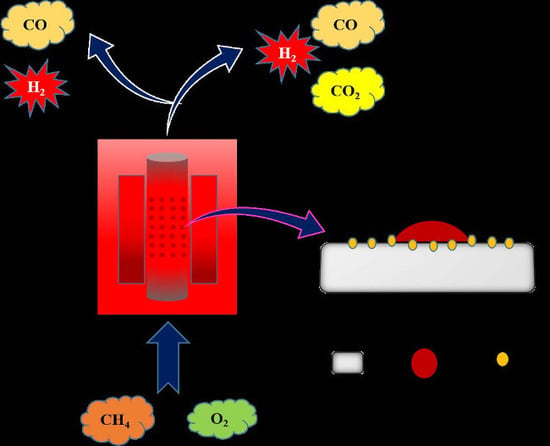
Highly Selective Syngas/H2 Production via Partial Oxidation of CH4 Using (Ni, Co and Ni–Co)/ZrO2–Al2O3 Catalysts: Influence of Calcination Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
2.3. Catalyst Testing
2.4. Catalyst Characterization
3. Results and Discussion
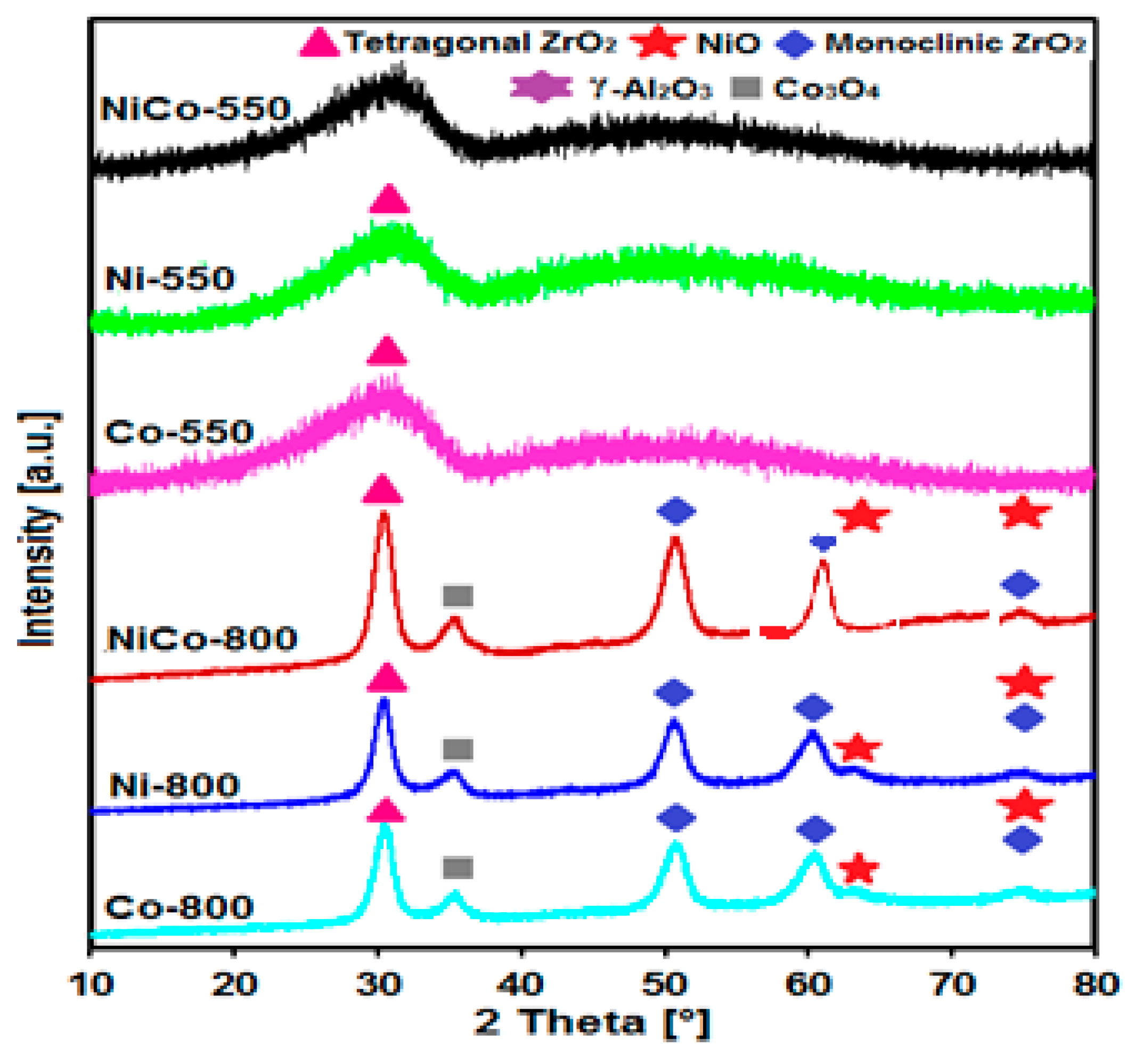
3.1. X-ray Diffraction (XRD)
3.2. Textural Properties
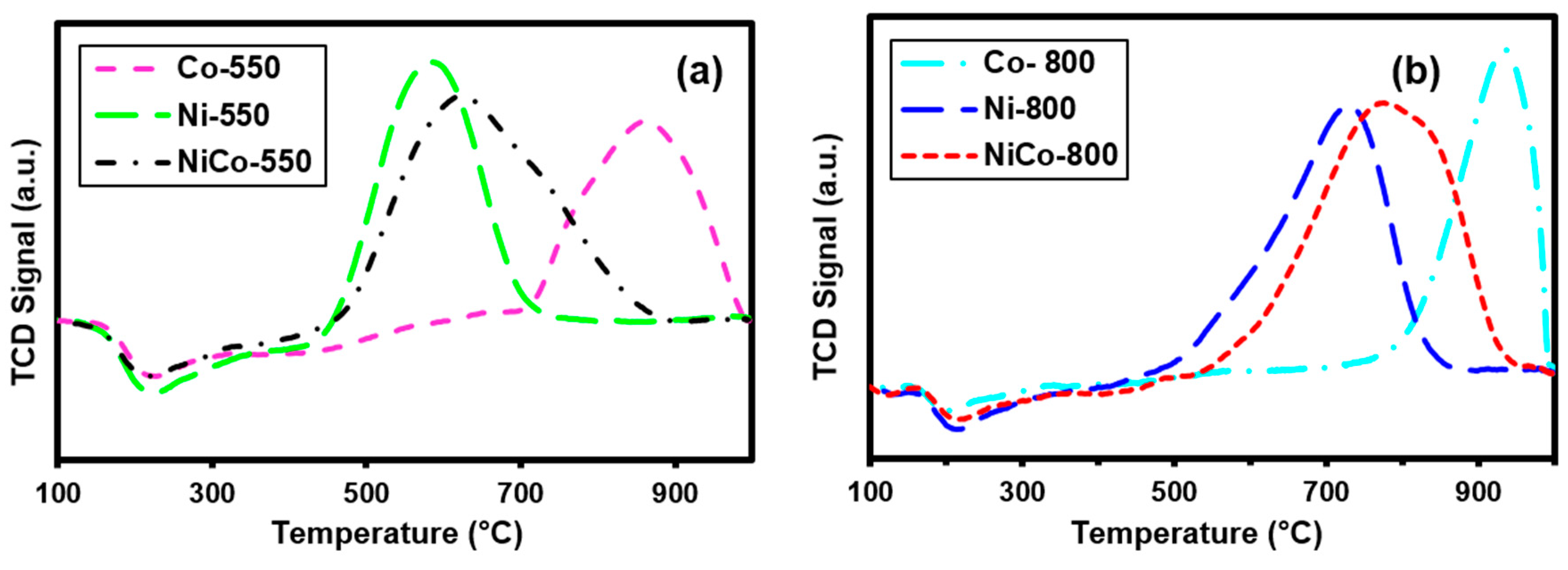
3.3. Temperature-Programmed Reduction (H2-TPR)
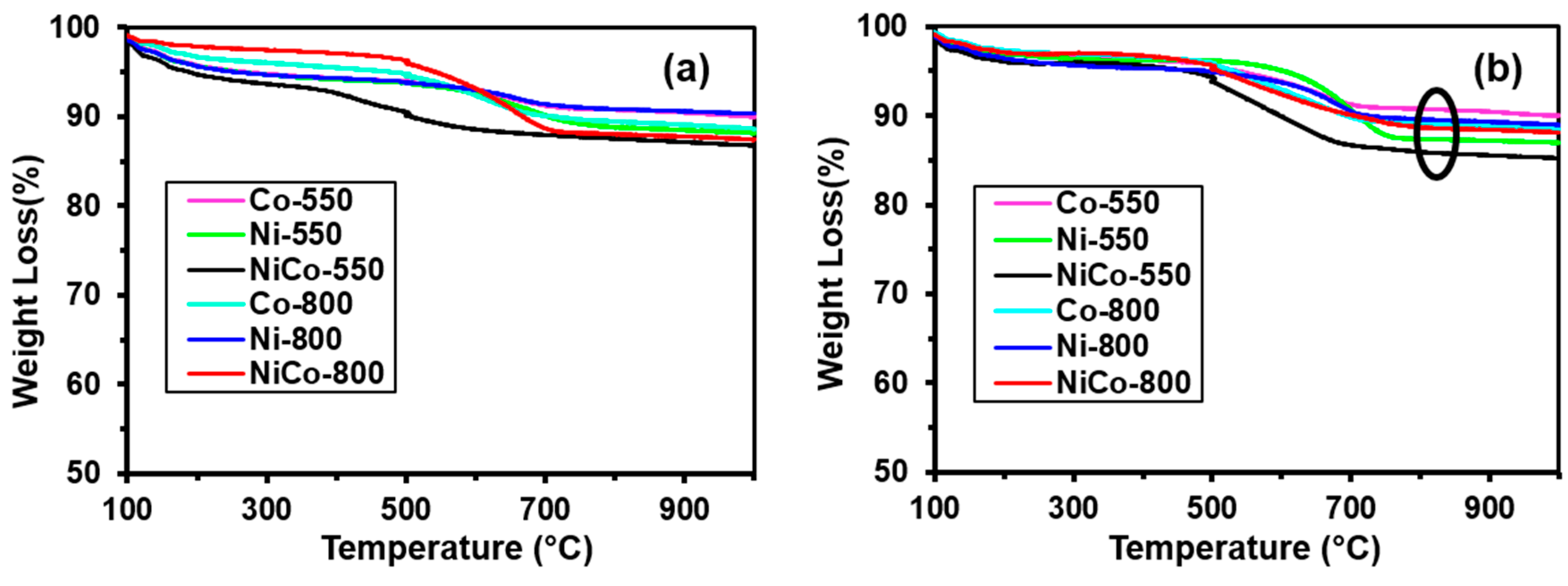
3.4. Thermal Analysis for Carbon Deposition
3.5. Temperature-Programmed Desorption of CO2 (CO2-TPD)
3.6. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
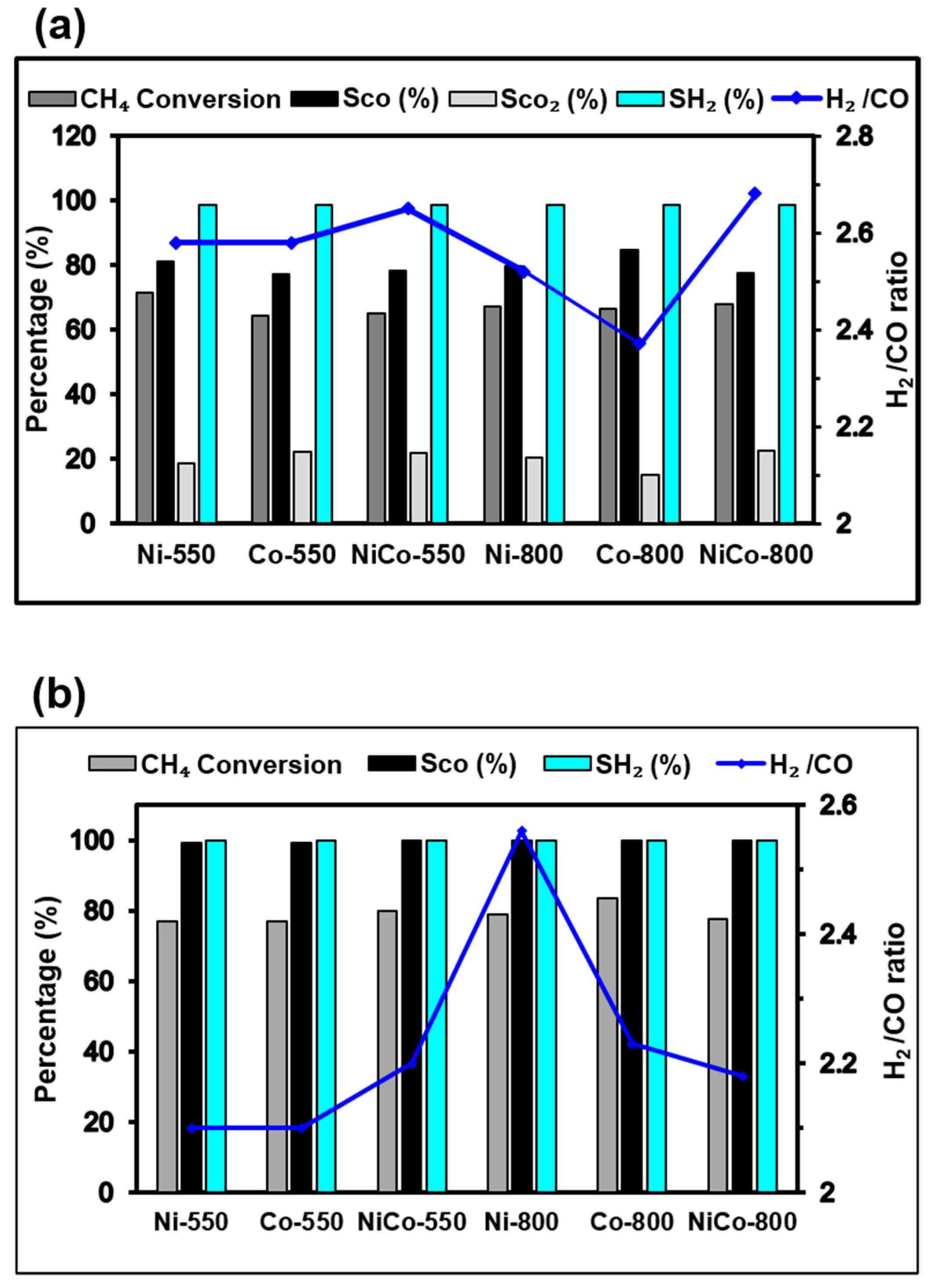
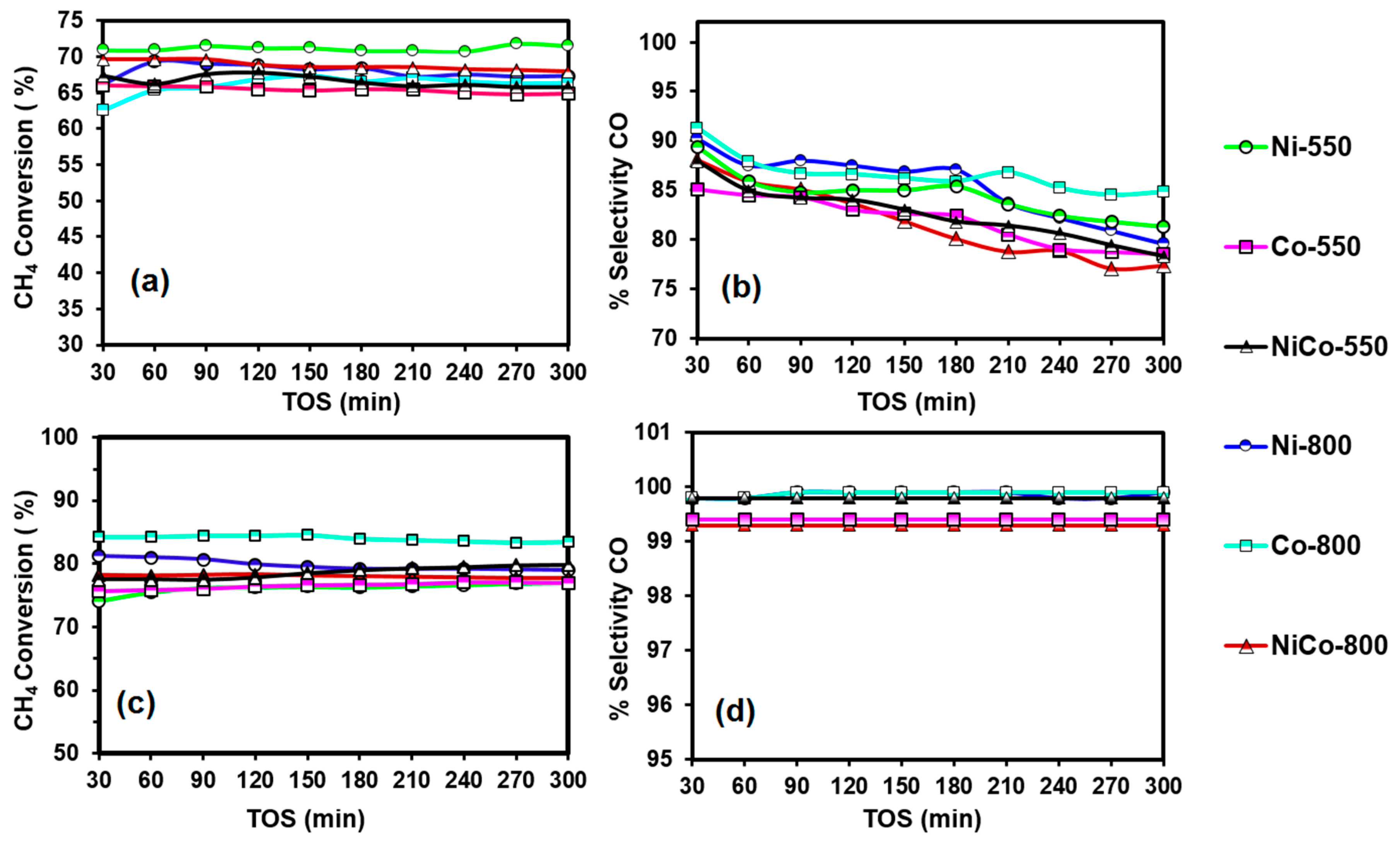
3.7. Catalytic Activity
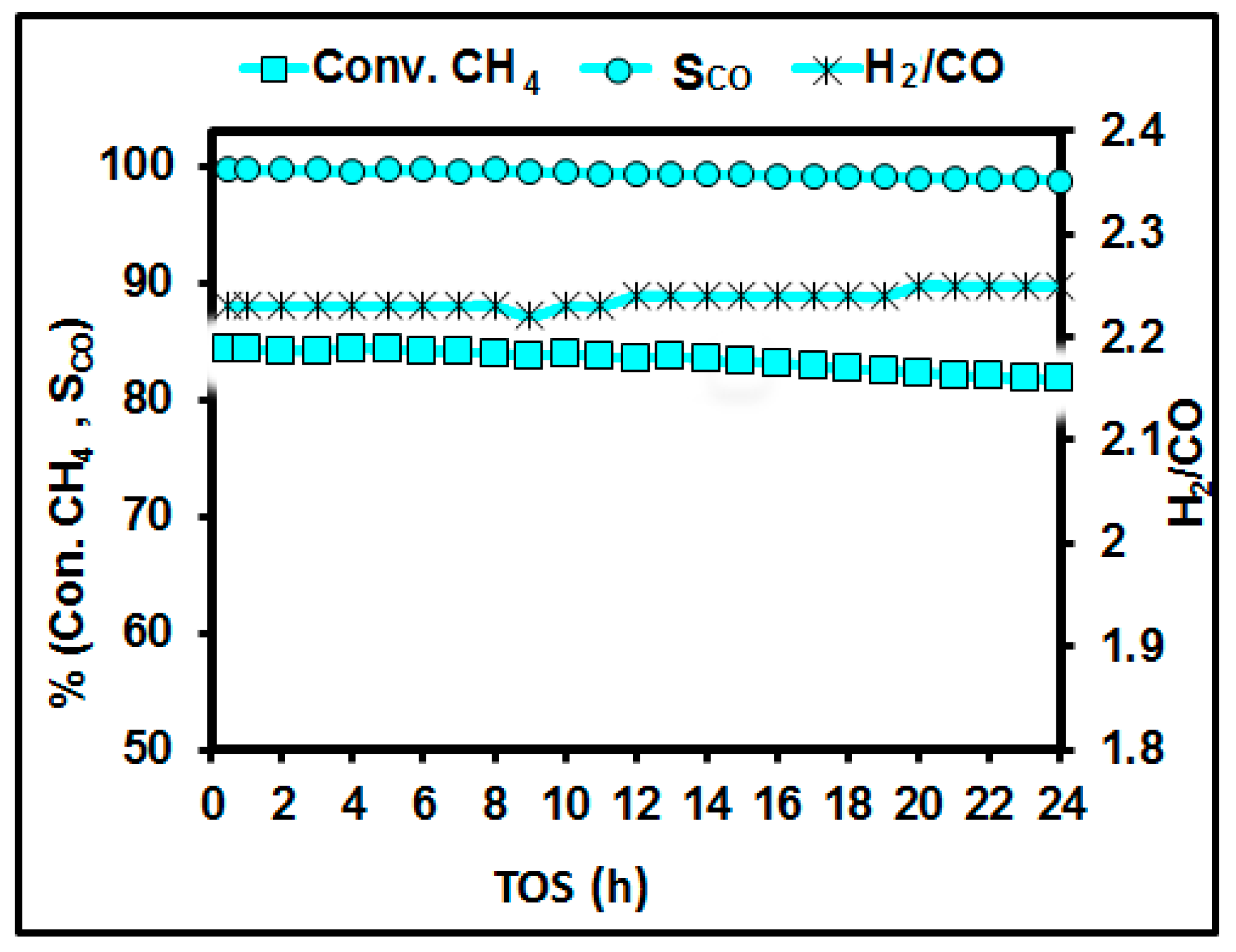
3.8. Long-Term Stability Test
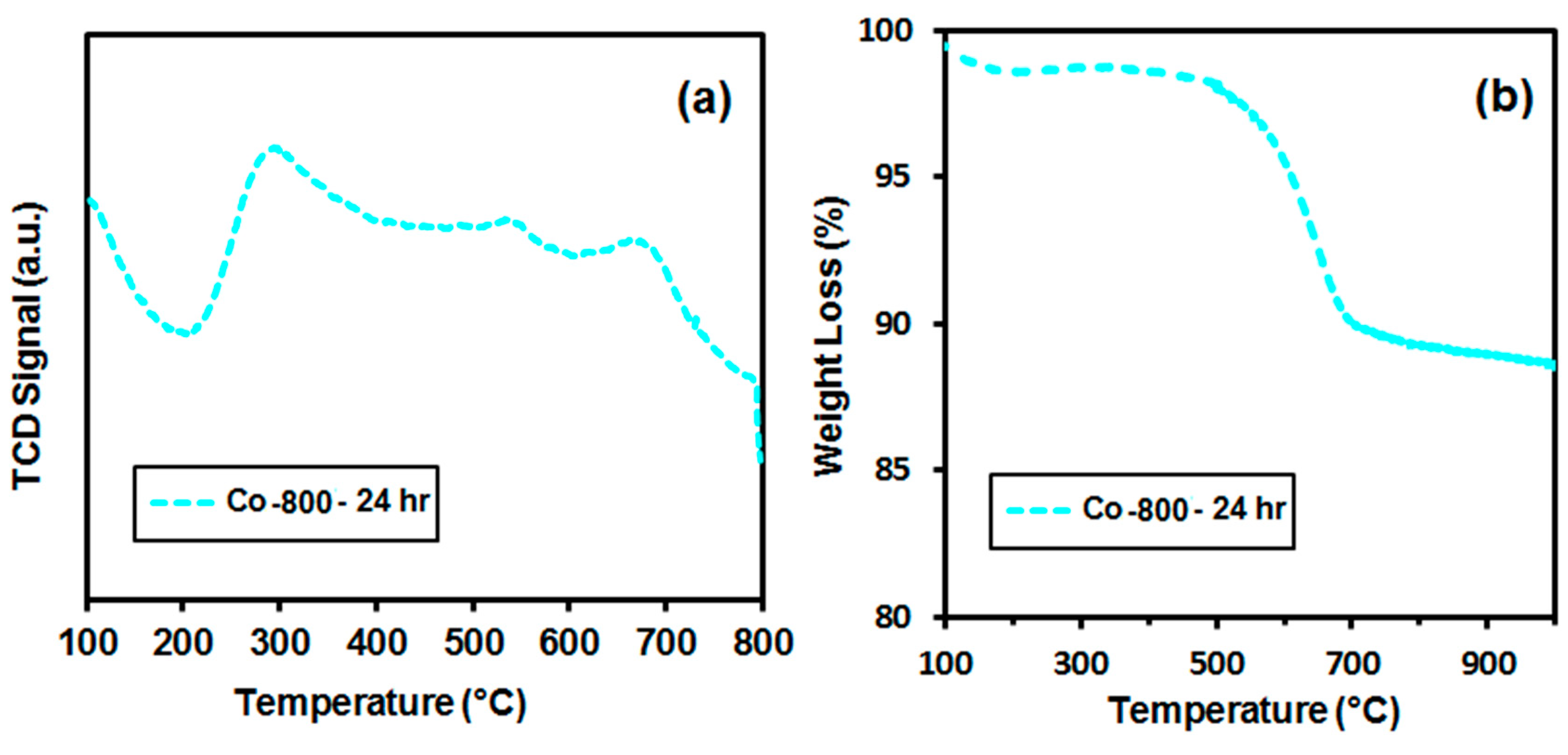
3.9. Post (Long Term Test) Characterizations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Wang, T.; Li, Q.; Wang, D. A Study of Acetylene Production by Methane Flaming in a Partial Oxidation Reactor. Chin. J. Chem. Eng. 2011, 19, 424–433. [Google Scholar] [CrossRef]
- Chibane, L.; Djellouli, B. Role of Periodic Input Composition and Sweeping Gas for Improvement of Hydrogen Production in a Palladium Membrane Reactor by Partial Oxidation of Methane. Chin. J. Chem. Eng. 2012, 20, 577–588. