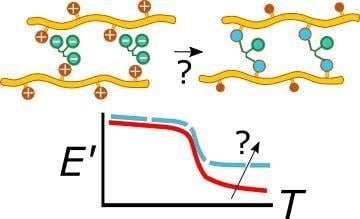
Viscoelastic Properties of Crosslinked Chitosan Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Dynamic Mechanical Analysis
3. Results and Discussion
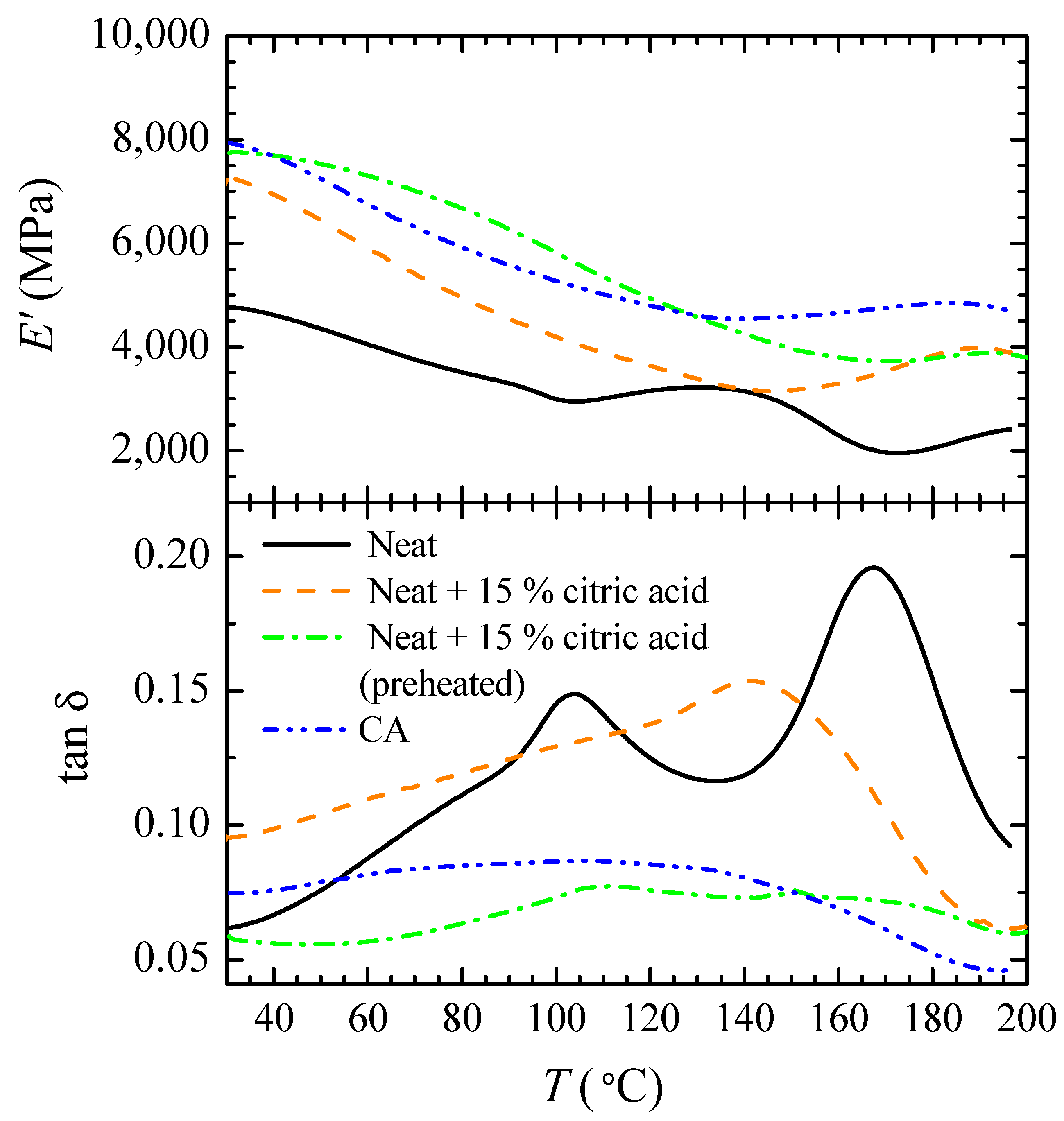
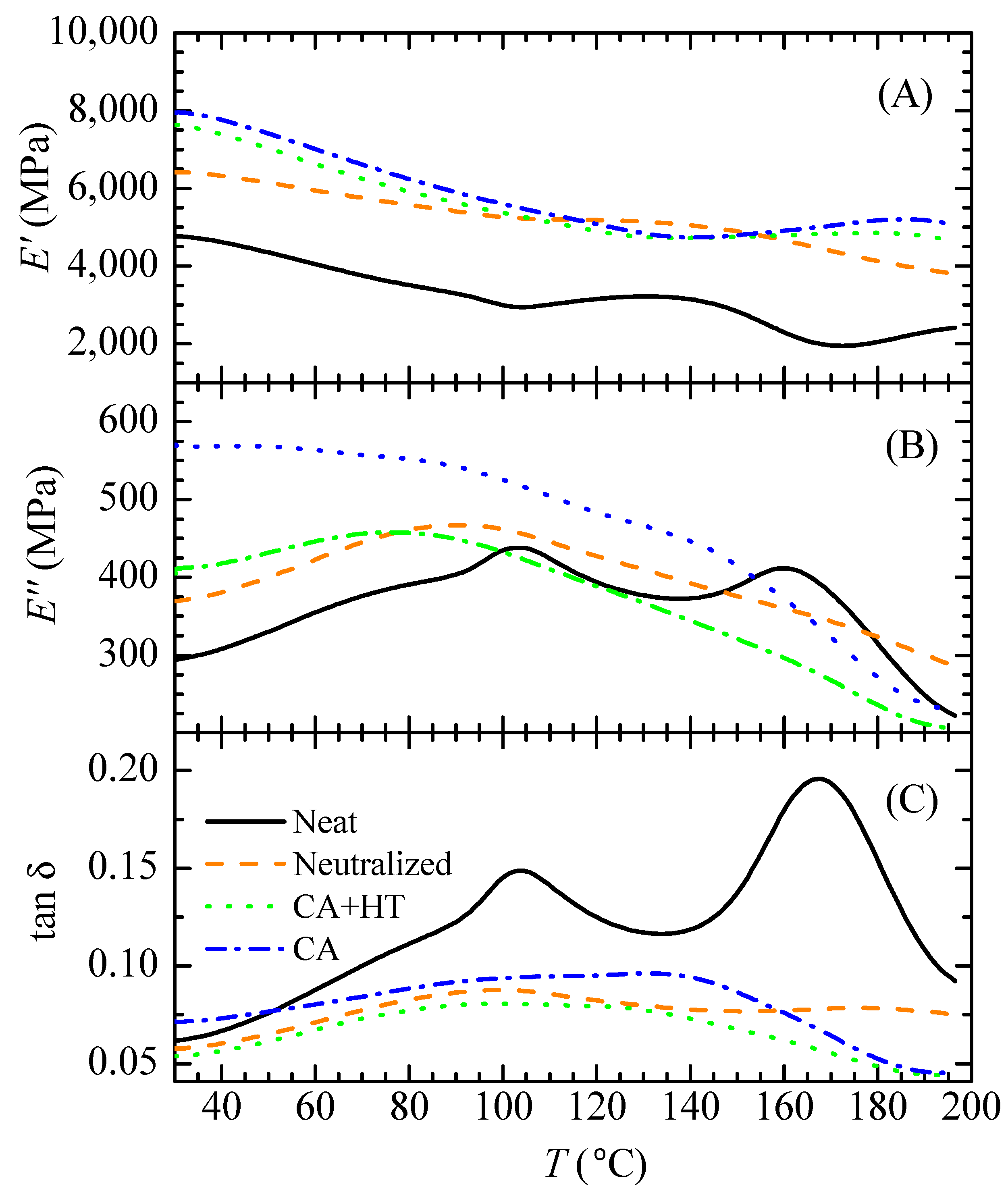
3.1. Description of Viscoelastic Behavior of Neat, Neutralized and CA Films
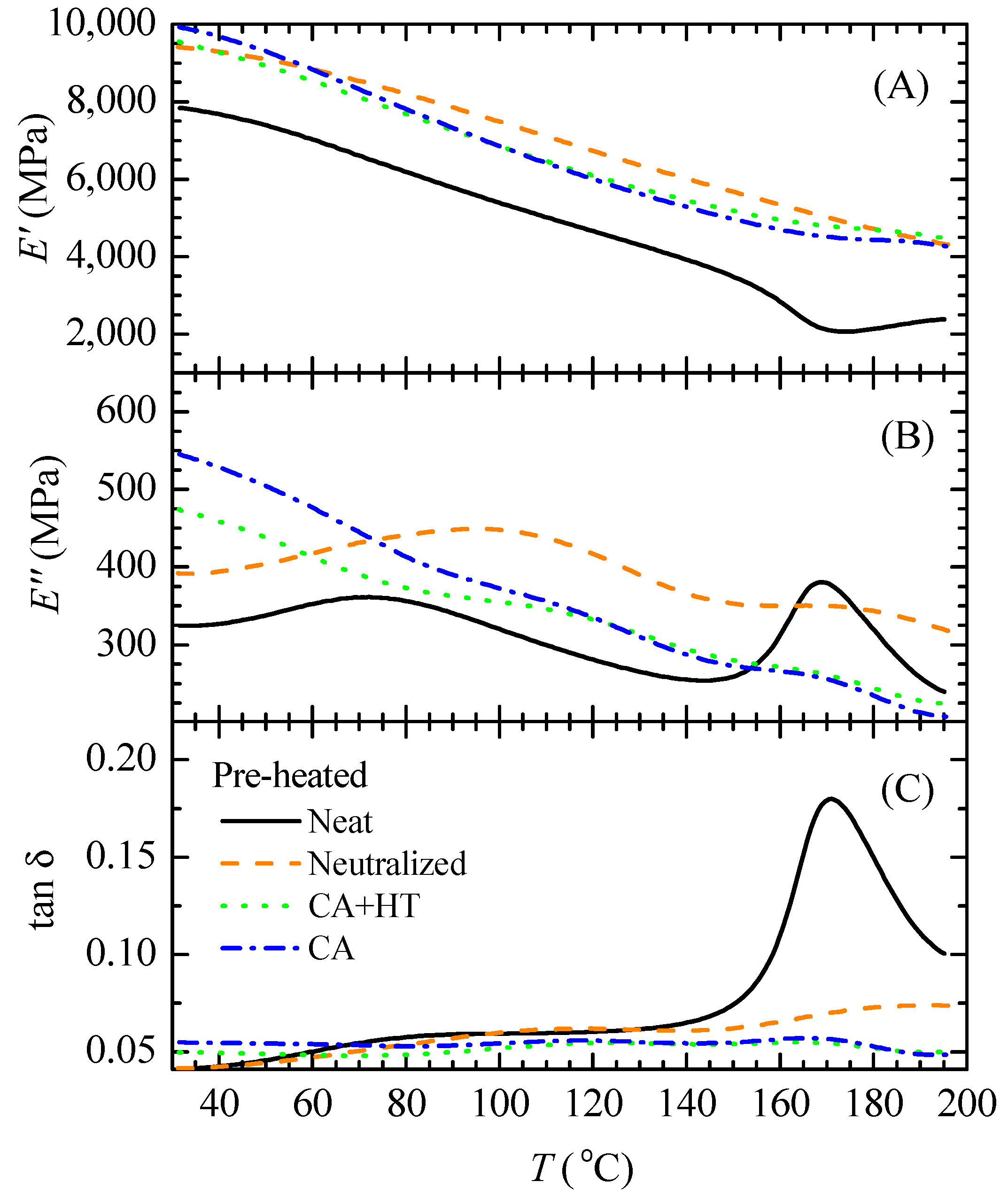
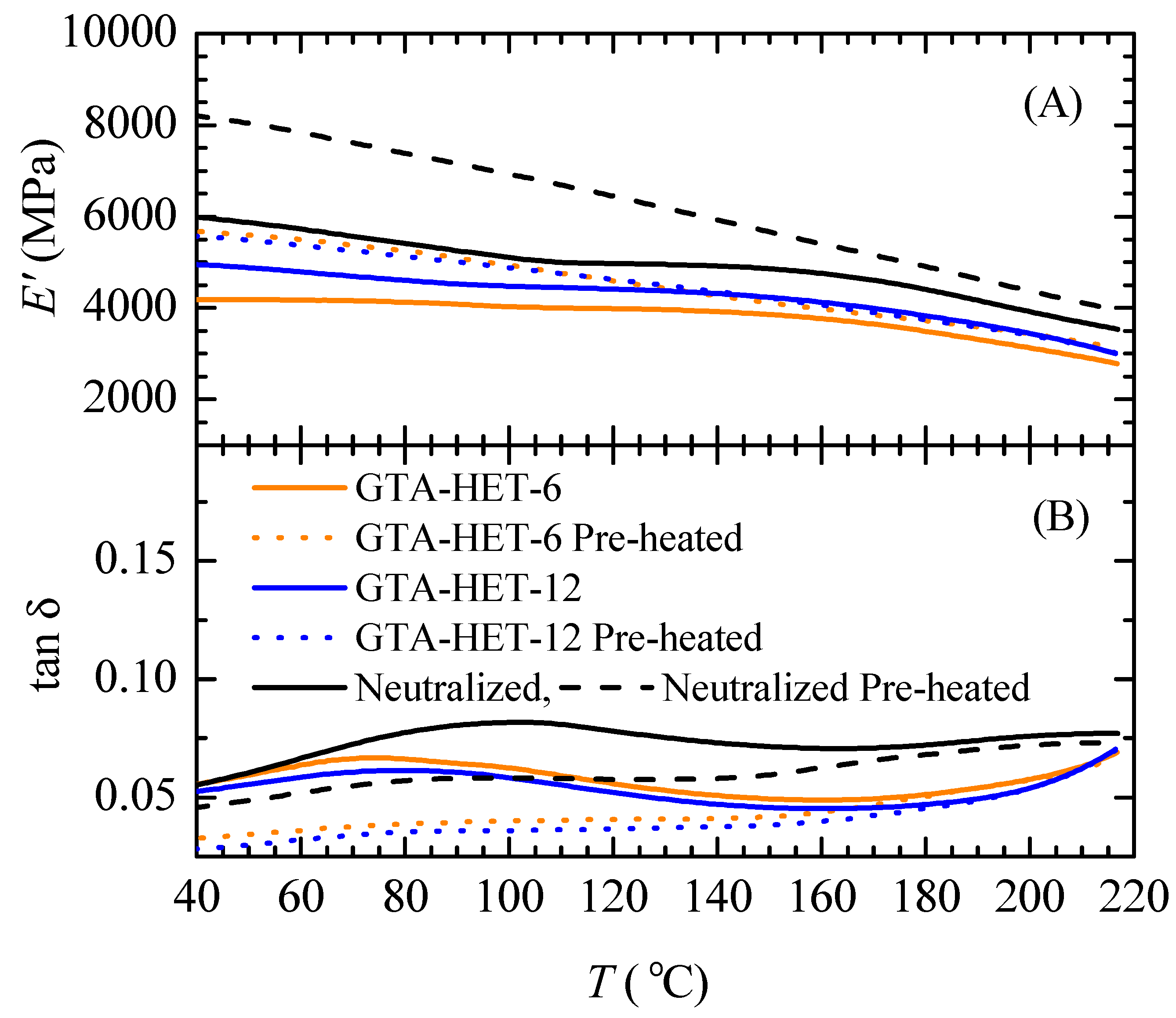
3.2. Effect of Preheating Film Specimens and Thermal Treatment on CA Films
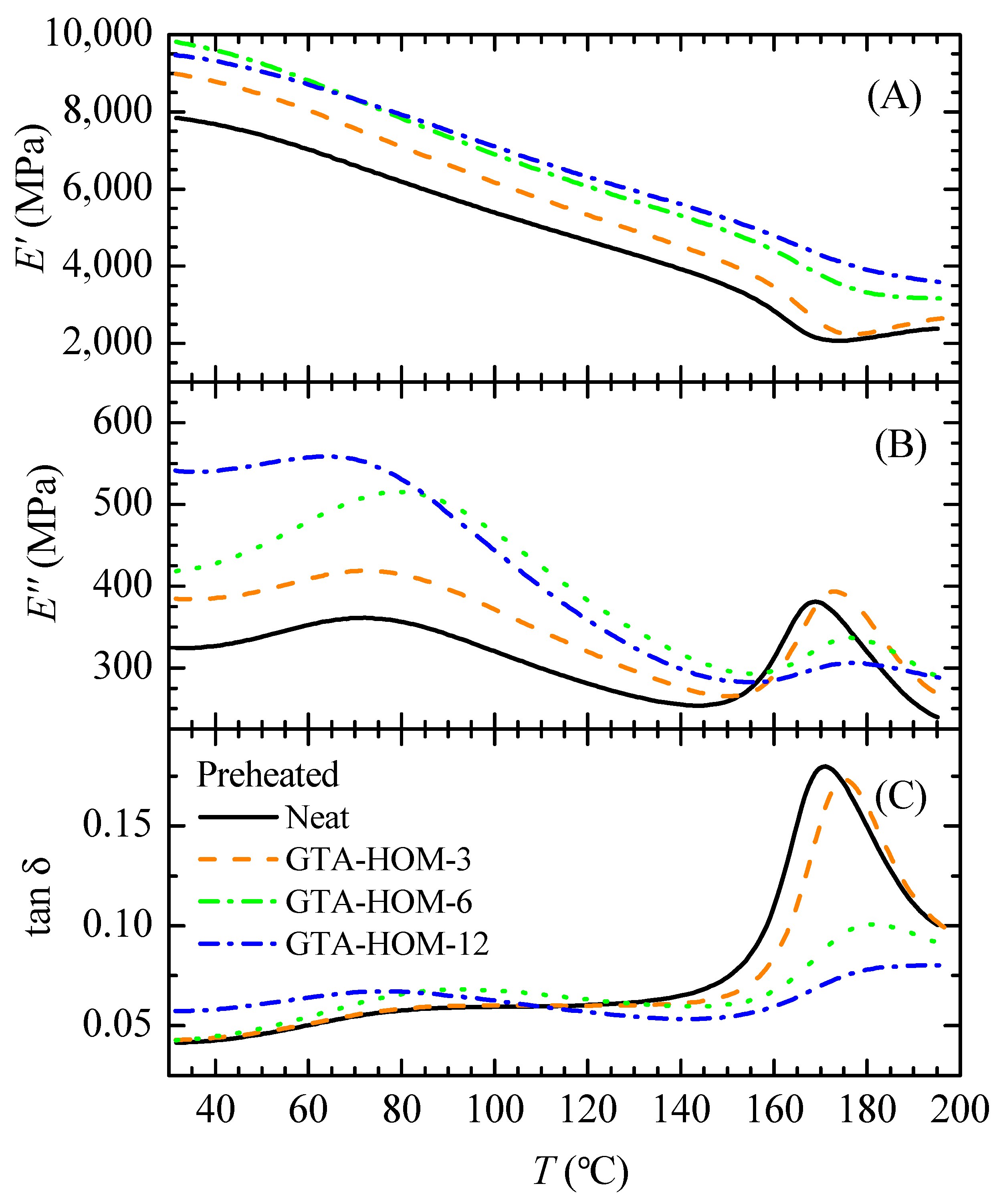
3.3. GTA-Crosslinked Films
3.4. Analysis of tanδ and Relation to Crosslinking
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.; Weller, C.; Ham, K. Characteristics of Chitosan Films as Affected by the Type of Solvent Acid. Food Sci. Food Biotechnol. 1998, 7, 263–268. [Google Scholar]
- Park, S.; Marsh, K.; Rhim, J. Characteristics of Different Molecular Weight Chitosan Films Affected by the Type of Organic Solvents. J. Food Sci. 2002, 67, 194–197. [Google Scholar] [CrossRef]
- Blair, H.; Guthrie, J.; Law, T.; Turkington, P. Chitosan and Modified Chitosan Membranes: I. Preparation and Characterisation. J. Appl. Polym. Sci. 1987, 33, 641–656. [Google Scholar] [CrossRef]
- Chen, R.; Lin, J.; Yang, M. Relationships Between the Chain Flexibilities of Chitosan Molecules and the Physical Properties of Their Casted Films. Carbohydr. Polym. 1994, 24, 41–46. [Google Scholar] [CrossRef]
- Wong, D.; Gastineau, F.; Gregorski, K.; Tillin, S.; Pavlath, A. Chitosan-Lipid Films: Microstructure and Surface Energy. J. Agric. Food Chem. 1992, 40, 540–544. [Google Scholar] [CrossRef]
- Srinivasa, P.; Ramesh, M.; Tharanathan, R. Effect of Plasticizers and Fatty Acids on Mechanical and Permeability Characteristics of Chitosan Films. Food Hydrocoll. 2007, 21, 1113–1122. [Google Scholar] [CrossRef]
- Mӧller, H.; Grelier, S.; Pardon, P.; Coma, V. Antimicrobial and Physicochemical Properties of Chitosan-HPMC-Based Films. J. Agric. Food Chem. 2004, 52, 6585–6591. [Google Scholar] [CrossRef] [PubMed]
- Bordenave, N.; Grelier, S.; Coma, V. Hydrophobization and Antimicrobial Activity of Chitosan and Paper-Based Packaging Material. Biomacromolecules 2010, 11, 88–96. [Google Scholar] [CrossRef]
