Preparation and Characterization of Furosemide-Silver Complex Loaded Chitosan Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
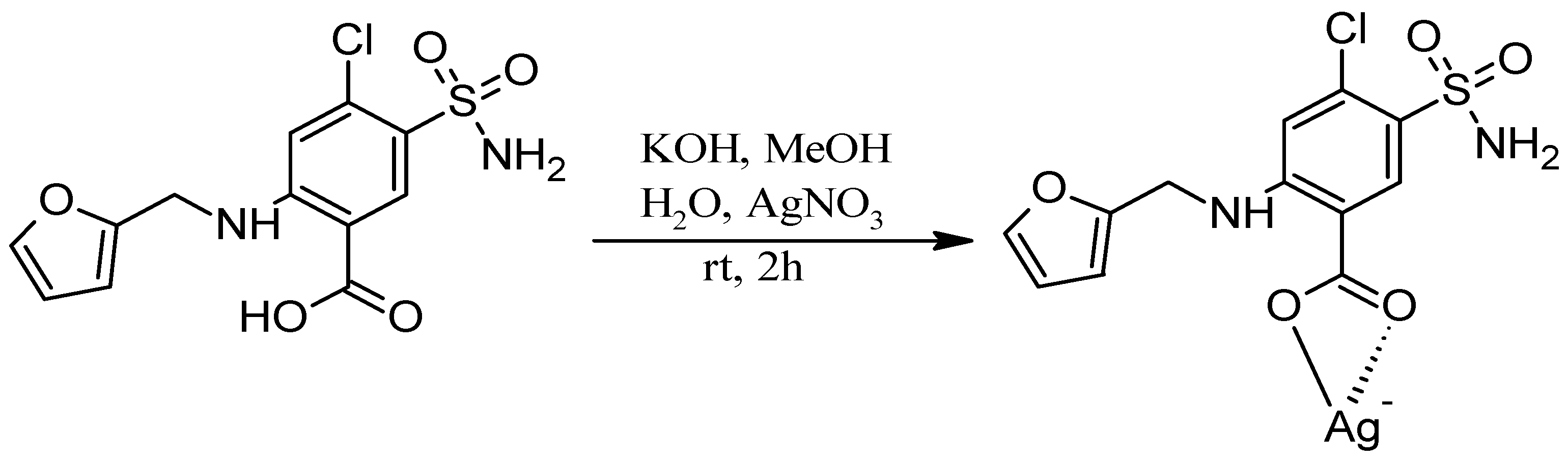
2.2.1. Synthesis of Ag-FSE Complex
2.2.2. Preparation of CSNPs
2.2.3. Determination of Size, Polydispersity Index (PDI), and Zeta Potential (ZP)