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, H.; Lin, W. Preparation and Characterization of a Perovskite-type Mixed Conducting SrFe0.6Cu0.3Ti0.1O3−δ Membrane for Partial Oxidation of Methane to Syngas. Chin. J. Chem. Eng. 2008, 16, 411–415. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Al-Fatesh, A.S.; Chowdhury, B.; Ibrahim, A.A.; Khan, W.U.; Hassan, S.; Sasudeen, K.; Abasaeed, A.E. Bi-metallic catalysts of mesoporous Al2O3 supported on Fe, Ni and Mn for methane decomposition: Effect of activation temperature. Chin. J. Chem. Eng. 2018, 26, 1904–1911. [Google Scholar] [CrossRef]
- Jun, J.H.; Lee, S.J.; Lee, S.H.; Lee, T.J.; Kong, S.J.; Lim, T.H.; Nam, S.W.; Hong, S.A.; Yoon, K.J. Characterization of a nickel-strontium phosphate catalyst for partial oxidation of methane. Korean J. Chem. Eng. 2003, 20, 829–834. [Google Scholar] [CrossRef]
- Nichio, N.; Casella, M.; Ferretti, O.; Gonzalez, M.; Nicot, C.; Moraweck, B.; Frety, R. Partial oxidation of methane to synthesis gas. Behaviour of different Ni supported catalysts. Catal. Lett. 1996, 42, 65–72. [Google Scholar] [CrossRef]
- Singha, R.K.; Shukla, A.; Yadav, A.; Konathala, L.S.; Bal, R. Effect of metal-support interaction on activity and stability of Ni-CeO2 catalyst for partial oxidation of methane. Appl. Catal. Environ. 2017, 202, 473–488. [Google Scholar] [CrossRef]
- Ding, C.; Ai, G.; Zhang, K.; Yuan, Q.; Han, Y.; Ma, X.; Wang, J.; Liu, S. Coking resistant Ni/ZrO2@SiO2 catalyst for the partial oxidation of methane to synthesis gas. Int. J. Hydrog. Energy 2015, 40, 6835–6843. [Google Scholar] [CrossRef]