- Hӧhne, S.; Frenzel, R.; Heppe, A.; Simon, F. Hydrophobic Chitosan Microparticles: Heterogeneous Phase Reaction with Hydrophobic Carbonyl Reagents. Biomacromolecules 2007, 8, 2051–2058. [Google Scholar] [CrossRef]
- Schreiber, S.; Bozell, J.; Hayes, D.; Zivanovic, S. Introduction of Primary Antioxidant Activity to Chitosan for Application as a Multifunctional Food Packaging Material. Food Hydrocoll. 2013, 33, 207–214. [Google Scholar] [CrossRef]
- Wu, C.; Tian, J.; Li, S.; Wu, T.; Hu, Y.; Chen, S.; Sugawara, T.; Ye, X. Structural Properties of Films and Rheology of Film-Forming Solutions of Chitosan Gallate for Food Packaging. Carbohydr. Polym. 2016, 146, 10–19. [Google Scholar] [CrossRef]
- Yoshida, C.; Oliveira, E.; Franco, T. Chitosan Tailor-Made Films: The Effects of Additives on Barrier and Mechanical Properties. Packag. Technol. Sci. 2009, 22, 161–170. [Google Scholar] [CrossRef]
- Bof, M.; Bordagaray, V.; Locaso, D.; García, M. Chitosan Molecular Weight Effect on Starch-Composite Film Properties. Food Hydrocoll. 2015, 51, 281–294. [Google Scholar] [CrossRef]
- Rubentheren, V.; Ward, T.; Chee, C.; Nair, P.; Salami, E.; Fearday, C. Effects of Heat Treatment on Chitosan Nanocomposite Film Reinforced with Nanocrystalline Cellulose and Tannic Acid. Carbohydr. Polym. 2016, 140, 202–208. [Google Scholar] [CrossRef]
- Jimenez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and Biodegradable Starch Films: A Review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Guilbert, S.; Gontard, N.; Gorris, L.G.M. Prolongation of the Shelf-Life of Perishable Food Products using Biodegradable Films and Coatings. LWT Food Sci. Technol. 1996, 29, 10–17. [Google Scholar] [CrossRef]
- Miller, K.S.; Krochta, J.M. Oxygen and Aroma Barrier Properties of Edible Films: A Review. Trends Food Sci. Technol. 1997, 8, 228–237. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.; Peppley, B.; Bui, V. Ionic Conductivity and Related Properties of Crosslinked Chitosan Membranes. J. Appl. Polym. Sci. 2003, 89, 306–317. [Google Scholar] [CrossRef]