2.2.4. Drug Encapsulation Efficiency (EE%)
2.2.5. Transmission Electron Microscopy (TEM)
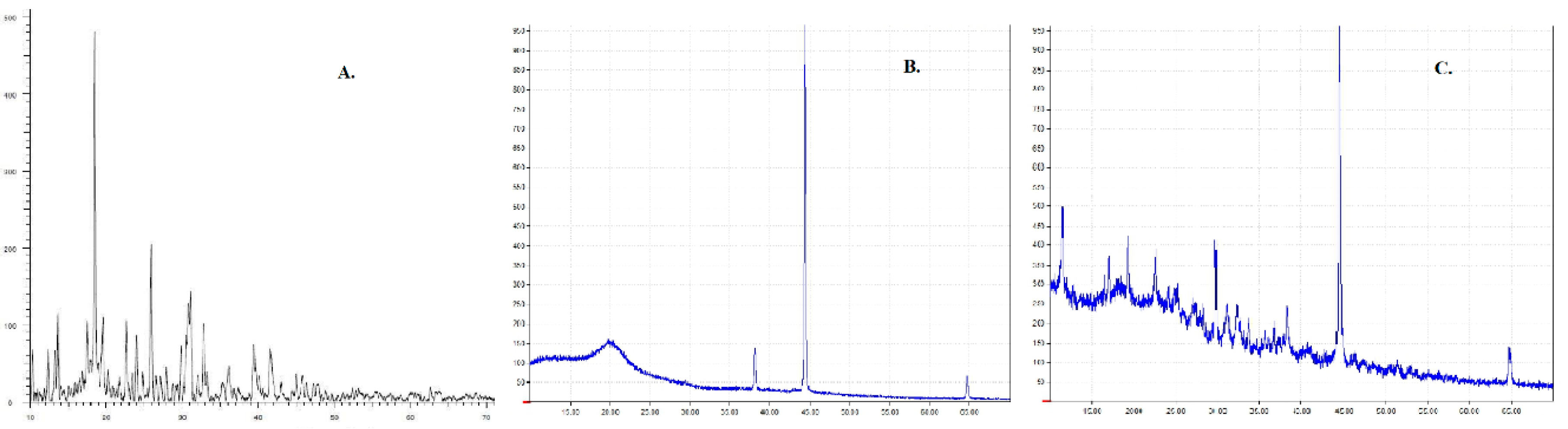
2.2.6. X-Ray Diffraction (XRD)
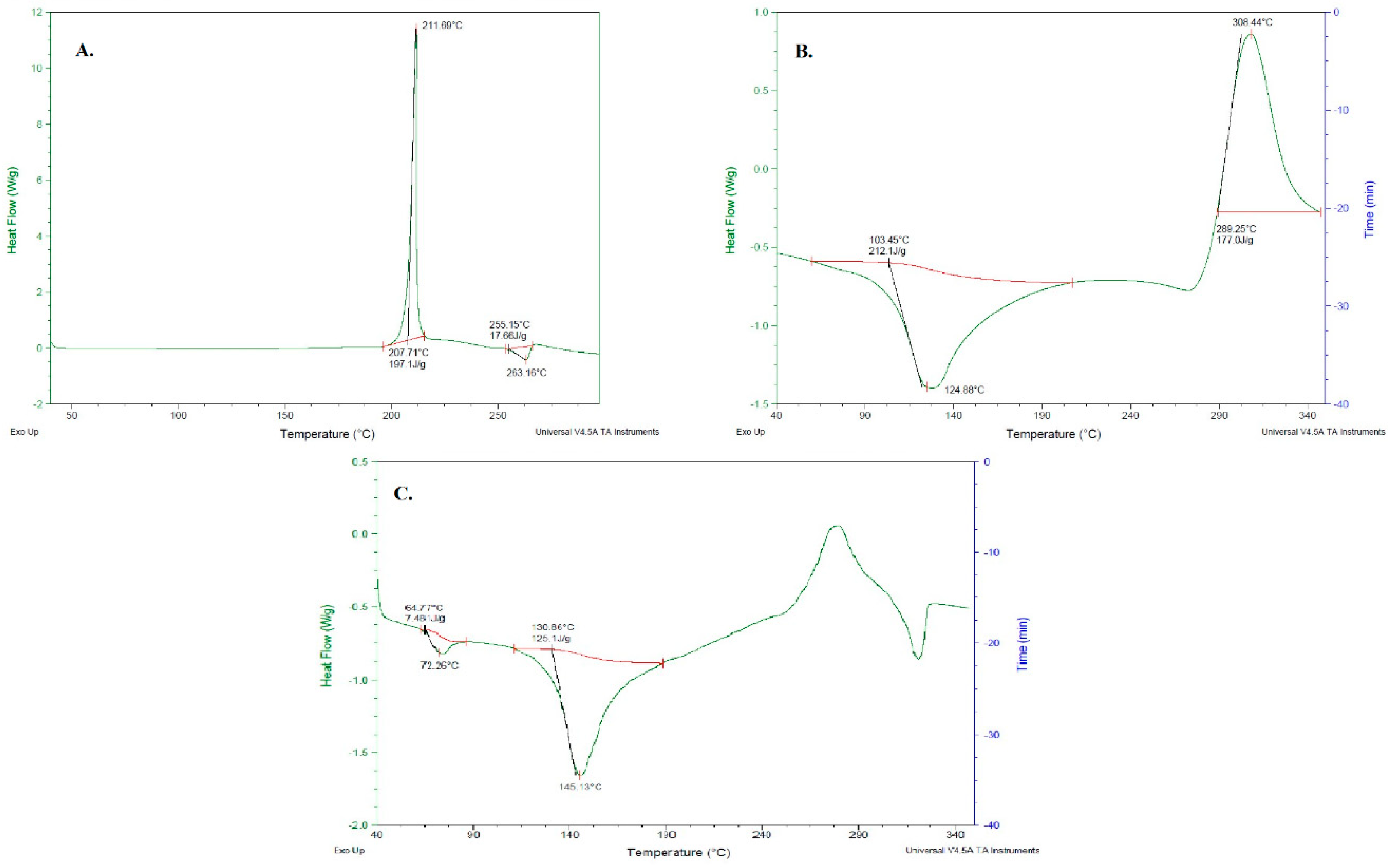
2.2.7. Differential Scanning Calorimetry (DSC)