- Wu, P.; Li, X.; Ji, S.; Lang, B.; Habimana, F.; Li, C. Steam reforming of methane to hydrogen over Ni-based metal monolith catalysts. Catal. Today 2009, 146, 82–86. [Google Scholar] [CrossRef]
- Berrocal, G.P.; Da Silva, A.L.; Assaf, J.M.; Albornoz, A.; do Carmo Rangel, M. Novel supports for nickel-based catalysts for the partial oxidation of methane. Catal. Today 2010, 149, 240–247. [Google Scholar] [CrossRef]
- Sharifi, M.; Haghighi, M.; Rahmani, F.; Karimipour, S. Syngas production via dry reforming of CH4 over Co-and Cu-Promoted Ni/Al2O3–ZrO2 nanocatalysts synthesized via sequential impregnation and sol-gel methods. J. Nat. Gas Sci. Eng. 2014, 21, 993–1004. [Google Scholar] [CrossRef]
- Therdthianwong, S.; Siangchin, C.; Therdthianwong, A. Improvement of coke resistance of Ni/Al2O3 catalyst in CH4/CO2 reforming by ZrO2 addition. Fuel Process. Technol. 2008, 89, 160–168. [Google Scholar] [CrossRef]
- Song, J.H.; Han, S.J.; Yoo, J.; Park, S.; Kim, D.H.; Song, I.K. Hydrogen production by steam reforming of ethanol over Ni–X/Al2O3–ZrO2 (X = Mg, Ca, Sr, and Ba) xerogel catalysts: Effect of alkaline earth metal addition. J. Mol. Catal. Chem. 2016, 415, 151–159. [Google Scholar] [CrossRef]
- Zagaynov, I.; Loktev, A.; Arashanova, A.; Ivanov, V.; Dedov, A.; Moiseev, I. Ni (Co)-Gd0.1Ti0.1Zr0.1Ce0.7O2 mesoporous materials in partial oxidation and dry reforming of methane into synthesis gas. Chem. Eng. J. 2016, 290, 193–200. [Google Scholar] [CrossRef]
