An Experimental Approach on Industrial Pd-Ag Supported α-Al2O3 Catalyst Used in Acetylene Hydrogenation Process: Mechanism, Kinetic and Catalyst Decay
Abstract
1. Introduction
1.1. Hydrogenation Catalysts
1.2. Hydrogenation Method
1.3. Reactor Arrangement
1.4. Research Outlook
2. Experimental Method
2.1. Catalyst Preparation
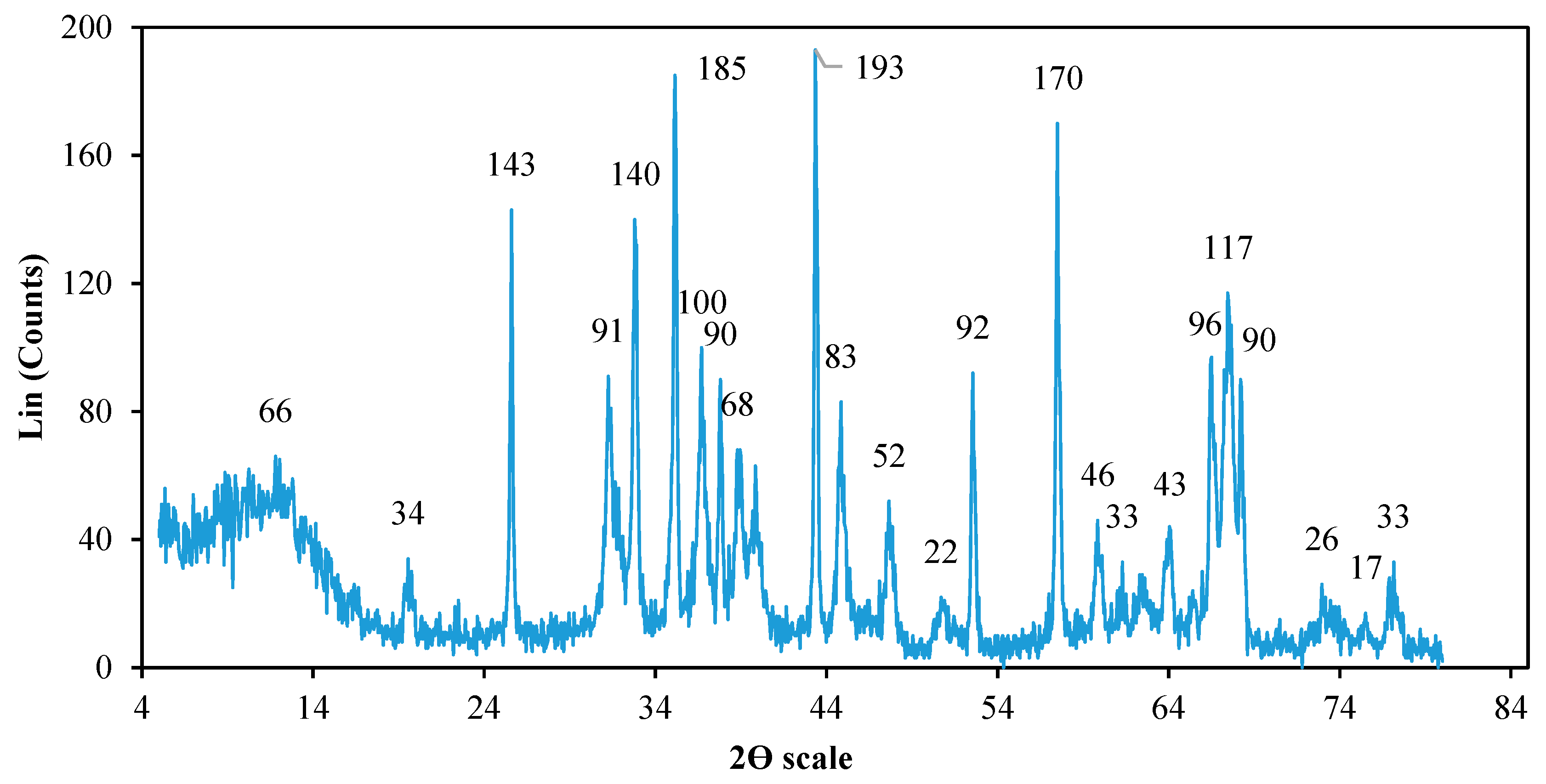
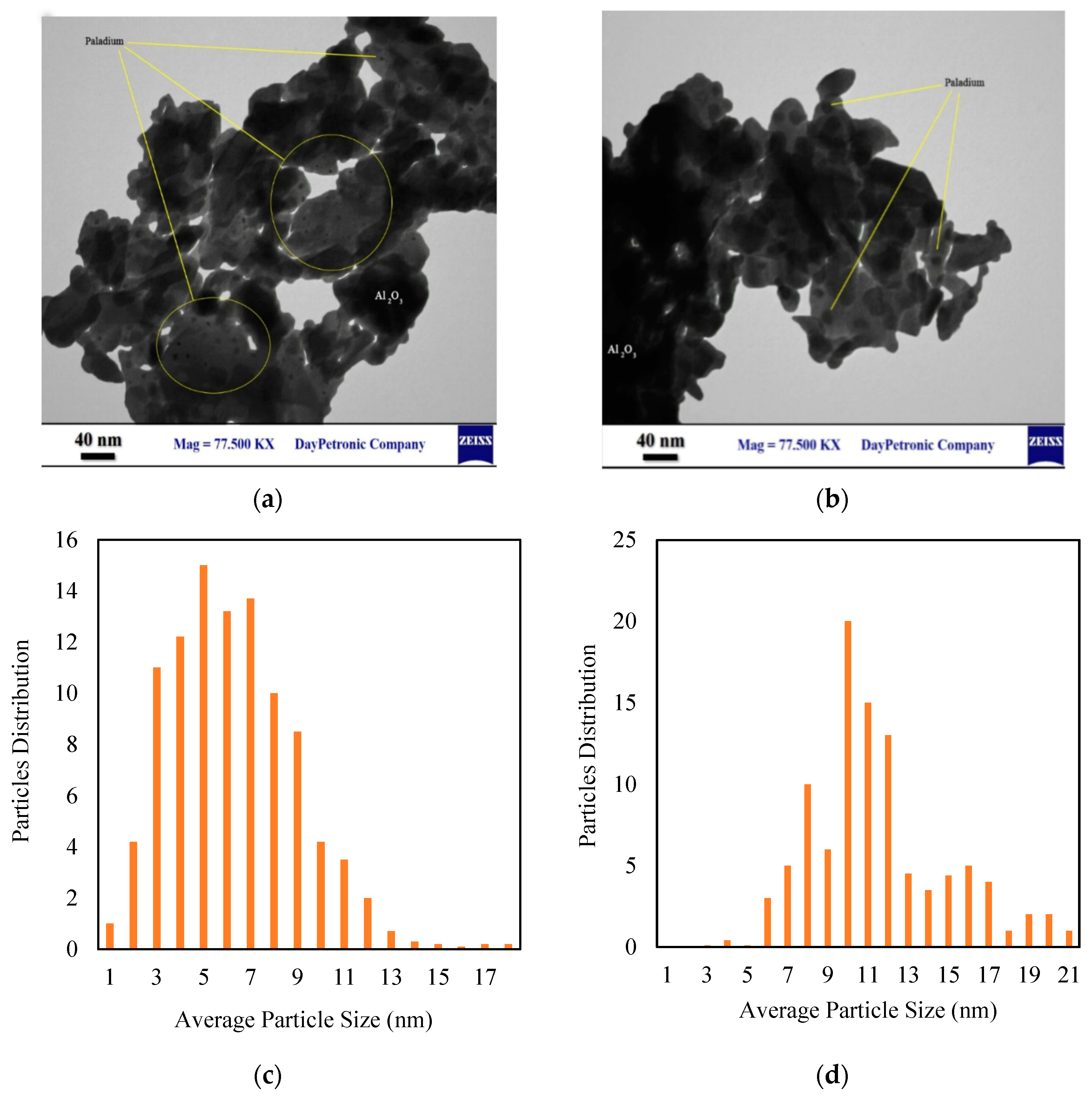
2.2. Catalyst Characterization
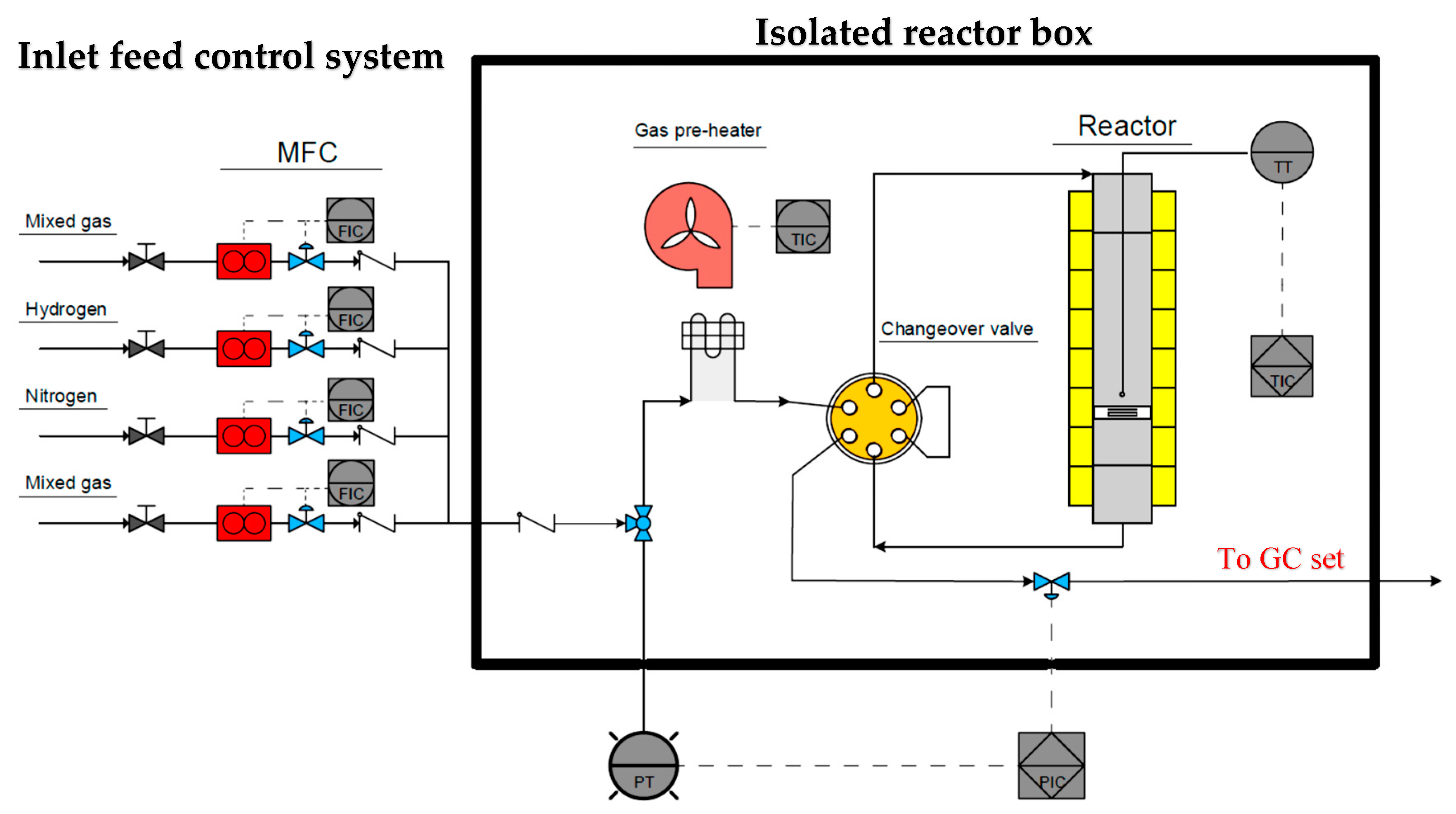
2.3. Experimental Apparatus
3. Kinetic Modeling
3.1. Experiment Design
3.2. Reaction Mechanism
3.3. Kinetic Model
3.4. Deactivation Model
4. Process Modeling
- Pseudo-steady state condition;
- the plug flow pattern in the reactor;
- negligible concentration and temperature gradients in the catalyst particle;
- negligible radial mass and energy diffusion;
- negligible mass and heat transfer in the longitudinal direction; and
- adiabatic conditions.
