Abstract
Pharmaceuticals are considered among the group of emerging contaminants. Paracetamol is a moderate painkiller, which has been detected in ground and surface water. Photodegradation of paracetamol at a wavelength of radiation of 254 nm with TiO2 nanotubes was studied by UV-spectroscopy, HPLC and measurement of the potential zeta in dependence of the solution pH. The efficiency of the photodegradation of paracetamol (20 mg L−1) was 99% after 100 min exposure. Application of the Langmuir-Hinshelwood equation allowed the evaluation of the rate constant. Non-organic by-products were detected under the conditions of the chromatographic analysis. The photoreaction was faster at pH 6.5, a value at which adsorption was favored, leading to higher efficiency.
1. Introduction
The rapid development of modern industry and society occurs hand in hand with the increasing generation of residual liquids that contain highly toxic compounds with limited biodegradability. The high usage of chemical products such as dyes, pesticides and pharmaceuticals, which have recently been called “emerging contaminants”, causes a growing problem because of their concentration and accumulation in the environment, particularly in aqueous effluents [1,2,3]. A type of water contaminants that is becoming more common are pharmaceuticals. They are mainly attributed to hospital and therapeutic waste, personal care products, and the pharmaceutical industry [4,5]. The presence of these products in drinking water is a public health problem because little is known about the potentially long-term toxic health effects that are associated with the ingestion of mixtures of these compounds in drinking water, even at very low concentrations [6,7,8].
Innovative treatments, such as Advanced Oxidation Processes (AOPs), are effective in drastically reducing the concentrations of pharmaceutical compounds in effluents. Generally, these processes are based on physicochemical methods that cause internal changes in the chemical structures of the contaminants to make them inert and/or decrease their toxicity. The concept of AOPs was initially established by Halling et al. [9], who defined them as processes that involve the generation and use of potential transitory species, especially the hydroxyl radical (HO•) because it is able to attack almost all organic compounds and can react up to 1012 times faster than other oxidants because of its very high oxidation potential of 2.80 eV.
Laboratory-scale reports and studies of treatment plants have found that paracetamol has no or very little biodegradability [10,11]. However, when it is subjected to a tertiary treatment, such as chloration, it produces highly toxic reaction by-products, such as 1,4-benzoquinone and N-acetyl-p-benzoquinone imine [12,13]. Thus, there is a need to investigate highly efficient processes, such as AOPs, that have been recently used to degrade paracetamol, including ozonation, electrocatalysis, photolysis, electrolysis, sonolysis, photo-Fenton oxidation [14,15,16,17,18], and also heterogeneous photocatalysis using nanomaterials of titanium oxide (TiO2) as photocatalysts [19,20].
It is particularly important to develop highly effective alternative methodologies that are harmless to the environment, such as heterogeneous photocatalysis, which employs nanocatalysts that are usually composed of TiO2 because of its low toxicity, low cost and high performance [21,22]. The photocatalytic activity of nanocatalysts is governed by many properties, including its specific surface area, pore volume and distribution, crystal structure, phase composition, particle size and morpholog [9,23,24]. In particular, the nanotubes that are synthesized by hydrothermal processes have high surface- weight ratios of up to 120 m2 per gram [2,25,26,27,28,29,30].
Many factors affect the reactions that have been proposed for the photodegradation of paracetamol in effluents, such as the catalyst and contaminant concentrations, the excitation wavelength of the catalyst, the oxygenation and the pH [31,32]. The latter has not been thoroughly investigated, which implies that the direct impact of pH on the reaction is efficiency is still uncertain. This investigation evaluates the relationship between the pH of paracetamol suspensions and the efficiency of the photocatalytic degradation using ultraviolet radiation with a wavelength of 254 nm in presence of nanostructured TiO2 catalysts with a nanotubular morphology.
2. Materials and Methods
Reagents: All the chemical reagents used were analytical-grade. Paracetamol (C8H9NO2), 99% purity, and titanium dioxide (P25), 99.5% were purchased from Aldrich. 99.1% Sodium hydroxide (NaOH) and 37% hydrochloric acid (HCl), from sigma Aldrich, were used. HPLC grade water was obtained using a Millipore Milli-Q system (Millipore Sigma Corporation, MA, USA).