- Weng, W.Z.; Pei, X.Q.; Li, J.M.; Luo, C.R.; Liu, Y.; Lin, H.Q.; Huang, C.J.; Wan, H.L. Effects of calcination temperatures on the catalytic performance of Rh/Al2O3 for methane partial oxidation to synthesis gas. Catal. Today 2006, 117, 53–61. [Google Scholar] [CrossRef]
- Sokolov, S.; Kondratenko, E.V.; Pohl, M.-M.; Rodemerck, U. Effect of calcination conditions on time on-stream performance of Ni/La2O3-ZrO2 in low-temperature dry reforming of methane. Int. J. Hydrog. Energy 2013, 38, 16121–16132. [Google Scholar] [CrossRef]
- Dedov, A.G.; Loktev, A.S.; Komissarenko, D.A.; Parkhomenko, K.V.; Roger, A.C.; Shlyakhtin, O.A.; Mazo, G.N.; Moiseev, I.I. High-selectivity partial oxidation of methane into synthesis gas: The role of the redox transformations of rare earth-alkali earth cobaltate-based catalyst components. Fuel Process. Technol. 2016, 148, 128–137. [Google Scholar] [CrossRef]
- Abasaeed, A.E.; Al-Fatesh, A.S.; Naeem, M.A.; Ibrahim, A.A.; Fakeeha, A.H. Catalytic performance of CeO2 and ZrO2 supported Co catalysts for hydrogen production via dry reforming of methane. Int. J. Hydrog. Energy 2015, 40, 6818–6826. [Google Scholar] [CrossRef]
- Enger, B.C.; Lødeng, R.; Anders Holmen, A. Modified cobalt catalysts in the partial oxidation of methane at moderate temperatures. J. Catal. 2009, 262, 188–198. [Google Scholar] [CrossRef]