- Pratt, D.; Wilson, L.; Kozinski, J. Preparation and Sorption Studies of Glutaraldehyde Cross-Linked Chitosan Copolymers. J. Colloid Interface Sci. 2013, 395, 205–211. [Google Scholar] [CrossRef]
- Rivero, S.; García, M.; Pinotti, A. Heat Treatment to Modify the Structural and Physical Properties of Chitosan-Based Films. J. Agric. Food Chem. 2012, 60, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R. Genipin-Crosslinked Chitosan Hydrogels as Biomedical and Pharmaceutical Aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Pandis, C.; Madeira, S.; Matos, J.; Kyritsis, A.; Mano, J.; Ribelles, J. Chitosan-Silica Hybrid Porous Membranes. Mater. Sci. Eng. C 2014, 42, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Coma, V.; Sebti, I.; Pardon, F.; Pichavant, F.; Deschamps, A. Film Properties from Crosslinking of Cellulosic Derivatives with a Polyfunctional Carboxylic Acid. Carbohydr. Polym. 2003, 51, 265–271. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A. Improving the Barrier and Mechanical Properties of Corn Starch-Based Edible Films: Effect of Citric Acid and Carboxymethyl Cellulose. Ind. Crops Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Sauraj; Kumar, B.; Negi, Y. Chitosan Film Incorporated with Citric Acid and Glycerol as an Active Packaging Material for Extension of Green Chilli Shelf Life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef]
- Welch, C.; Andrews, B. Ester Crosslinks: A Route to High Performance Nonformaldehyde Finishing of Cotton. Text. Chem. Color. 1989, 21, 13–17. [Google Scholar]
- Gawish, S.; Abo El-Ola, S.; Ramadan, A.; Abou El-Kheir, A. Citric Acid Used as a Crosslinking Agent for the Grafting of Chitosan onto Woolen Fabric. J. Appl. Polym. Sci. 2012, 123, 3345–3353. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel Superabsorbent Cellulose-Based Hydrogels Crosslinked with Citric Acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Fahmy, H.; Fouda, M. Crosslinking of Alginic Acid/Chitosan Matrices Using Polycarboxylic Acids and Their Utilization for Sodium Diclofenac Release. Carbohydr. Polym. 2008, 73, 606–611. [Google Scholar] [CrossRef]