5. Optimization Problem
6. Results and Discussions
6.1. Catalyst Characteristics
6.2. Results of Kinetic Model
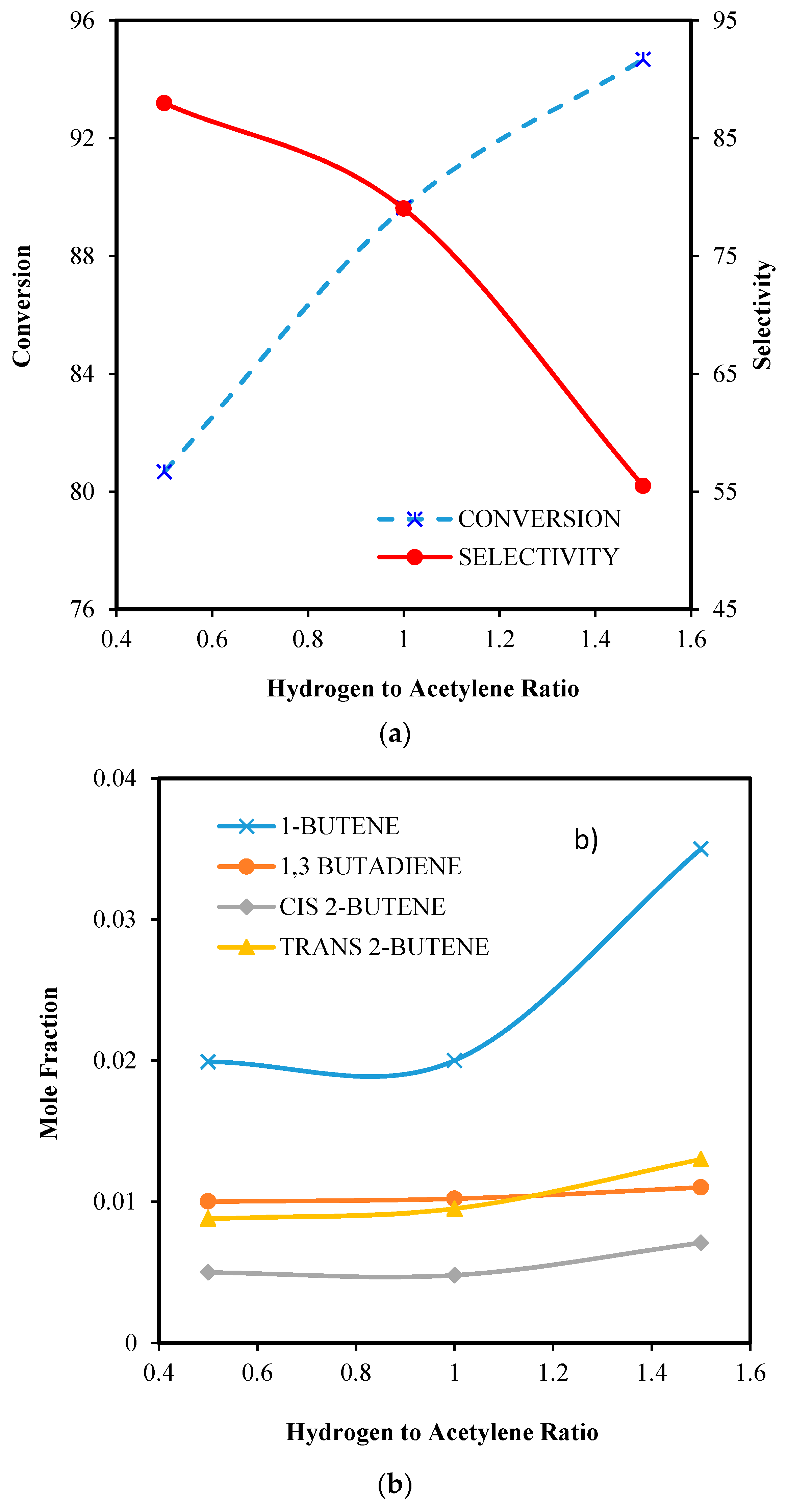
6.2.1. Effect of GHSV
6.2.2. Effect of Pressure
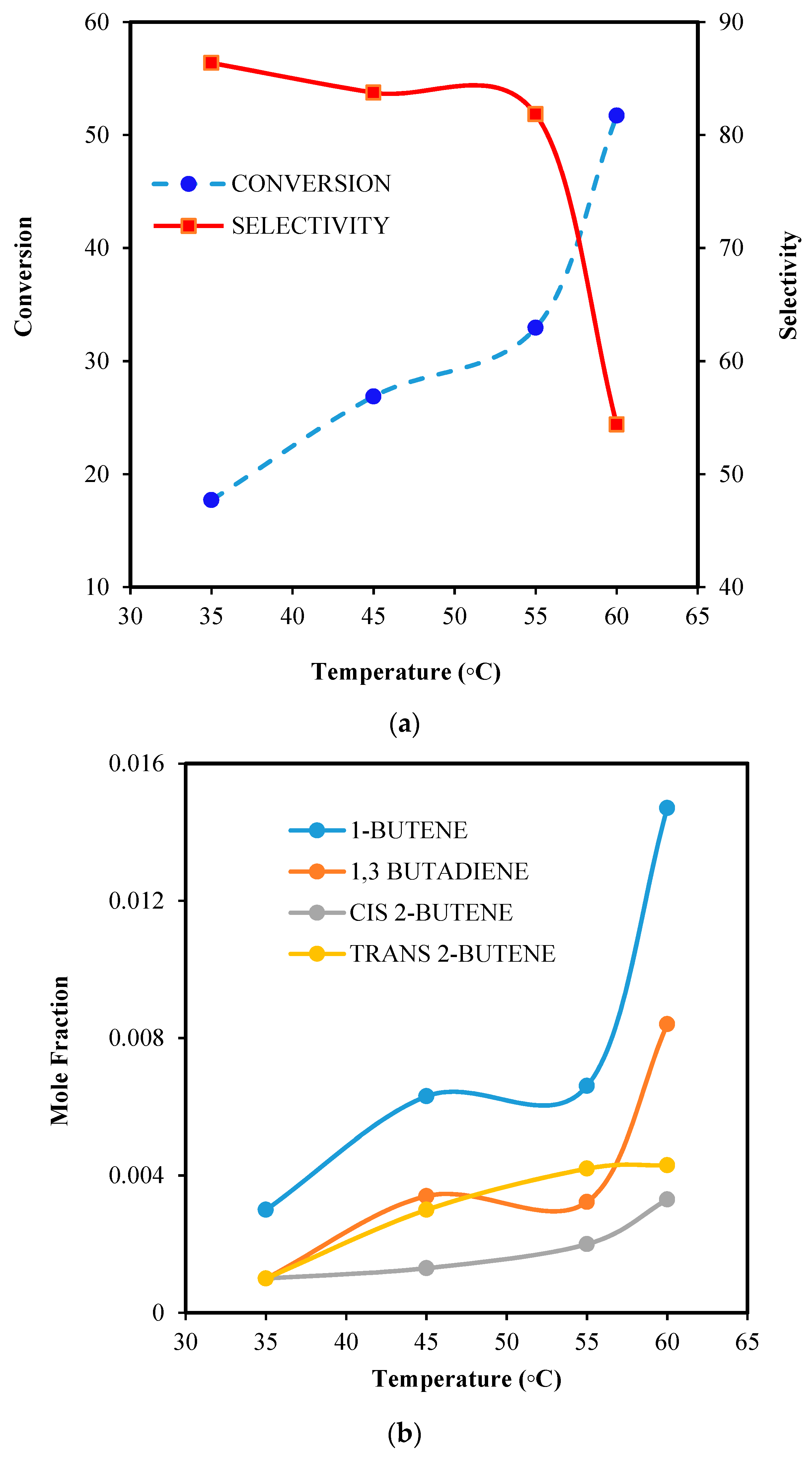
6.2.3. Effect of Temperature
6.2.4. Effect of Hydrogen to Acetylene Ratio
6.2.5. Developed Kinetic and Decay Models
6.3. Results of Process Simulation
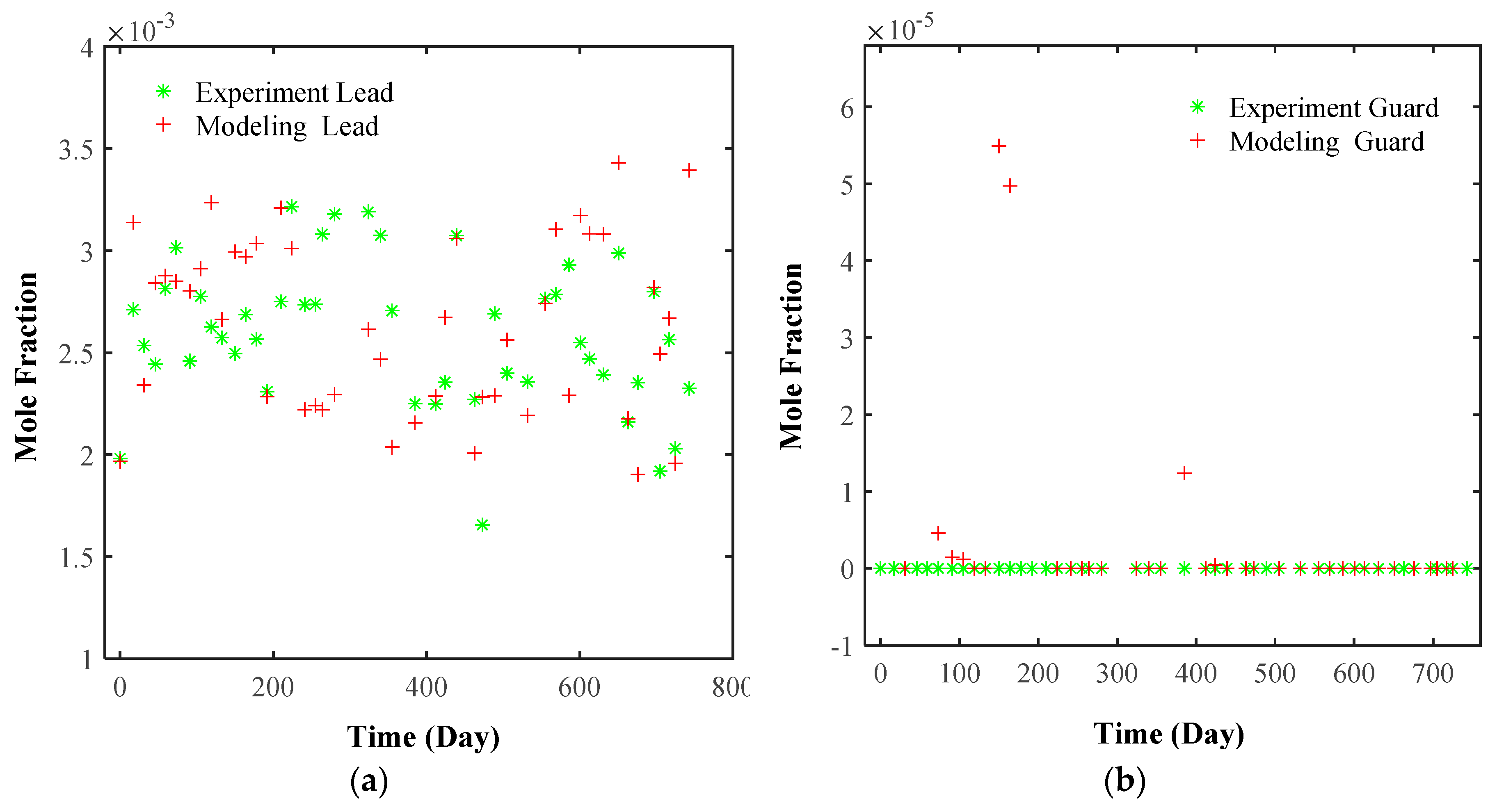
6.3.1. Model Validation
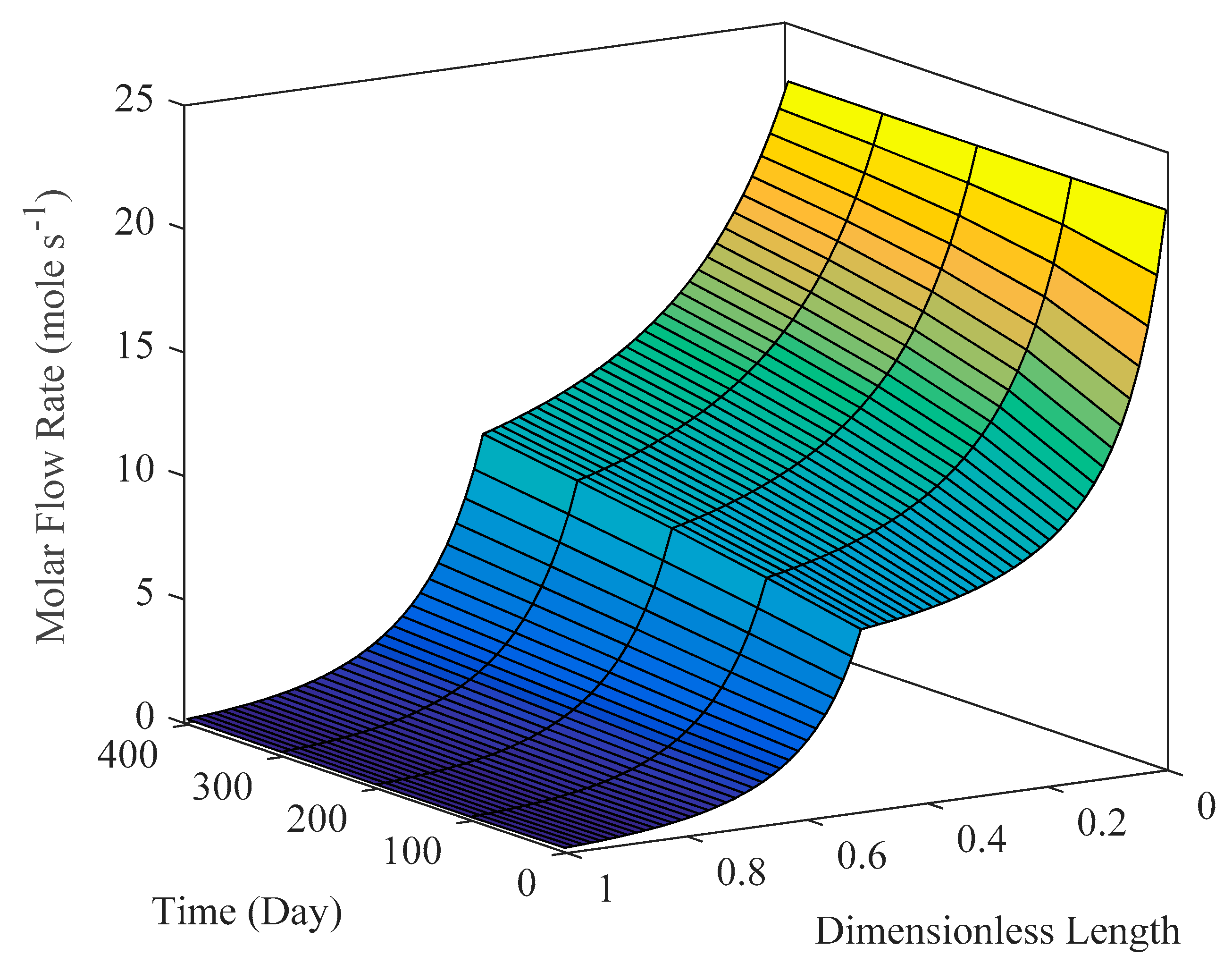
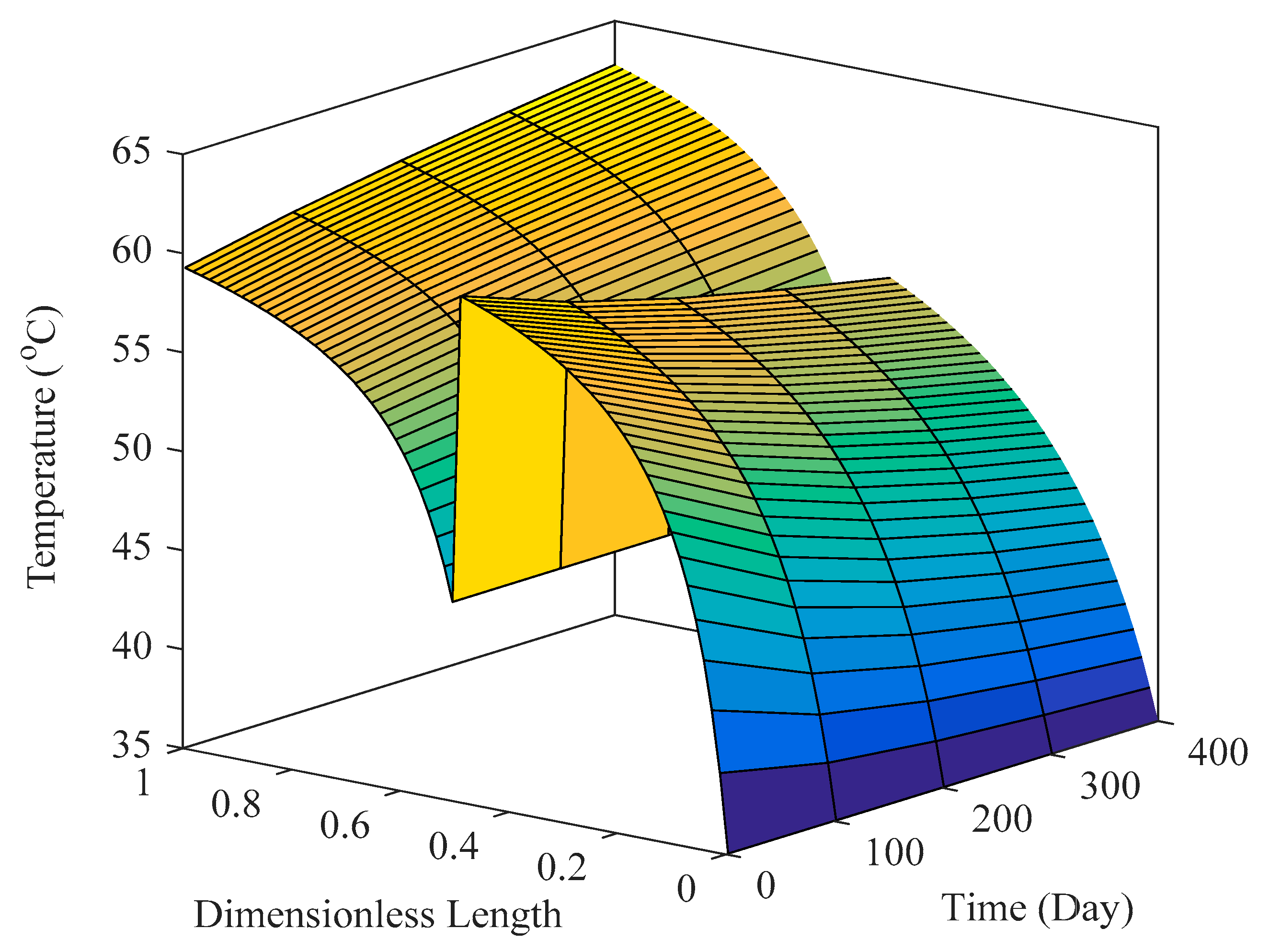
6.3.2. Reactor Simulation
7. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
Nomenclature
| ΔH | enthalpy of reaction |
| MFC | mass flow control |
| TIC | temperature indicator controller |
| PIC | pressure indicator controller |
| FIC | flow indicator controller |
| Re | Reynolds number |
| L | reactor length |
| D | reactor diameter |
| dp | catalyst diameter |
| Qg | gas flow rate (experimental) |
| GHSV | gas hourly space velocity |
| ri | overall rate of reaction |
| k | constant of reaction |
| K | constant of adsorption |
| P | pressure |
| n | power of reaction rate nominator |
| α | power of reaction rate denominator |
| A0 | Arrenius type constant |
| Aij | constant of adsorption |
| R | gas constant |
| Rj | local (component) rate of reaction |
| Vcat | volume of catalyst |
| a | activity of catalyst |
| T | temperature |
| t | time |
| E | activation energy |
| Ed | activation energy of deactivation equation |
| kd | constant of deactivation equation |
| GC | gas chromatography |
| mi | power of nominator and denominator of reaction rate equation (i = 1–12) |
| ni | power of nominator of deactivation equation (i = 1–2) |
| MW | molecular weight |
| TC | critical temperature |