- Lødeng, R.; Bjørgum, E.; Christian, B.; Enger Eilertsen, J.L.; Anders Holmen, A.; Krogh, B.; Rønnekleiv, M.; Erling Rytter, E. Catalytic partial oxidation of CH4 to H2 over cobalt catalysts at moderate temperatures. Appl. Catal. 2007, 333, 11–23. [Google Scholar] [CrossRef]
- Silver, R.G.; Hou, C.J.; Ekerdt, J.G. The role of lattice anion vacancies in the activation of CO and as the catalytic site for methanol synthesis over zirconium dioxide and yttria-doped zirconium dioxide. J. Catal. 1989, 118, 400–416. [Google Scholar] [CrossRef]
- Li, G.; Li, W.; Zhang, M.; Tao, K. Morphology and hydrodesulfurization activity of CoMo sulfide supported on amorphous ZrO2 nanoparticles combined with Al2O3. Appl. Catal. 2004, 273, 233–238. [Google Scholar] [CrossRef]
- Naeem, M.A.; Al-Fatesh, A.S.; Abasaeed, A.E.; Fakeeha, A.H. Activities of Ni-based nano catalysts for CO2-CH4 reforming prepared by polyol process. Fuel Process. Technol. 2014, 122, 141–152. [Google Scholar] [CrossRef]
- Klein, J.C.; Hercules, D.M. Surface characterization of model Urushibara catalysts. J. Catal. 1983, 82, 424–441. [Google Scholar] [CrossRef]
- Mile, B.; Stirling, D.; Zammitt, M.A.; Lowell, A.; Webb, M. The location of nickel oxide and nickel in silica-supported catalysts: Two forms of “NiO” and the assignment of temperature-programmed reduction profiles. J. Catal. 1998, 114, 217–229. [Google Scholar] [CrossRef]
- Kim, P.; Kim, Y.; Kim, H.; Song, I.K.; Yi, J. Synthesis and characterization of mesoporous alumina with nickel incorporated for use in the partial oxidation of methane into synthesis gas. Appl. Catal. 2004, 272, 157–166. [Google Scholar] [CrossRef]