Synthesis of TiO2 nanotubes: The TiO2 nanotubes were produced using a chemical process similar to that described by López et al. [30], TiO2 powder (P25) with a crystal structure of 80% anatase and 20% rutile and an average particle size of 25 nm was used as a precursor for the synthesis of the nanotubes. To perform the synthesis, 0.3 g of TiO2 powder was added to 30 mL of 10 M aqueous NaOH solution, and the suspension was vigorously agitated for 2 h at room temperature (30 °C). To perform the hydrothermal treatment, the mixture was transferred to an autoclave, and the temperature was increased to 110 °C for 72 h. The solid precipitate was separated by centrifugation at 10,000 rpm and re-suspended in 200 mL of a 0.1 M HCl solution with constant agitation for 3 h and then centrifuged again. The precipitated samples were washed with distilled water twice until the pH of the solution reached 6.6, which is approximately the pH value of the distilled water that was used. The washed samples were dried in a vacuum oven at 80 °C for 24 h
Preparation of paracetamol samples: An aqueous paracetamol stock solution (volume of 1.5 L) with a concentration of 20 mg L−1 was prepared. The solution was kept under constant magnetic agitation for 120 min on a stirring plate (LabTech model LMS-1003-Gemini BV Lab, Apeldoorn, The Netherlands) until complete solubilization of paracetamol. Six 250 mL aliquots were taken from the stock solution. The pH of each solution was modified using NaOH and HCl solutions to reach the desired pH values of 2.5, 4.5, 6.5, 8.5 and 10.5. To each aliquot 250 mL of the paracetamol solution, 100 mg of the TiO2 nanotubes were added, and the suspension was then agitated for 30 min until it was completely dispersed.
Monitoring of the photodegradation of paracetamol by UV-Vis spectrophotometry: Batch cylindrical stainless steel photoreactor was used to perform the degradation of the samples. The photoreactor used a high-pressure Hg candle lamp as the energy source, which provided UV light with a wavelength of 254 nm and an intensity of 25 W cm−2. The temperature was kept constant at 25 °C by means of a cooling system and was measured during each sampling during the 3 h of irradiation. The lamp was encapsulated in a quartz tube that was submerged in the center of the reactor along with the aqueous solution (Figure 1). The solution that surrounded the reaction system underwent constant agitation at air pressure and room temperature. The reaction kinetics inside the reactor was monitored by taking 1 mL samples every 10 min and analyzing them with a UV-Vis spectrometer (Hach model DR 5000-Loveland, CO, USA) within the range of 200 to 400 nm with 0.5 nm steps. The final paracetamol concentration Cf was determined with the aid of a calibration curve (m = 55.737, R2 = 0.9995).
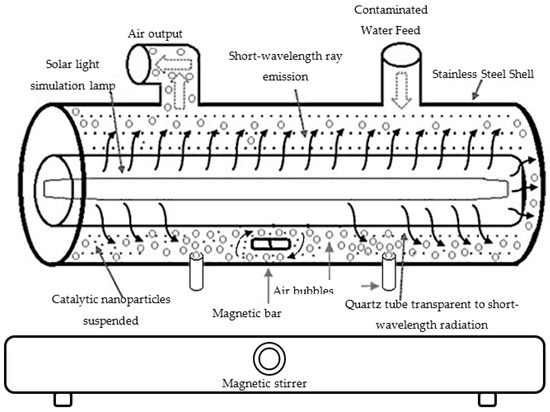
Figure 1.
Experimental setup of the heterogeneous photocatalysis reactor.
Detection of photodegradation by-products by HPLC: The most efficient paracetamol photodegradation reaction was identified using a high-pressure liquid chromatograph (Agilent 1200- Santa Clara, CA, USA) with a C18 reversed-phase column. The chromatographic conditions were similar to those reported by López et al. [33,34]. However, they were optimized for paracetamol and its by-products by using a mobile phase of methanol and acetic acid in 1% water with a v/v ratio of 40/60. The equipment was operated at 25 °C with a flowrate of 1.5 mL min−1. A diode array detector (DAD) was used with a principal wavelength of 254 nm, and intervals of 15 nm were used for the detection.