2.2.8. In Vitro Drug Release Studies
2.2.9. In Vitro Antibacterial Activity
2.2.10. Stability Studies
2.2.11. Statistical Analysis
3. Results and Discussion
3.1. Characterization of CSNPs
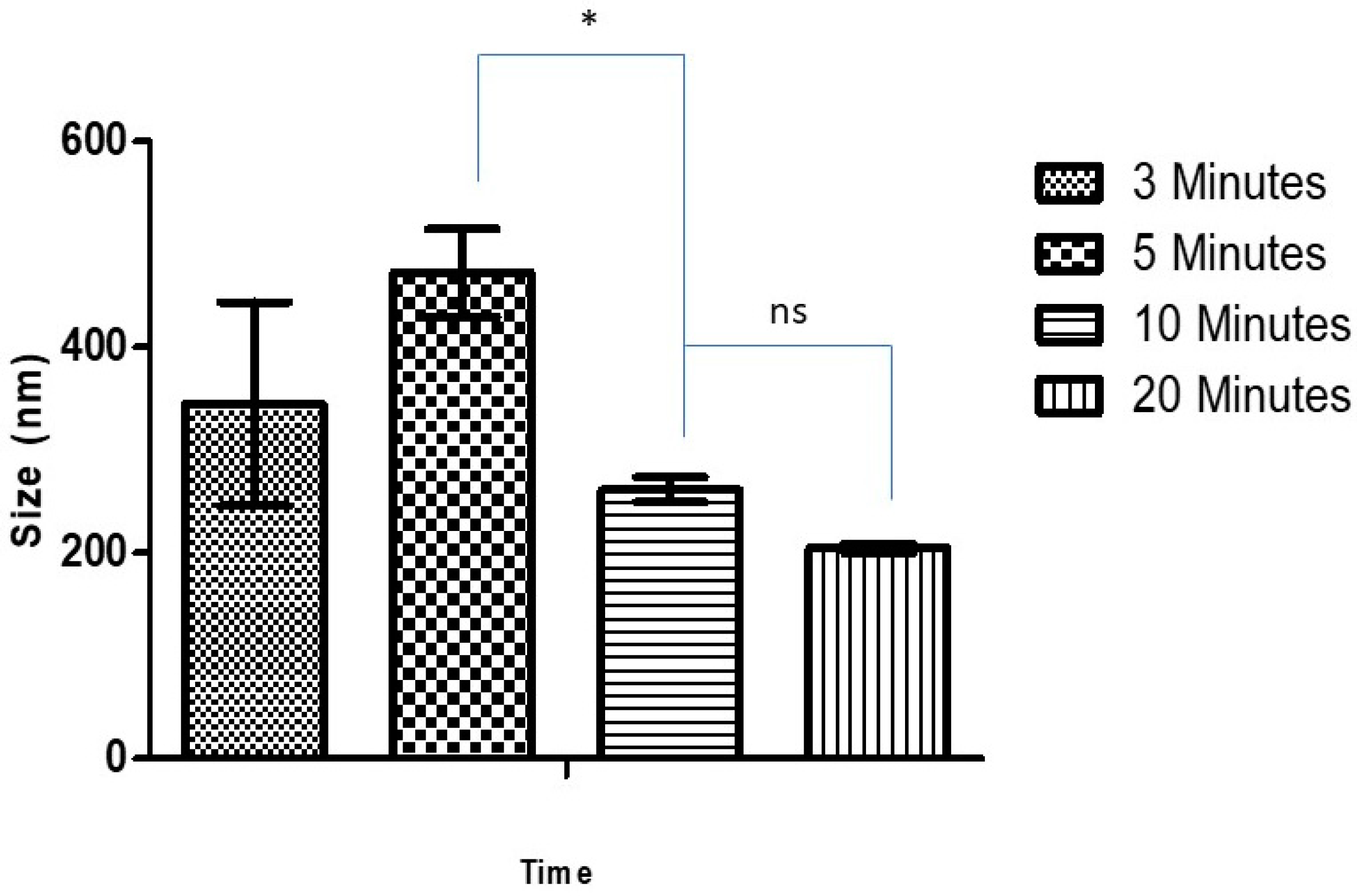
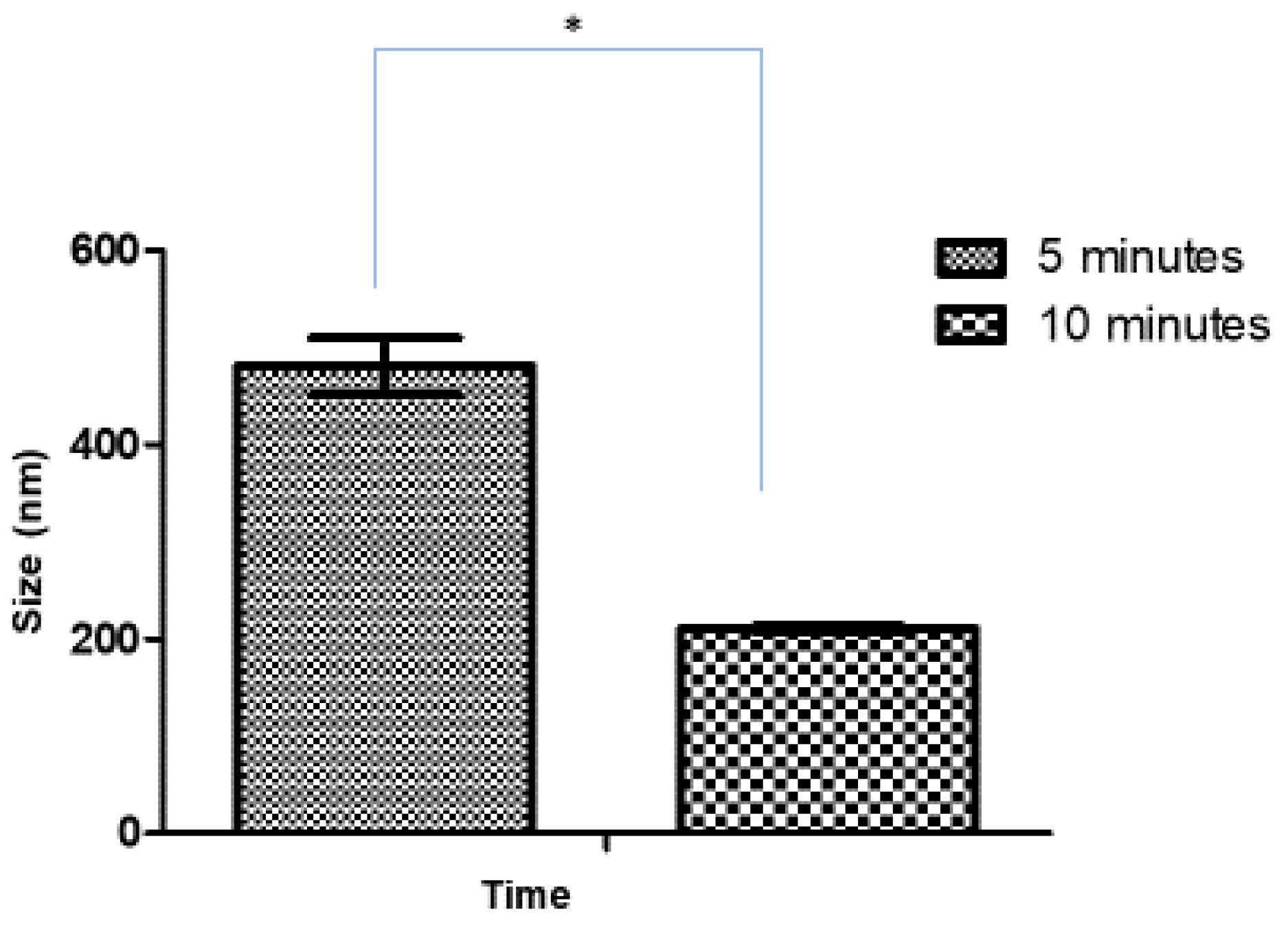