| PC | critical pressure |
| Cp | heat capacity |
| A,B,C,D | constant of heat capacity equation |
| μcr | critical viscosity |
| μ | viscosity |
| A | surface area |
| Z | length |
| NA | mole flux |
| ρb | bulk density |
| ε | porosity |
| MRE | mean relative error (N: number of component), (exp: experiment) |
| Z | z factor |
| S | vacant site |
| s | Entropy |
References
- Adams, D.; Blankenship, S.; Geyer, I.; Takenaka, T. Front end & back end acetylene converter catalysts. In Proceedings of the 3rd Asian Ethylene Symposium on Catalyst and Processes, Yokohama, Japan, 4–6 October 2000; pp. 4–6. [Google Scholar]
- Barazandeh, K.; Dehghani, O.; Hamidi, M.; Aryafard, E.; Rahimpour, M.R. Investigation of coil outlet temperature effect on the performance of naphtha cracking furnace. Chem. Eng. Res. Des. 2015, 94, 307–316. [Google Scholar] [CrossRef]
- Huang, W.; McCormick, J.R.; Lobo, R.F.; Chen, J.G. Selective hydrogenation of acetylene in the presence of ethylene on zeolite-supported bimetallic catalysts. J. Catal. 2007, 246, 40–51. [Google Scholar] [CrossRef]
- Miller, S.A. Acetylene: Its Properties, Manufacture, and Uses; Academic Press: Cambridge, MA, USA, 1966; Volume 2. [Google Scholar]
- Kadiva, A.; Sadeghi, M.T.; Sotudeh-Gharebagh, R.; Mahmudi, M. Estimation of kinetic parameters for hydrogenation reactions using a genetic algorithm. Chem. Eng. Technol. 2009, 32, 1588–1594. [Google Scholar] [CrossRef]
- Mansoornejad, B.; Mostoufi, N.; Jalali-Farahani, F. A hybrid GA–SQP optimization technique for determination of kinetic parameters of hydrogenation reactions. Comput. Chem. Eng. 2008, 32, 1447–1455. [Google Scholar] [CrossRef]