Measurement of the zeta potential: To measure the zeta potential of the TiO2 nanotubes, an aqueous sample of paracetamol was prepared at a concentration of 0.5 mg L−1. Adjustment of the pH was performed with 0.01 M HCl and 0.01 M NaOH solutions using a particle charge titration analyzer Stabino from Microtrac.
Data treatment: Generally, the photochemical oxidation reactions of organic compounds have a first-order kinetics behavior [35]. This is the case for the photocatalytic degradation of paracetamol [36]. The Langmuir-Hinshelwood model (Equation (1)) was applied because in heterogeneous catalysis processes, this model considers the decomposition at the catalyst surface to be the determining step. So, the first-order reaction rate is assumed to be the product of the kinetic constant that is associated with the decomposition and the adsorbate concentration [37,38].
where k is the rate constant, which depends on the light intensity, K is the adsorption constant of the nanocatalyst, and C is the concentration of paracetamol. For low adsorption magnitudes and concentrations, (KC < 1). So, Equation (1) reduces to the first-order kinetics according to Equation (2).
Separating variables and integrating between the initial conditions t = 0 and C = C0 at time t and if Kk = k′, where k′ is the rate constant for the photocatalytic degradation
When plotting Equation (3), the slope is the value of the reaction rate constant k′ and has units of min−1. The half-life (t1/2), which is an important parameter of photocatalytic degradation because it is the time that is required to reduce the paracetamol concentration by half (i.e., C = C0/2 in Equation (3), is given by:
The efficiency of the photocatalytic degradation of the paracetamol was quantified using Equation (5).
where (Cf) and (C0) are the paracetamol concentrations in the initial and final solutions, respectively.
3. Results and Discussion
3.1. Photodegradation of Paracetamol
Figure 2 shows the absorption spectrum of the paracetamol in the stock solution. The maximum absorption intensity was observed at a wavelength of approximately 243 nm. This change in absorbance at the wavelength of λmax is consistent with the maximum absorption band of paracetamol observed found in previous studies [39]. The absorbance at this wavelength was used to monitor the changes in concentration and to determine the photodegradation reaction rate constant k′ from the experimental data.
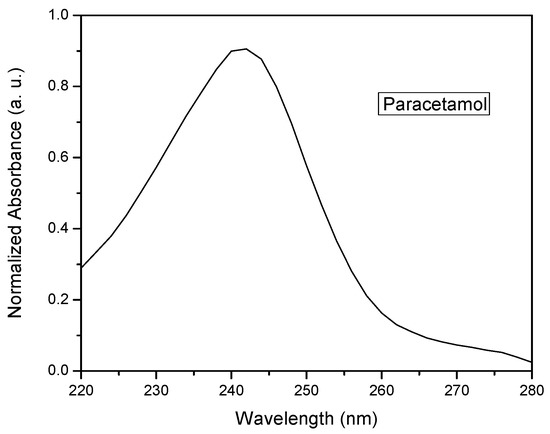
Figure 2.
UV-vis absorption spectrum of normalized paracetamol.
Figure 3 shows the normalized decrease of the paracetamol concentration with the reaction time at different pH values. At acidic pH values between 2.5 and 4.5, the reaction rate constants are low because the •OH in the reagent medium interacts weakly with the paracetamol molecules because of the possible recombination of free H+ protons and hydronium ions (H3O+) with the •OH that is generated in the photocatalytic process to form water molecules.
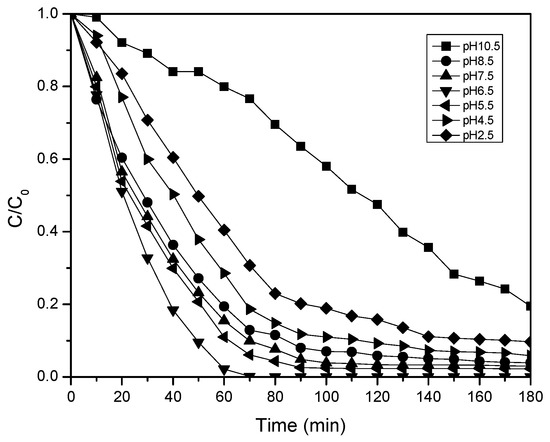
Figure 3.