3.1.1. Effect of Sonication Time on Blank and Ag-FSE CSNPs
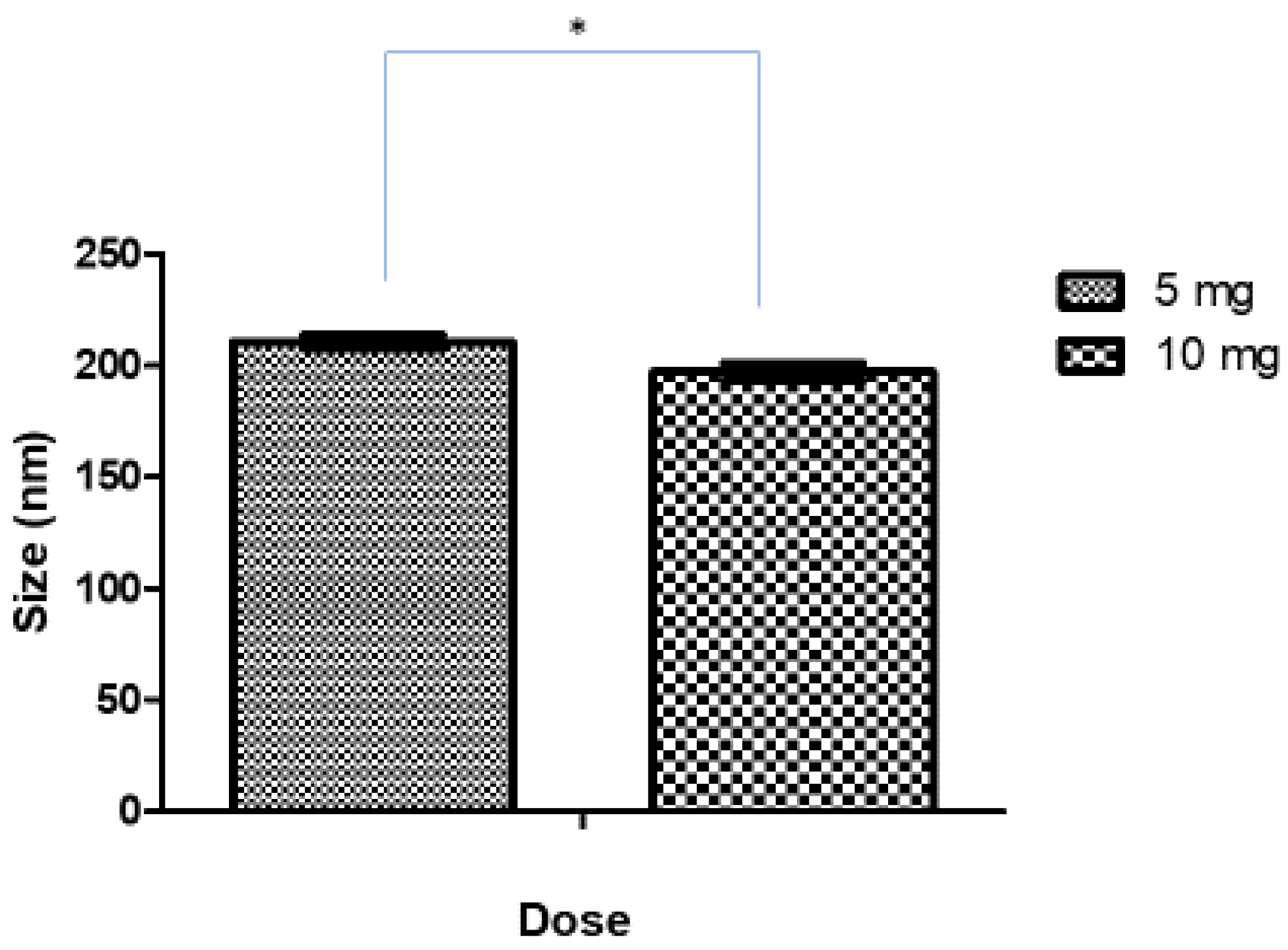
3.1.2. Effect of Drug Loading on Size, PDI, and ZP of Ag-FSE CSNPs
3.2. Drug Encapsulation Efficiency (EE%)
3.3. Transmission Electron Microscopy
3.4. XRD Analysis
3.5. DSC of Ag-FSE CSNPs
3.6. In Vitro Drug Release Studies
3.7. In Vitro Antibacterial Activity
3.8. Stability Studies
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention, Antibiotic/Antimicrobial Resistance, (n.d.). Available online: https://www.cdc.gov/drugresistance/index.html (accessed on 19 July 2018).