- Varshosaz, J.; Alinagari, R. Effect of Citric Acid as Cross-Linking Agent on Insulin Loaded Chitosan Microspheres. Iran. Polym. J. 2005, 7, 647–656. [Google Scholar]
- Cui, Z.; Beach, E.; Anastas, P. Modification of Chitosan Films with Environmentally Benign Reagents for Increased Water Resistance. Green Chem. Lett. Rev. 2011, 4, 35–40. [Google Scholar] [CrossRef]
- Salam, A.; Venditti, R.; Pawlak, J.; El-Tahlawy, K. Crosslinked Hemicellulose Citrate-Chitosan Aerogel Foams. Carbohydr. Polym. 2011, 84, 1221–1229. [Google Scholar] [CrossRef]
- Olsson, E.; Hedenqvist, M.; Johansson, C.; Järnstrӧm, L. Influence of Citric Acid and Curing on Moisture Sorption, Diffusion and Permeability of Starch Films. Carbohydr. Polym. 2013, 94, 765–772. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric Acid Cross-Linking of Starch Films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Ritthidej, G.; Phaechamud, T.; Koizumi, T. Moist Heat Treatment on Physicochemical Change of Chitosan Salt Films. Int. J. Pharm. 2002, 232, 11–22. [Google Scholar] [CrossRef]
- Tual, C.; Espuche, E.; Escoubes, M.; Domard, A. Transport Properties of Chitosan Membranes: Influence of Crosslinking. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 1521–1529. [Google Scholar] [CrossRef]
- Hsien, T.; Rorrer, G. Heterogeneous Cross-Linking of Chitosan Gel Beads: Kinetics, Modeling, and Influence on Cadmium Ion Adsorption Capacity. Ind. Eng. Chem. Res. 1997, 36, 3631–3638. [Google Scholar] [CrossRef]
- Park, J.; Park, J.; Ruckenstein, E. Thermal and Dynamic Mechanical Analysis of PVA/MC Blend Hydrogels. Polymer 2001, 42, 4271–4280. [Google Scholar] [CrossRef]
- Galietta, G.; di Gioia, L.; Guilbert, S.; Cuq, B. Mechanical and Thermomechanical Properties of Films Based on Whey Proteins as Affected by Plasticizer and Crosslinking Agents. J. Dairy Sci. 1998, 81, 3123–3130. [Google Scholar] [CrossRef]
- Demirgӧz, D.; Elvira, C.; Mano, J.; Cunha, A.; Piskin, E.; Reis, R. Chemical Modification of Starch Based Biodegradable Polymeric Blends: Effects on Water Uptake, Degradation Behaviour and Mechanical Properties. Polym. Degrad. Stab. 2000, 70, 161–170. [Google Scholar] [CrossRef]
