |
| 2015 | Free cyanide degradation by the oxidative pathway in synthetic wastewater. | Demonstrated the free cyanide degradation in more than 10 h, using LEDs at UVA, UVB, and UVC. The last one was the most effective in photooxidation [21]. |
| 2017 | Methylene blue degradation in a mini-CPC photoreactor. | Evaluated two systems in a coupled mini CPC and a traditional beaker with external UV-A LED illumination. Demonstrated the capability of the mini CPC in harvesting LED Light in the degradation [129]. |
| 2018 | CFD simulation to enhance LED light utilization and evaluation in iron cyano-metalic complexes. | Demonstrated the utilization of a baffled plat plate photoreactor is useful for UV-LED light harvesting [89]. |
| 2018 | Iron cyanocomplexes degraded in anoxic conditions using a mini-CPC UV LED photoreactor | Achieved the photoreduction of iron and free cyanide liberation as a strategy of recovery instead remediation for this iron cyanocomplex [88]. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betancourt-Buitrago, L.A.; Hernandez-Ramirez, A.; Colina-Marquez, J.A.; Bustillo-Lecompte, C.F.; Rehmann, L.; Machuca-Martinez, F. Recent Developments in the Photocatalytic Treatment of Cyanide Wastewater: An Approach to Remediation and Recovery of Metals. Processes 2019, 7, 225. https://doi.org/10.3390/pr7040225
Betancourt-Buitrago LA, Hernandez-Ramirez A, Colina-Marquez JA, Bustillo-Lecompte CF, Rehmann L, Machuca-Martinez F. Recent Developments in the Photocatalytic Treatment of Cyanide Wastewater: An Approach to Remediation and Recovery of Metals. Processes. 2019; 7(4):225. https://doi.org/10.3390/pr7040225
Chicago/Turabian StyleBetancourt-Buitrago, Luis Andrés, Aracely Hernandez-Ramirez, Jose Angel Colina-Marquez, Ciro Fernando Bustillo-Lecompte, Lars Rehmann, and Fiderman Machuca-Martinez. 2019. "Recent Developments in the Photocatalytic Treatment of Cyanide Wastewater: An Approach to Remediation and Recovery of Metals" Processes 7, no. 4: 225. https://doi.org/10.3390/pr7040225
APA StyleBetancourt-Buitrago, L. A., Hernandez-Ramirez, A., Colina-Marquez, J. A., Bustillo-Lecompte, C. F., Rehmann, L., & Machuca-Martinez, F. (2019). Recent Developments in the Photocatalytic Treatment of Cyanide Wastewater: An Approach to Remediation and Recovery of Metals. Processes, 7(4), 225. https://doi.org/10.3390/pr7040225