- Mostoufi, N.; Ghoorchian, A.; Sotudeh-Gharebagh, R. Hydrogenation of acetylene: Kinetic studies and reactor modeling. In. J. Chem. React. Eng. 2005, 3. [Google Scholar] [CrossRef]
- Ravanchi, M.T.; Sahebdelfar, S.; Komeili, S. Acetylene selective hydrogenation: A technical review on catalytic aspects. Rev. Chem. Eng. 2018, 34, 215–237. [Google Scholar] [CrossRef]
- Bos, A.; Botsma, E.; Foeth, F.; Sleyster, H.; Westerterp, K. A kinetic study of the hydrogenation of ethyne and ethene on a commercial Pd/Al2O3 catalyst. Chem. Eng. Process. Process Intensif. 1993, 32, 53–63. [Google Scholar] [CrossRef]
- Borodziński, A. Hydrogenation of acetylene–ethylene mixtures on a commercial palladium catalyst. Catal. Lett. 1999, 63, 35–42. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Liu, X.; Zhu, Q. Synergetic effect of pd and ag dispersed on Al2O3 in the selective hydrogenation of acetylene. Appl. Catal. A Gen. 2000, 197, 221–228. [Google Scholar] [CrossRef]
- Sarkany, A.; Horvath, A.; Beck, A. Hydrogenation of acetylene over low loaded pd and pd-au/SiO2 catalysts. Appl. Catal. A Gen. 2002, 229, 117–125. [Google Scholar] [CrossRef]
- Schbib, N.S.; García, M.A.; Gígola, C.E.; Errazu, A.F. Kinetics of front-end acetylene hydrogenation in ethylene production. Ind. Eng. Chem. Res. 1996, 35, 1496–1505. [Google Scholar] [CrossRef]
- Flick, K.; Herion, C.; Allmann, H.-M. Supported Palladium Catalyst for Selective Catalytic Hydrogenation of Acetylene in Hydrocarbonaceous Streams. U.S. Patent US5856262A, 5 January 1999. [Google Scholar]
- Khan, N.A.; Shaikhutdinov, S.; Freund, H.-J. Acetylene and ethylene hydrogenation on alumina supported pd-ag model catalysts. Catal. Lett. 2006, 108, 159–164. [Google Scholar] [CrossRef]