- Silva, S.; Caridade, S.; Mano, J.; Reis, R. Effect of Crosslinking in Chitosan/Aloe Vera-Based Membranes for Biomedical Applications. Carbohydr. Polym. 2013, 98, 581–588. [Google Scholar] [CrossRef]
- Casey, L.; Wilson, L. Investigation of Chitosan-PVA Composite Films and Their Adsorption Properties. J. Geosci. Environ. Prot. 2015, 3, 78–84. [Google Scholar] [CrossRef]
- Roberts, G.; Taylor, K. The Formation of Gels by Reaction of Chitosan with Glutaraldehyde. Macromol. Chem. Phys. 1989, 190, 951–960. [Google Scholar] [CrossRef]
- Pizzoli, M.; Ceccorulli, G.; Scandola, M. Molecular Motions of Chitosan in the Solid State. Carbohydr. Polym. 1991, 222, 205–213. [Google Scholar] [CrossRef]
- Viciosa, M.; Dionisio, M.; Silva, R.; Reis, R.; Mano, J. Molecular Motions in Chitosan Studied by Dielectric Relaxation Spectroscopy. Biomacromolecules 2004, 5, 2073–2078. [Google Scholar] [CrossRef]
- Nielsen, L.; Landel, R. Mechanical Properties of Polymers and Composites, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 1994. [Google Scholar]
- Krongauz, V. Diffusion in Polymers Dependence on Crosslink Density. J. Therm. Anal. Calorim. 2010, 102, 435–445. [Google Scholar] [CrossRef]
- Mitchell, J. The Rheology of Gels. J. Texture Stud. 1980, 11, 315–337. [Google Scholar] [CrossRef]
- Gartner, C.; Lόpez, B.; Sierra, L.; Graf, R.; Spiess, H.; Gaborieau, M. Interplay between Structure and Dynamics in Chitosan Films Investigated with Solid-State NMR, Dynamic Mechanical Analysis, and X-ray Diffraction. Biomacromolecules 2011, 12, 1380–1386. [Google Scholar] [CrossRef]
- Sakurai, K.; Takugi, M.; Takahashi, T. Crystal Structure of Chitosan. I. Unit Cell Parameters. Sen’i Gakkaishi 1984, 40, 246–253. [Google Scholar] [CrossRef]
- Ferry, J. Viscoelastic Properties of Polymers, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1980. [Google Scholar]
- Mattei, G.; Ahluwalia, A. Sample, testing and analysis variables affecting liver mechanical properties: A review. Acta Biomater. 2016, 45, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, L.; Iannuzzi, D.; Mattei, G. Comparison of frequency and strain-rate domain mechanical characterization. Sci. Rep. 2018, 8, 13697. [Google Scholar] [CrossRef] [PubMed]