Photocatalytic activity of TiO2 nanotubes in paracetamol solution at different pHs as function of irradiation time.
Table 1 shows that at a pH values of 6.5, the magnitudes of the reaction rate constant are higher, and correspondingly of the photodegradation efficiency. This can be attributed to the greater formation of hydroxyl radicals because at high pH values, more •OH groups are available at the nanocatalyst surface, and they can easily oxidize [40].

Table 1.
Values of the photodegradation parameters calculated from Equations (3)–(5).
The graph of the zeta potential (Figure 4) shows the variation of the zeta potential of paracetamol and the catalyst, with the pH of the medium. The curves intersect at pH 6.5; a value at which the paracetamol molecule is not ionized and the surface charge of TiO2 is close to zero [41].
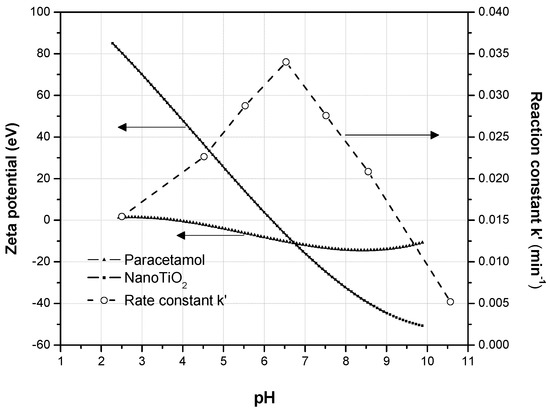
Figure 4.
Graph of the zeta potential of the substrate and the photocatalyst with the variation of pH.
Specific interactions of the polar groups and the aromatic moiety of paracetamol with TiO2 lead to good adsorption, whereas the free generated and available •OH species have more interactions with the paracetamol molecules to degrade them. This explains why the reaction rate constant is higher at pH 6.5 (hollow points in Figure 4). The behavior of the rate constant depicted in Figure 4 also corresponds to the maximum value of the zeta potential. Also, the smaller the charge difference (intersect in Figure 4) between the substrate and the catalyst, the higher k.
This result is consistent with the Langmuir-Hinshelwood kinetic model because it suggests that the adsorption-desorption equilibrium between the surface of the semiconductor and the fluid phase is indispensable for initiating the photodegradation of any substrate. Once this equilibrium is established, the photodegradation of the molecule starts through a mechanism that involves radicals. This adsorption-desorption equilibrium is produced precisely when the value of the zeta potential of paracetamol and the nanocatalyst are similar.
At the pH values of 8.5 to 10.5, the photodegradation rate decreases (Figure 4) because 10% paracetamol exists as anion already at pH 8.5 and 92% at pH 10.5 at higher pH values (pH > pKa). Thus, the increase in the pH gradually increases the electrostatic repulsion between the surface of the nanocatalyst and paracetamol, this considerably reduces the adsorption of paracetamol. Consequently, the reaction rate constant for the photodegradation of paracetamol is expected to decrease gradually at pH values higher than 8.5 [42].
3.2. Paracetamol Photodegradation at pH 6.5
To explore the intermediate products that result from the photodegradation of paracetamol, HPLC was used to analyze the treated solution after 100 min of sample treatment at pH 6.5. Only one peak is observed at a retention time of 0.43 min, both for the initial paracetamol sample and after of the photodegradation (Figure 5). This indicates that in our study, the molecule degrades into small molecules or non-detectable compounds under these measurement conditions.
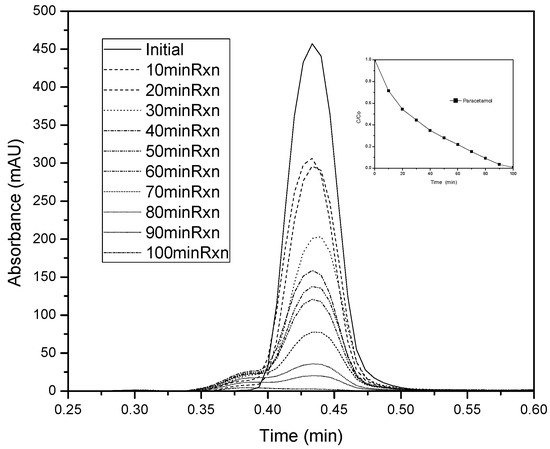
Figure 5.