- Adedeji, W.A. The Treasure Called Antibiotics. Ann. Ib. Postgrad. Med. 2016, 14, 56–57. [Google Scholar] [PubMed]
- Richardson, L.A. Understanding and overcoming antibiotic resistance. PLoS Biol. 2017, 15, 1–5. [Google Scholar] [CrossRef]
- Faya, M.; Kalhapure, R.S.; Kumalo, H.M.; Waddad, A.Y.; Omolo, C.; Govender, T. Conjugates and nano-delivery of antimicrobial peptides for enhancing therapeutic activity. J. Drug Deliv. Sci. Technol. 2018, 44, 153–171. [Google Scholar] [CrossRef]
- Keller, A.A.; Adeleye, A.S.; Conway, J.R.; Garner, K.L.; Zhao, L.; Cherr, G.N.; Hong, J.; Gardea-Torresdey, J.L.; Godwin, H.A.; Hanna, S.; et al. Comparative environmental fate and toxicity of copper nanomaterials. NanoImpact 2017, 7, 28–40. [Google Scholar] [CrossRef]
- Clement, J.L.; Jarrett, P.S. Antibacterial Silver. Met. Based Drugs 1994, 1, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Kalhapure, R.S.; Sonawane, S.J.; Sikwal, D.R.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Govender, T. Solid lipid nanoparticles of clotrimazole silver complex: An efficient nano antibacterial against Staphylococcus aureus and MRSA. Colloids Surf. B Biointerfaces 2015, 136, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Lustri, B.C.; Massabni, A.C.; Silva, M.A.C.; Nogueira, F.A.R.; Aquino, R.; Amaral, A.C.; Massabni, A.C.; Barud, H.d. Spectroscopic characterization and biological studies in vitro of a new silver complex with furosemide: Prospective of application as an antimicrobial agent. J. Mol. Struct. 2017, 1134, 386–394. [Google Scholar] [CrossRef]
- Shakhashiri, B.Z.; Dirreen, G.E.; Juergens, F. Color, solubility, and complex ion equilibria of nickel(II) species in aqueous solution. J. Chem. Educ. 1980, 57, 900. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B. 2015, 5, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Bolla, P.K.; Kalhapure, R.S.; Rodriguez, V.A.; Ramos, D.V.; Dahl, A.; Renukuntla, J. Preparation of solid lipid nanoparticles of furosemide-silver complex and evaluation of antibacterial activity. J. Drug Deliv. Sci. Technol. 2019, 49, 6–13. [Google Scholar] [CrossRef]
- Renukuntla, J. FSE–Ag complex NS: Preparation and evaluation of antibacterial activity. Iet Nanobiotechnology 2018, 1–5. [Google Scholar] [CrossRef]
- Ghadi, A.; Mahjoub, S.; Tabandeh, F.; Talebnia, F. Synthesis and optimization of chitosan nanoparticles: Potential applications in nanomedicine and biomedical engineering. Casp. J. Intern. Med. 2014, 5, 156–161. [Google Scholar]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Wieckiewicz, M.; Boening, K.W.; Grychowska, N.; Paradowska-Stolarz, A. Clinical Application of Chitosan in Dental Specialities. Mini Rev. Med. Chem. 2017, 17, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Kmiec, M.; Pighinelli, L.; Tedesco, M.F.; Silva, M.M.; Reis, V. Chitosan-Properties and Applications in Dentistry. Adv. Tissue Eng. Regen. Med. Open Access. 2017, 2, 205–211. [Google Scholar] [CrossRef]
- Uraz, A.; Boynueğri, D.; Özcan, G.; Karaduman, B.; Uç, D.; Şenel, S.; Pehlivan, S.; Öğüs, E.; Sultan, N. Two percent chitosan mouthwash: A microbiological and clinical comparative study. J. Dent. Sci. 2012, 7, 342–349. [Google Scholar] [CrossRef]
- Chitosan, N.A. Chitosan Nanoparticles, and Chlorhexidine Gluconate, as Intra Canal Medicaments in Primary Teeth, (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03588351 (accessed on 3 July 2018).