- Oyen, M.L. Nanoindentation of biological and biomimetic materials. Exp. Technol. 2013, 37, 73–87. [Google Scholar] [CrossRef]
- van Hoorn, H.; Kurniawan, N.A.; Koenderink, G.H.; Iannuzzi, D. Local dynamic mechanical analysis for heterogeneous soft matter using ferrule-top indentation. Soft Matter. 2016, 12, 3066–3073. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Khor, E.; Ling, C. Effects of Dry Heat and Saturated Steam on the Physical Properties of Chitosan. J. Biomed. Mater. Res. 1999, 48, 111–116. [Google Scholar] [CrossRef]
- Neto, C.; Giacometti, J.; Job, A.; Ferreira, F.; Fonseca, J. Thermal Analysis of Chitosan Based Networks. Carbohydr. Polym. 2005, 62, 97–103. [Google Scholar] [CrossRef]
- Correia, D.; Gámiz-González, M.; Botelho, G.; Vidaurre, A.; Gomez Ribelles, J.; Lanceros-Mendez, A.; Sencadas, V. Effect of Neutralization and Cross-Linking on the Thermal Degradation of Chitosan Electrospun Membranes. J. Therm. Anal. Calorim. 2014, 117, 123–130. [Google Scholar] [CrossRef]
- Sakurai, K.; Maegawa, T.; Takahashi, T. Glass Transition Temperature of Chitosan and Miscibility of Chitosan/Poly(N-Vinyl Pyrrolidone) Blends. Polymer 2000, 41, 7051–7056. [Google Scholar] [CrossRef]
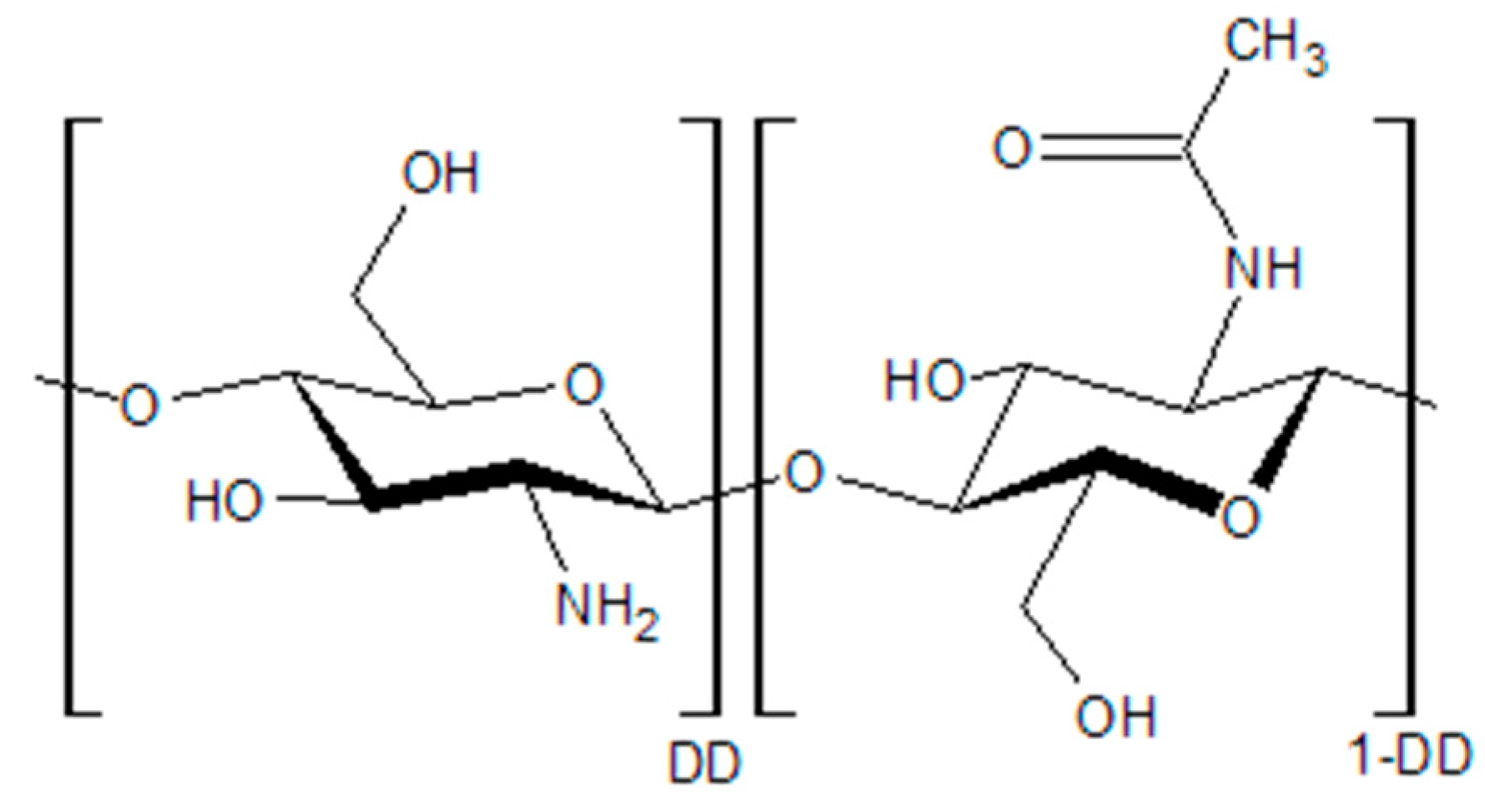
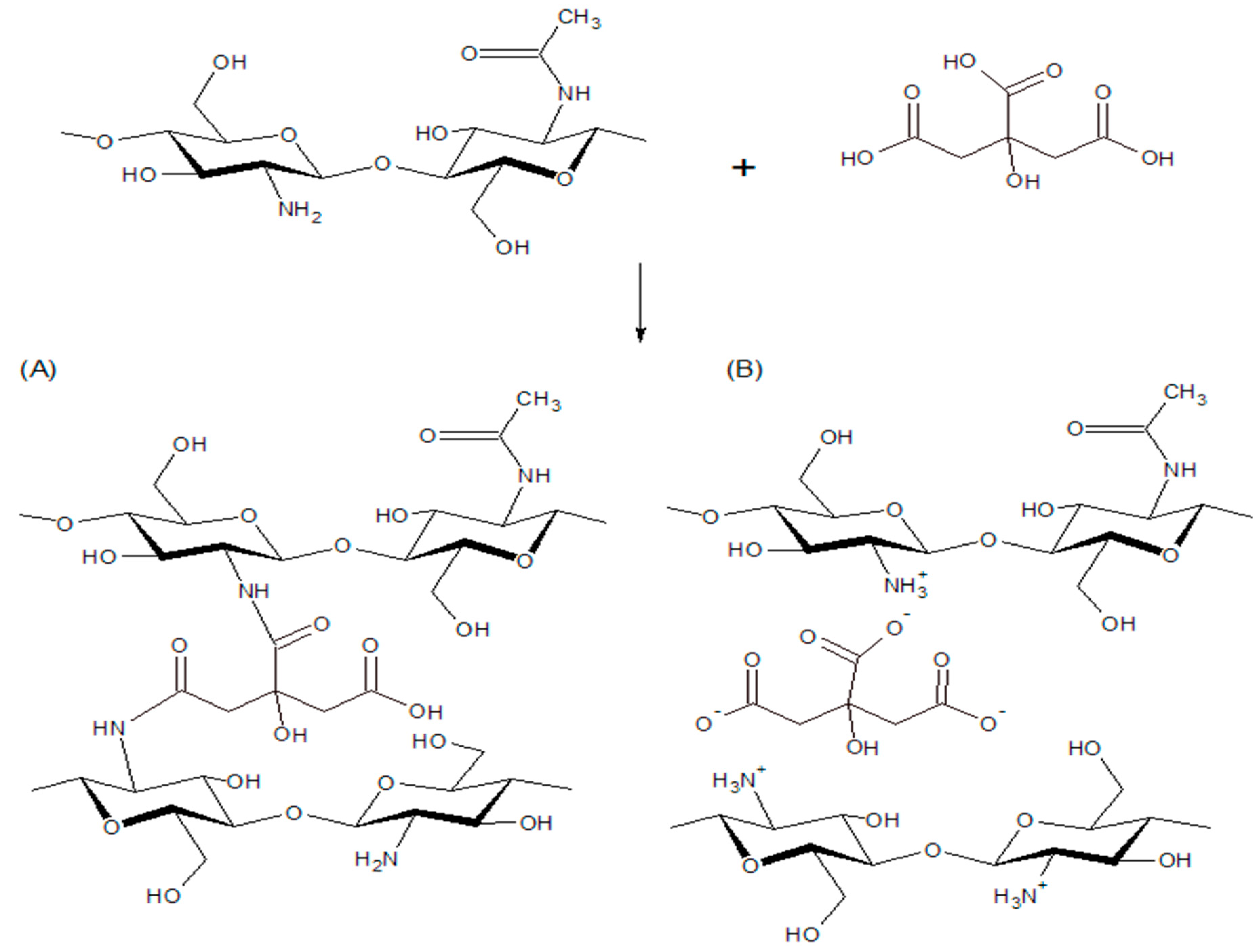
| Property | Chitosan | Common Plastics |
|---|---|---|
| Water Vapor Permeability (WVP) (g/(m·s·Pa)) | 1–10 × 10−11 [2,3,6,7,15] | 0.01–1 × 10−11 [16,17,18] |
| Oxygen Gas Permeability (OP) (cm3/(m·s·Pa)) | 1 × 10−15–1 × 10−13 [3,6,7,15] | 1 × 10−13–1× 10−12 [16,17,18] |
| Tensile Strength (TS) (MPa) | 1–100 [2,3,4,7,15] | 1–100 [16] |
| Elongation Before Break (EBB) (%) | 1–50 [2,3,4,7], 100 (with plasticizer) [2,7] | 1–500 [16] |
| Young’s Modulus (E) (GPa) | 0.1–3 [7,15] | 1–10 |
| Film | Code Name |
|---|---|
| Neat | Neat |
| Neutralized | Neutralized |
| Homogeneously crosslinked with 3% (w/w) glutaraldehyde (GTA) | GTA-HOM-3 |
| Homogeneously crosslinked with 6% (w/w) glutaraldehyde | GTA-HOM-6 |
| Homogeneously crosslinked with 12% (w/w) glutaraldehyde | GTA-HOM-12 |
| Heterogeneously crosslinked with 6% (w/w) glutaraldehyde | GTA-HET-6 |