Paracetamol photodegradation kinetics with HPLC data for the samples at pH 6.5.
4. Conclusions
Paracetamol photodegradation in an aqueous solution using radiation with a wavelength of 254 nm is feasible. The best results were obtained at a pH of 6.5 and resulted in 99% photodegradation of paracetamol by catalytic oxidation under the studied conditions. The photodegradation of paracetamol can be performed at concentrations lower than 20 mg L−1 with relatively short exposure times (100 min) by using TiO2 nanotubes. No traces of the metabolites were detected. With measurement of the zeta potential it was demonstrated that the most rapid photoreaction takes place at the pH, at which adsorption is favored. The pH of the photocatalytic process may influence the efficiency of the photodegradation.
Author Contributions
Designed the experiments, performed the experiments, analyzed and interpreted the data, wrote the paper, S.A.L.-M.; writing—review and editing, analyzed and interpreted the data, wrote the paper, G.M.; conceived and designed the experiments, contributed reagents, materials, analysis tools or data, M.Á.L.Z.; writing, methodology, formal analysis of the investigation, A.A.-S.; writing, methodology, formal analysis of the investigation and writing—review and editing, F.M.-M.
Funding
The authors are grateful to Universidad del Valle for the financial support to produce this work (GRANT 1004. Síntesis y evaluación de la actividad fotocatalítica TiO2/WO3 en el tratamiento de aguas residuales de la industria minera y la mineralización de contaminantes emergentes farmacéuticos).
Acknowledgments
The authors thank the support of the Consorcio del Agua FORDECYT No. 297116 of the National Council of Science and Technology of México (Consejo Nacional de Ciencia y Tecnología de México, CONACYT) and the Universidad del Valle of Cali Colombia for support during the internship. Arce-Sarria Thanks to Colciencias-Colombia for supporting his PhD Scolarship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ. Pollut. 2017, 231, 954–970. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.S.; Yoshimura, C. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2composites: A critical review of recent literature. Water Res. 2018, 142, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Uheida, A.; Mohamed, A.; Belaqziz, M.; Nasser, W.S. Photocatalytic degradation of Ibuprofen, Naproxen, and Cetirizine using PAN-MWCNT nanofibers crosslinked TiO2-NH2 nanoparticles under visible light irradiation. Sep. Purif. Technol. 2019, 212, 110–118. [Google Scholar] [CrossRef]
- Tobajas, M.; Belver, C.; Rodriguez, J.J. Degradation of emerging pollutants in water under solar irradiation using novel TiO2-ZnO/clay nanoarchitectures. Chem. Eng. J. 2017, 309, 596–606. [Google Scholar] [CrossRef]
- Zhao, L.; Deng, J.; Sun, P.; Liu, J.; Ji, Y.; Nakada, N.; Qiao, Z.; Tanaka, H.; Yang, Y. Nanomaterials for treating emerging contaminants in water by adsorption and photocatalysis: Systematic review and bibliometric analysis. Sci. Total Environ. 2018, 627, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Richardson, S.D.; Kimura, S.Y. Emerging environmental contaminants: Challenges facing our next generation and potential engineering solutions. Environ. Technol. Innov. 2017, 8, 40–56. [Google Scholar] [CrossRef]