- Kalhapure, R.S.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Singh, S.; Renukuntla, J.; Govender, T. pH-responsive chitosan nanoparticles from a novel twin-chain anionic amphiphile for controlled and targeted delivery of vancomycin. Colloids Surf. B Biointerfaces 2017, 158, 650–657. [Google Scholar] [CrossRef]
- Abdelkader, A.; El-Mokhtar, M.A.; Abdelkader, O.; Hamad, M.A.; Elsabahy, M.; El-Gazayerly, O.N. Ultrahigh antibacterial efficacy of meropenem-loaded chitosan nanoparticles in a septic animal model. Carbohydr. Polym. 2017, 174, 1041–1050. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Y.; Zhou, Y.; Guo, D.; Fan, Y.; Guo, F.; Zheng, Y.; Chen, W. Preparation of 5-fluorouracil-loaded chitosan nanoparticles and study of the sustained release in vitro and in vivo. Asian J. Pharm. Sci. 2017, 12, 418–423. [Google Scholar] [CrossRef]
- Pan, Y.; Li, Y.J.; Zhao, H.Y.; Zheng, J.M.; Xu, H.; Wei, G.; Hao, J.S.; de Cui, F. Bioadhesive polysaccharide in protein delivery system: Chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int. J. Pharm. 2002, 249, 139–147. [Google Scholar] [CrossRef]
- Liu, H.; He, J. Simultaneous release of hydrophilic and hydrophobic drugs from modified chitosan nanoparticles. Mater. Lett. 2015, 161, 415–418. [Google Scholar] [CrossRef]
- Tang, E.S.K.; Huang, M.; Lim, L.Y. Ultrasonication of chitosan and chitosan nanoparticles. Int. J. Pharm. 2003, 265, 103–114. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z. Lead sorption from aqueous solutions on chitosan nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2004, 251, 183–190. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Vicente, S.; Neto, C.; Castro, P.M.; Veiga, M.; Madureira, R.; Tavaria, F.; Pintado, M.M. Chitosan nanoparticles as alternative anti-staphylococci agents: Bactericidal, antibiofilm and antiadhesive effects. Mater. Sci. Eng. C 2017, 79, 221–226. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar] [CrossRef]
- López-León, T.; Carvalho, E.L.S.; Seijo, B.; Ortega-Vinuesa, J.L.; Bastos-González, D. Physicochemical characterization of chitosan nanoparticles: Electrokinetic and stability behavior. J. Colloid Interface Sci. 2005, 283, 344–351. [Google Scholar] [CrossRef]


| Time (min) | Size (nm) | PDI | ZP (mV) |
|---|---|---|---|
| 3 | 344.6 ± 98.9 | 0.517 ± 0.107 | 44.1 ± 2.21 |
| 5 | 472.1 ± 42.8 | 0.507 ± 0.139 | 42.9 ± 3.86 |
| 10 | 261.3 ± 12.2 | 0.195 ± 0.023 | 42.8 ± 1.31 |
| 20 | 204.8 ± 4.4 | 0.205 ± 0.019 | 36.0 ± 2.54 |
| Time (min) | Size (nm) | PDI | ZP (mV) |
|---|---|---|---|