| Heterogeneously crosslinked with 12% (w/w) glutaraldehyde | GTA-HET-12 |
| Heterogeneously prepared with 15% (w/w) citric acid (CA) | CA |
| Heterogeneously prepared with 15% (w/w) citric acid, heat treated | CA-HT |
| Film | Parameter | E′ (35 °C) | E′ (195 °C) | tanδ Peak 1 | tanδ Peak 2 | ||
|---|---|---|---|---|---|---|---|
| T | tanδ | T | tanδ | ||||
| (MPa) | (MPa) | (°C) | (°C) | ||||
| Neat | mean | 5071 a | 2382 a | 102.0 a | 0.129 a | 164.8 a | 0.216 a |
| ± | 432 | 127 | 2.8 | 0.015 | 2.8 | 0.045 | |
| COV * (%) | 8.5 | 5.3 | 2.7 | 11.7 | 1.7 | 21.1 | |
| n | 6 | 6 | 6 | 6 | 6 | 6 | |
| Neutralized | mean | 6665 b | 4143 b | 106.7 a | 0.089 b | 187.6 b | 0.078 b |
| ± | 728 | 334 | 12.9 | 0.003 | 9.8 | 0.001 | |
| COV * (%) | 11 | 8 | 12.1 | 3.4 | 5.2 | 1.7 | |
| n | 5 | 5 | 5 | 5 | 4 | 5 | |
| CA | mean | 7588 c | 4727 c | 106.9 a | 0.099 b | 130.2 c | 0.097 b,c |
| ± | 489 | 349 | 8.9 | 0.016 | 8.4 | 0.026 | |
| COV * (%) | 6.4 | 7.4 | 8.3 | 16.4 | 6.5 | 26.3 | |
| n | 3 | 3 | 3 | 3 | 2 | 2 | |
| CA+HT | mean | 7605 c | 4479 b,c | 104.2 a | 0.088 b | 131.1 c | 0.096 c |
| ± | 871 | 635 | 4.6 | 0.005 | - | - | |
| COV * (%) | 11.5 | 14.2 | 4.4 | 5.5 | - | - | |
| n | 4 | 4 | 4 | 4 | 1 | 1 | |
| GTA-HOM-3 | n = 1 | 5123 | 2475 | 106.5 | 0.156 | 165.7 | 0.201 |
| GTA-HOM-6 | n = 1 | 6222 | 3367 | 96.1 | 0.110 | 168.9 | 0.110 |
| GTA-HOM-12 | n = 1 | 5882 | 3160 | 97.3 | 0.090 | 167.0 | 0.091 |
| Film | Parameter | E′ (35 °C) | E′ (195 °C) | tanδ Peak 1 | tanδ Peak 2 | ||
|---|---|---|---|---|---|---|---|
| T | tanδ | T | tanδ | ||||
| (MPa) | (MPa) | (°C) | (°C) | ||||
| Neat | mean | 7826 a | 2289 a | - | - | 169.3 a | 0.213 a |
| s | 427 | 152 | - | - | 4.2 | 0.034 | |
| COV * (%) | 5.5 | 6.6 | - | - | 2.5 | 16.0 | |
| n | 4 | 4 | 0 | 0 | 4 | 4 | |
| Neutralized | mean | 9384 b | 4162 b | 124.1 a | 0.059 a | 187.2 b | 0.078 b |
| s | 859 | 385 | 3.8 | 0.002 | 5.5 | 0.003 | |
| COV (%) | 6.7 | 6.8 | 2.5 | 3.4 | 2.3 | 2.6 | |
| n | 6 | 6 | 6 | 6 | 6 | 6 | |
| CA | mean | 9982 b | 4348 b | 117.8 b | 0.059 a,b | 166.5 a | 0.061c |
| s | 220 | 93 | 1.0 | 0.005 | 1.1 | 0.005 | |