- Arone, S.; Bagnasco, G.; Busca, G.; Lisi, L.; Russo, G.; Turco, M. Catalytic combustion of methane over transition metal oxides. Stud. Surf. Sci. Catal. 1998, 119, 65–70. [Google Scholar]
- Al-Fatesh, A.S.; Arafat, Y.; Ibrahim, A.A.; Atia, H.; Fakeeha, A.H.; Armbruster, U.; Abasaeed, A.E.; Frusteri, F. Evaluation of Co-Ni/Sc-SBA-15 as a novel coke resistant catalyst for syngas production via CO2 reforming of methane. Appl. Catal. 2018, 567, 102–111. [Google Scholar] [CrossRef]
- Jongsomjit, B.; Panpranot, J.; Goodwin, J.G. Effect of zirconia-modified alumina on the properties of Co/γ-Al2O3 catalysts. J. Catal. 2003, 215, 66–77. [Google Scholar] [CrossRef]
- Jongsomjit, B.; Sakdamnuson, C.; Goodwin, J.G.; Praserthdam, P. Co-support compound formation in titania-supported cobalt catalyst. Catal. Lett. 2004, 94, 209–215. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Nascente, P.A.P.; Assaf, E.M. Partial oxidation of methane on NiO-MgO-ZrO2 catalysts. Fuel 2012, 97, 630–637. [Google Scholar] [CrossRef]
- Harrison, P.G.; Ball, I.K.; Daniell, W.; Lukinskas, P.; Céspedes, M.A.; Miró, E.E.; Ulla, M.A.A. Cobalt catalysts for the oxidation of diesel soot particulate. Chem. Eng. J. 2003, 95, 47–55. [Google Scholar] [CrossRef]
- Rui, Z.; Feng, D.; Chen, H.; Ji, H. Anodic TiO2 nanotube array supported nickel-noble metal bimetallic catalysts for activation of CH4 and CO2 to syngas. Int. J. Hydrog. Energy 2014, 39, 16252–16261. [Google Scholar] [CrossRef]