- Aduriz, H.; Bodnariuk, P.; Dennehy, M.; Gigola, C. Activity and selectivity of Pd/α-Al2O3 for ethyne hydrogenation in a large excess of ethene and hydrogen. Appl. Catal. 1990, 58, 227–239. [Google Scholar] [CrossRef]
- Pachulski, A.; Schödel, R.; Claus, P. Performance and regeneration studies of Pd–Ag/Al2O3 catalysts for the selective hydrogenation of acetylene. Appl. Catal. A Gen. 2011, 400, 14–24. [Google Scholar] [CrossRef]
- Tejeda-Serrano, M.A.; Mon, M.; Ross, B.; Gonell, F.; Ferrando-Soria, J.S.; Corma, A.; Leyva-Pérez, A.; Armentano, D.; Pardo, E. Isolated Fe (iii)–o sites catalyze the hydrogenation of acetylene in ethylene flows under front-end industrial conditions. J. Am. Chem. Soc. 2018, 140, 8827–8832. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, R.; Soares, R.d.P.; Lansarin, M.A.; Secchi, A.R.; Ferreira, J.M.P. Modeling, simulation, and optimization of a front-end system for acetylene hydrogenation reactors. Braz. J. Chem. Eng. 2004, 21, 545–556. [Google Scholar] [CrossRef]
- Aeowjaroenlap, H.; Chotiwiriyakun, K.; Tiensai, N.; Tanthapanichakoon, W.; Spatenka, S.; Cano, A. Model-based optimization of an acetylene hydrogenation reactor to improve overall ethylene plant economics. Ind. Eng. Chem. Res. 2018, 57, 9943–9951. [Google Scholar] [CrossRef]
- Samavati, M.; Ebrahim, H.A.; Dorj, Y. Effect of the operating parameters on the simulation of acetylene hydrogenation reactor with catalyst deactivation. Appl. Catal. A Gen. 2018, 567, 45–55. [Google Scholar] [CrossRef]
- Khold, O.D.; Parhoudeh, M.; Rahimpour, M.R.; Raeissi, S. A new configuration in the tail-end acetylene hydrogenation reactor to enhance catalyst lifetime and performance. J. Taiwan Inst. Chem. Eng. 2016, 65, 8–21. [Google Scholar] [CrossRef]