| 5 | 481.4 ± 28.9 | 0.520 ± 0.100 | 42.2 ± 2.44 |
| 10 | 210.5 ± 3.11 | 0.232 ± 0.009 | 41.5 ± 3.39 |
| Ag-FSE loading (mg) | Size (nm) | PDI | ZP (mV) |
|---|---|---|---|
| 5 | 210.5 ± 3.11 | 0.232 ± 0.009 | 41.5 ± 3.39 |
| 10 | 197.1 ± 3.88 | 0.234 ± 0.018 | 36.7 ± 1.78 |
| Formulation | Minimum Inhibition Concentration (µg/mL) | |
|---|---|---|
| E. coli | S. aureus | |
| Blank CSNPs | NA | NA |
| Ag-FSE CSNPs | 166.5 | 41.63 |
| Ag-FSE 10% DMSO | 500 | 250 |
| 10% DMSO | NA | NA |
| Storage Time | Size (nm) | PDI | ZP (mV) | |||
|---|---|---|---|---|---|---|
| 4 °C | 25 °C | 4 °C | 25 °C | 4 °C | 25 °C | |
| Day 0 | 290.3 ± 162.6 | 0.395 ± 0.222 | 35.5 ± 1.52 | |||
| Day 30 | 239.8 ± 9.8 | 241.5 ± 5.6 | 0.224 ± 0.023 | 0.191 ± 0.021 | 33.7 ± 1.70 | 35.6 ± 1.08 |
| Day 60 | 229.7 ± 3.2 | 232.0 ± 11.2 | 0.217 ± 0.008 | 0.242 ± 0.018 | 39.0 ± 2.09 | 35.5 ± 8.90 |
| Day 120 | 296.8 ± 2.8 | 331.8 ± 16.1 | 0.236 ± 0.014 | 0.288 ± 0.038 | 29.8 ± 2.03 | 30.7 ± 0.66 |
| Storage Time | Size (nm) | PDI | ZP (mV) | |||
|---|---|---|---|---|---|---|
| 4 °C | 25 °C | 4 °C | 25 °C | 4 °C | 25 °C | |
| Day 0 | 216.6 ± 26.8 | 0.194 ± 0.021 | 25.8 ± 3.05 | |||
| Day 30 | 224.6 ± 13.8 | 176.5 ± 10.5 | 0.217 ± 0.025 | 0.272 ± 0.030 | 30.5 ± 11.1 | 30.3 ± 5.45 |
| Day 60 | 217.4 ± 5.3 | 207.3 ± 9.9 | 0.232 ± 0.017 | 0.248 ± 0.018 | 37.3 ± 3.79 | 36.2 ± 6.61 |
| Day 120 | 324.8 ± 50.7 | 321.7 ± 33.5 | 0.587 ± 0.162 | 0.407 ± 0.021 | 39.7 ± 1.92 | 37.3 ± 2.40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, V.A.; Bolla, P.K.; Kalhapure, R.S.; Boddu, S.H.S.; Neupane, R.; Franco, J.; Renukuntla, J. Preparation and Characterization of Furosemide-Silver Complex Loaded Chitosan Nanoparticles. Processes 2019, 7, 206. https://doi.org/10.3390/pr7040206
Rodriguez VA, Bolla PK, Kalhapure RS, Boddu SHS, Neupane R, Franco J, Renukuntla J. Preparation and Characterization of Furosemide-Silver Complex Loaded Chitosan Nanoparticles. Processes. 2019; 7(4):206. https://doi.org/10.3390/pr7040206
Chicago/Turabian StyleRodriguez, Victor A., Pradeep Kumar Bolla, Rahul S. Kalhapure, Sai Hanuman Sagar Boddu, Rabin Neupane, Julian Franco, and Jwala Renukuntla. 2019. "Preparation and Characterization of Furosemide-Silver Complex Loaded Chitosan Nanoparticles" Processes 7, no. 4: 206. https://doi.org/10.3390/pr7040206
APA StyleRodriguez, V. A., Bolla, P. K., Kalhapure, R. S., Boddu, S. H. S., Neupane, R., Franco, J., & Renukuntla, J. (2019). Preparation and Characterization of Furosemide-Silver Complex Loaded Chitosan Nanoparticles. Processes, 7(4), 206. https://doi.org/10.3390/pr7040206