- Djinović, P.; Črnivec, I.G.O.; Erjavec, B.; Pintar, A. Influence of active metal loading and oxygen mobility on coke-free dry reforming of Ni–Co bimetallic catalysts. Appl. Catal. 2012, 125, 259–270. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Atia, H.; Ibrahim, A.A.; Manh Ha, Q.L.; Schneider, M.; Pohl, M.M.; Fakeeha, A.H. CO2-reforming of methane to produce syngas over Co-Ni/SBA-15 catalyst: Effect of support modifiers (Mg, La and Sc) on catalytic stability. J. CO2 Util. 2017, 21, 395–404. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Shen, S. Design of stable Ni catalysts for partial oxidation of methane to synthesis gas. J. Catal. 1998, 177, 386–388. [Google Scholar] [CrossRef]
- Pompeo, F.; Nichio, N.N.; Ferretti, O.A.; Resasco, D. Study of Ni catalysts on different supports to obtain synthesis gas. Int. J. Hydrog Energy 2005, 30, 1399–1405. [Google Scholar] [CrossRef]
- Souza, M.; Aranda, D.; Scmal, M. Reforming of Methane with Carbon Dioxide over Pt/ZrO2/Al2O3 Catalysts. J. Catal. 2001, 204, 498–511. [Google Scholar] [CrossRef]
- Zhang, Z.; Verykios, X. Carbon dioxide reforming of methane to synthesis gas over supported Ni catalysts. Catal. Today 1994, 21, 589–595. [Google Scholar] [CrossRef]



| Catalysts | BET Surface Area (m2/g) | P.V. (cm3/g) | P.D. (Å) |
|---|---|---|---|
| Ni-550 | 212 | 0.51 | 100 |
| Co-550 | 230 | 0.44 | 82 |
| Ni–Co-550 | 216 | 0.61 | 114 |
| Ni-800 | 105 | 0.37 | 134 |
| Co-800 | 130 | 0.38 | 104 |