- Kirk, R.E. Experimental design. In The Blackwell Encyclopedia of Sociology; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Bos, A.; Westerterp, K. Mechanism and kinetics of the selective hydrogenation of ethyne and ethene. Chem. Eng. Process. Process Intensif. 1993, 32, 1–7. [Google Scholar] [CrossRef]
- Oudar, J. Deactivation and Poisoning of Catalysts; CRC Press: Boca Raton, FL, USA, 1985; Volume 20. [Google Scholar]
- Fogler, H.S. Elements of Chemical Reaction Engineering; Prentice Hall: Upper Sanddle River, NJ, USA, 1999. [Google Scholar]
- Bartholomew, C. Catalyst deactivation and regeneration. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Sarkany, A.; Guczi, L.; Weiss, A.H. On the aging phenomenon in palladium catalysed acetylene hydrogenation. Appl. Catal. 1984, 10, 369–388. [Google Scholar] [CrossRef]
- Robinson, D. Catalyst regeneration, metal catalysts. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Anderson-Cook, C.M. Practical Genetic Algorithms; Taylor & Francis: Abingdon, UK, 2005. [Google Scholar]
- Boudart, M.; Djéga-Mariadassou, G. Kinetics of Heterogeneous Catalytic Reactions; Princeton University Press: Princeton, NJ, USA, 2014; Volume 767. [Google Scholar]





| Bulk Density (kg m−3) | 720 |
| Size (mm) | 2–4 |
| Shape | Sphere |
| Pd content (ppm) | 300 |
| Ag to Pd ratio | 6 |
| Particle porosity (%) | 60–70% |
| Particle tortuosity | 2.5 |
| BET Surface Area (m2 g−1) | 30.1062 |
| BJH Adsorption average pore diameter (Å) | 291.218 |
| Thermal conductivity of catalyst (W m−1 k−1) | 0.29 |
| Methane | 0.014 |
| Acetylene | 0.738 |
| Ethylene | 64.594 |
| Propane | 0.002 |
| Propylene | 0.199 |
| Ethane | 34.449 |
| Other C4s | 0.0023 |
| MAPD | 0.0005 |
| Cyclopropane | <0.0001 |
| C5+ Hydrocarbons | <0.0001 |
| 1,3 Butadiene | <0.0001 |