| COV (%) | 2.2 | 2.1 | 0.8 | 8.3 | 0.7 | 9.0 | |
| n | 2 | 2 | 2 | 2 | 2 | 2 | |
| CA+HT | mean | 9351 b | 4612 b | 125.8 a,b | 0.050 b | 165.3 a | 0.051 c |
| s | 609 | 244 | 10 | 0.004 | 3.2 | 0.004 | |
| COV (%) | 6.5 | 5.2 | 7.9 | 8.0 | 1.9 | 8.8 | |
| n | 3 | 3 | 3 | 3 | 3 | 3 | |
| GTA-HOM-3 | mean | 9119 | 2749 | 97.3 | 0.060 | 175.3 | 0.167 |
| s | 282.1 | 159.3 | 5.8 | 0.000 | 0.5 | 0.008 | |
| n | 2 | 2 | 2 | 2 | 2 | 2 | |
| GTA-HOM-6 | n = 1 | 9740 | 3162 | 92.4 | 0.068 | 181.6 | 0.101 |
| GTA-HOM-12 | n = 1 | 9413 | 3584 | 77.9 | 0.067 | 191.8 | 0.080 |
| Film Type | Preheat | Parameter | Baseline | Center (°C) | FWHM | Height |
|---|---|---|---|---|---|---|
| Neat | N | mean | 0.068 a | 166.0 a | 36.5 a | 0.130 a |
| ± | 0.005 | 2.9 | 2.6 | 0.034 | ||
| Neat | Y | mean | 0.052 b | 177.2 b | 29.9 a | 0.116 a |
| ± | 0.008 | 6.5 | 4.4 | 0.047 | ||
| Neutralized | N | mean | 0.057 b | 188.8 c | 105.5 b | 0.021 b,c |
| ± | 0.002 | 12.2 | 29.1 | 0.002 | ||
| Neutralized | Y | mean | 0.029 c | 188.5 c | 67.9 c | 0.048 c |
| ± | 0.006 | 3.4 | 4.8 | 0.008 | ||
| CA+HT | N | mean | 0.046 b | 137.6 d | 56.8 c | 0.013 b |
| ± | 0.002 | 5.7 | 3.4 | 0.002 | ||
| CA+HT | Y | mean | 0.045 b | 168.3 a | 32.2 a | 0.006 b |
| ± | 0.006 | 4.1 | 7.6 | 0.004 | ||
| CA | N | mean | 0.046 b | 146.2 d | 44.7 a | 0.024 b |
| ± | 0.004 | 2.0 | 5.5 | 0.004 | ||
| CA | Y | mean | 0.045 b | 173.3 a,b | 44.1 a | 0.012 b |
| ± | 0.005 | 13.3 | 27.8 | 0.008 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khouri, J.; Penlidis, A.; Moresoli, C. Viscoelastic Properties of Crosslinked Chitosan Films. Processes 2019, 7, 157. https://doi.org/10.3390/pr7030157
Khouri J, Penlidis A, Moresoli C. Viscoelastic Properties of Crosslinked Chitosan Films. Processes. 2019; 7(3):157. https://doi.org/10.3390/pr7030157
Chicago/Turabian StyleKhouri, Joseph, Alexander Penlidis, and Christine Moresoli. 2019. "Viscoelastic Properties of Crosslinked Chitosan Films" Processes 7, no. 3: 157. https://doi.org/10.3390/pr7030157
APA StyleKhouri, J., Penlidis, A., & Moresoli, C. (2019). Viscoelastic Properties of Crosslinked Chitosan Films. Processes, 7(3), 157. https://doi.org/10.3390/pr7030157