| Ni–Co-800 | 123 | 0.47 | 142 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza Fakeeha, A.; Arafat, Y.; Aidid Ibrahim, A.; Shaikh, H.; Atia, H.; Elhag Abasaeed, A.; Armbruster, U.; Sadeq Al-Fatesh, A. Highly Selective Syngas/H2 Production via Partial Oxidation of CH4 Using (Ni, Co and Ni–Co)/ZrO2–Al2O3 Catalysts: Influence of Calcination Temperature. Processes 2019, 7, 141. https://doi.org/10.3390/pr7030141
Hamza Fakeeha A, Arafat Y, Aidid Ibrahim A, Shaikh H, Atia H, Elhag Abasaeed A, Armbruster U, Sadeq Al-Fatesh A. Highly Selective Syngas/H2 Production via Partial Oxidation of CH4 Using (Ni, Co and Ni–Co)/ZrO2–Al2O3 Catalysts: Influence of Calcination Temperature. Processes. 2019; 7(3):141. https://doi.org/10.3390/pr7030141
Chicago/Turabian StyleHamza Fakeeha, Anis, Yasir Arafat, Ahmed Aidid Ibrahim, Hamid Shaikh, Hanan Atia, Ahmed Elhag Abasaeed, Udo Armbruster, and Ahmed Sadeq Al-Fatesh. 2019. "Highly Selective Syngas/H2 Production via Partial Oxidation of CH4 Using (Ni, Co and Ni–Co)/ZrO2–Al2O3 Catalysts: Influence of Calcination Temperature" Processes 7, no. 3: 141. https://doi.org/10.3390/pr7030141
APA StyleHamza Fakeeha, A., Arafat, Y., Aidid Ibrahim, A., Shaikh, H., Atia, H., Elhag Abasaeed, A., Armbruster, U., & Sadeq Al-Fatesh, A. (2019). Highly Selective Syngas/H2 Production via Partial Oxidation of CH4 Using (Ni, Co and Ni–Co)/ZrO2–Al2O3 Catalysts: Influence of Calcination Temperature. Processes, 7(3), 141. https://doi.org/10.3390/pr7030141