- Sanganyado, E.; Gwenzi, W. Antibiotic resistance in drinking water systems: Occurrence, removal, and human health risks. Sci. Total Environ. 2019, 669, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Halling-Sørensen, B.; Nielsen, S.N.; Lanzky, P.F.; Ingerslev, F.; Lützhøft, H.C.H.; Jørgensen, S.E. Occurrence, fate and effects of pharmaceutical substances in the environment-A review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef]
- Barber, L.B.; Keefe, S.H.; Brown, G.K.; Furlong, E.T.; Gray, J.L.; Kolpin, D.W.; Meyer, M.T.; Sandstrom, M.W.; Zaugg, S.D. Persistence and potential effects of complex organic contaminant mixtures in wastewater-impacted streams. Environ. Sci. Technol. 2013, 47, 2177–2188. [Google Scholar] [CrossRef]
- Shaheen, S.O.; Newson, R.B.; Ring, S.M.; Rose-Zerilli, M.J.; Holloway, J.W.; Henderson, A.J. Prenatal and infant acetaminophen exposure, antioxidant gene polymorphisms, and childhood asthma. J. Allergy Clin. Immunol. 2010, 126. [Google Scholar] [CrossRef] [PubMed]
- Bedner, M.; MacCrehan, W.A. Transformation of acetaminophen by chlorination produces the toxicants 1,4-benzoquinone and N-acetyl-p-benzoquinone imine. Environ. Sci. Technol. 2006, 40, 516–522. [Google Scholar] [CrossRef]
- Xagoraraki, I.; Hullman, R.; Song, W.; Li, H.; Voice, T. Effect of pH on degradation of acetaminophen and production of 1,4-benzoquinone in water chlorination. J. Water Supply Res. Technol.-AQUA 2008, 57, 381–390. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Marotta, R.; Vogna, D. Paracetamol oxidation from aqueous solutions by means of ozonation and H2O2/UV system. Water Res. 2003, 37, 993–1004. [Google Scholar] [CrossRef]
- Valdez, H.C.A.; Jiménez, G.G.; Granados, S.G.; de León, C.P. Degradation of paracetamol by advance oxidation processes using modified reticulated vitreous carbon electrodes with TiO2 and CuO/TiO2/Al2O3. Chemosphere 2012, 89, 1195–1201. [Google Scholar] [CrossRef]
- Jagannathan, M.; Grieser, F.; Ashokkumar, M. Sonophotocatalytic degradation of paracetamol using TiO2 and Fe3+. Sep. Purif. Technol. 2013, 103, 114–118. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Fu, W.; Tsang, D.C.W.; Yang, X. The roles of halides in the acetaminophen degradation by UV/H2O2 treatment: Kinetics, mechanisms, and products analysis. Chem. Eng. J. 2015, 271, 214–222. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Zhou, S.; Xiao, Y.; Zhuang, Z. Kinetic study of acetaminophen degradation by UV-based advanced oxidation processes. Chem. Eng. J. 2014, 253, 229–236. [Google Scholar] [CrossRef]
- Su, C.C.; Cada, C.A.; Dalida, M.L.P.; Lu, M.C. Effect of UV light on acetaminophen degradation in the electro-Fenton process. Sep. Purif. Technol. 2013, 120, 43–51. [Google Scholar] [CrossRef]
- Martinez-Haya, R.; Miranda, M.A.; Marin, M.L. Type I vs Type II photodegradation of pollutants. Catal. Today 2018, 313, 161–166. [Google Scholar] [CrossRef]
- Scott, J.P.; Ollis, D.F. Integration of chemical and biological oxidation processes for water treatment: Review and recommendations. Environ. Prog. 1995, 14, 88–103. [Google Scholar] [CrossRef]
- Verlicchi, P.; al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment-A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Baird, C.; Cann, M. Enviromental Chemistry, 5th ed.; W. H. Freeman and Company: New York, NY, USA, 2012. [Google Scholar]
- Wen, Z.H.; Chen, L.; Meng, X.Z.; Duan, Y.P.; Zhang, Z.S.; Zeng, E.Y. Occurrence and human health risk of wastewater-derived pharmaceuticals in a drinking water source for Shanghai, East China. Sci. Total Environ. 2014, 490, 987–993. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of Titanium Oxide Nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Idakiev, V.; Yuan, Z.-Y.; Tabakova, T.; Su, B.-L. Titanium oxide nanotubes as supports of nano-sized gold catalysts for low temperature water-gas shift reaction. Appl. Catal. A Gen. 2005, 281, 149–155. [Google Scholar] [CrossRef]