| Lower | Upper | Number of Levels | |
|---|---|---|---|
| Hydrogen to acetylene ratio | 0.5 | 1.5 | 3 |
| Pressure (Bar) | 15 | 20 | 3 |
| Temperature | 35 | 60 | 4 |
| GHSV | 2600 | 6200 | 6 |
| Fresh | Spent | |
|---|---|---|
| BET surface area (m2 g−1) | 24.75 | 30.11 |
| Langmuir surface area (m2 g−1) | 34.13 | 41.86 |
| External surface area (m2 g−1) | 20.15 | 27.27 |
| Micro pore area (m2 g−1) | 4.6018 | 2.84 |
| Adsorption average pore width (Å) | 235.52 | 191.17 |
| Adsorption cumulative volume of pores (cm3 g−1) | 0.229 | 0.218 |
| Reaction | Proposed Kinetic Model |
|---|---|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehghani, O.; Rahimpour, M.R.; Shariati, A. An Experimental Approach on Industrial Pd-Ag Supported α-Al2O3 Catalyst Used in Acetylene Hydrogenation Process: Mechanism, Kinetic and Catalyst Decay. Processes 2019, 7, 136. https://doi.org/10.3390/pr7030136
Dehghani O, Rahimpour MR, Shariati A. An Experimental Approach on Industrial Pd-Ag Supported α-Al2O3 Catalyst Used in Acetylene Hydrogenation Process: Mechanism, Kinetic and Catalyst Decay. Processes. 2019; 7(3):136. https://doi.org/10.3390/pr7030136
Chicago/Turabian StyleDehghani, Ourmazd, Mohammad Reza Rahimpour, and Alireza Shariati. 2019. "An Experimental Approach on Industrial Pd-Ag Supported α-Al2O3 Catalyst Used in Acetylene Hydrogenation Process: Mechanism, Kinetic and Catalyst Decay" Processes 7, no. 3: 136. https://doi.org/10.3390/pr7030136
APA StyleDehghani, O., Rahimpour, M. R., & Shariati, A. (2019). An Experimental Approach on Industrial Pd-Ag Supported α-Al2O3 Catalyst Used in Acetylene Hydrogenation Process: Mechanism, Kinetic and Catalyst Decay. Processes, 7(3), 136. https://doi.org/10.3390/pr7030136