- Ou, H.H.; Lo, S.L. Review of titania nanotubes synthesized via the hydrothermal treatment: Fabrication, modification, and application. Sep. Purif. Technol. 2007, 58, 179–191. [Google Scholar] [CrossRef]
- Guo, Y.; Lee, N.H.; Oh, H.J.; Yoon, C.R.; Park, K.S.; Lee, W.H.; Li, Y.; Lee, H.G.; Lee, K.S.; Kim, S.J. Preparation of titanate nanotube thin film using hydrothermal method. Thin Solid Films 2008, 516, 8363–8371. [Google Scholar] [CrossRef]
- Li, H.L.; Luo, W.L.; Tian, W.Y.; Chen, T.; Li, C.; Sun, M.; Zhu, D.; Liu, R.R.; Zhao, Y.L.; Liu, C.L. Fabrication and Photocatalytic Activity of Pt-Inserted Titania Nanotubes. Spectrosc. Spectr. Anal. 2009, 29, 1623–1626. [Google Scholar] [CrossRef]
- Zavala, M.L.; Morales, S.L. Synthesis of stable TiO2 nanotubes: Effect of hydrothermal treatment, acid washing and annealing temperature. Heliyon 2017, 3, e00456. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, K.E.; Pernas, E.; Saiers, J. Influence of mineralization products on the coagulation of TiO2 photocatalyst. Langmuir 1999, 15, 2071–2076. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.E.; Ray, M.B. Photocatalytic oxidation of paracetamol: Dominant reactants, intermediates, and reaction mechanisms. Environ. Sci. Technol. 2009, 43, 460–465. [Google Scholar] [CrossRef]
- Zavala, M.Á.L.; Lara, C.R.J. Degradation of Paracetamol and Its Oxidation Products in Surface Water by Electrochemical Oxidation. Environ. Eng. Sci. 2018, 35, 1248–1254. [Google Scholar] [CrossRef]
- Zavala, M.Á.L.; Estrada, E.E. Degradation of acetaminophen and its transformation products in aqueous solutions by using an electrochemical oxidation cell with stainless steel electrodes. Water 2016, 8, 383. [Google Scholar] [CrossRef]
- Metcalf & Eddy, Inc.; Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Fundamentals of Biological Treatment. In Wastewater Engineering Treatment Reuse; McGraw-Hill: New York, NY, USA, 2003; pp. 611–615, 619–621. [Google Scholar]
- Zhang, X.; Wu, F.; Wu, X.; Chen, P.; Deng, N. Photodegradation of acetaminophen in TiO2 suspended solution. J. Hazard. Mater. 2008, 157, 300–307. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Langmuir-Hinshelwood kinetics-A theoretical study. Catal. Commun. 2008, 9, 82–84. [Google Scholar] [CrossRef]
- Ollis, D.F. Kinetics of liquid phase photocatalyzed reactions: An illuminating approach. J. Phys. Chem. B 2005, 109, 2439–2444. [Google Scholar] [CrossRef]
- El-shahawy, A. DFT Cancer Energy Barrier and Spectral Studies of Aspirin, Paracetamol and Some Analogues. Comput. Chem. 2014, 2, 6–17. [Google Scholar] [CrossRef]
- Shourong, Z.; Qingguo, H.; Jun, Z.; Bingkun, W. A study on dye photoremoval in TiO2 suspension solution. J. Photochem. Photobiol. A Chem. 1997, 108, 235–238. [Google Scholar] [CrossRef]
- Kulkarni, M.; Flašker, A.; Lokar, A.; Mrak-Poljšak, M.; Mazare, A.; Artenjak, A.; Čučnik, S.; Kralj, S.; Velikonja, A.; Schmuki, P.; et al. Binding of plasma proteins to titanium dioxide nanotubes with different diameters. Int. J. Nanomed. 2015, 10, 1359–1373. [Google Scholar] [CrossRef]
- Borges, M.; García, D.; Hernández, T.; Ruiz-Morales, J.; Esparza, P. Supported Photocatalyst for Removal of Emerging Contaminants from Wastewater in a Continuous Packed-Bed Photoreactor Configuration. Catalysts 2015, 5, 77–87. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).